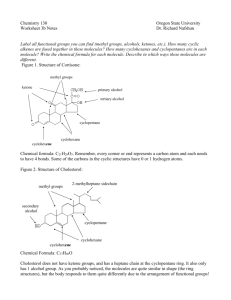
Methyl eugenol
advertisement

Screening Assessment for the Challenge Benzene, 1,2-dimethoxy-4-(2-propenyl)(Methyl eugenol) Chemical Abstracts Service Registry Number 93-15-2 Environment Canada Health Canada September 2010 Screening Assessment CAS RN 93-15-2 Synopsis The Ministers of the Environment and of Health have conducted a screening assessment of benzene, 1,2-dimethoxy-4-(2-propenyl)-, (commonly called methyl eugenol), Chemical Abstracts Service Registry Number 93-15-2. Methyl eugenol was identified in the categorization of the Domestic Substances List as a high priority for action under the Ministerial Challenge, as it was considered to pose an intermediate potential for exposure of individuals in Canada and it had been classified by the United States National Toxicology Program on the basis of carcinogenicity. This substance was not considered to be a high priority for assessment of potential risks to the environment as it did not meet the ecological categorization criteria for persistence, bioaccumulation potential or inherent toxicity to aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of human health. Methyl eugenol is an organic substance that occurs naturally in the essential oils of several plant species. These oils are extracted for use principally as flavour ingredients in food and beverages and as fragrance ingredients and emollients in personal care products. Methyl eugenol can be a component of citronella oil, which is registered as an active ingredient in personal insect repellent in Canada. Based on information reported pursuant to section 71 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), methyl eugenol was not reported to be manufactured in Canada in 2006, and less than 100 kg of the substance was imported into the country in the same calendar year. Methyl eugenol is considered to be ubiquitous in air and water at very low concentrations. The predominant source of exposure to the general population is expected to be its naturally occurring presence in food and beverages, with smaller contributions from the use of personal care products and citronella oil based personal insect repellents. Based principally on the weight of evidence–based assessments of international or other national agencies, a critical effect for the characterization of risk to human health for methyl eugenol is carcinogenicity. In the standard 2-year carcinogenicity studies with rats and mice, methyl eugenol induced multiple types of tumours in both males and females in a dose-related manner. Of note, the significantly increased incidences of liver tumours were observed at the lowest dose tested in both rats and mice in the chronic studies. Methyl eugenol was genotoxic in a range of in vivo and in vitro assays, although it was not mutagenic in bacterial cells. Methyl eugenol bound to liver DNA and formed DNA adducts in vivo and in vitro. In addition, methyl eugenol caused gene mutation in the liver of transgenic animals and induced mutation of β-catenin gene in mouse liver tumours. While the mode of induction of tumours has not been fully elucidated, based on genotoxicity of methyl eugenol, it cannot be precluded that methyl eugenol induces tumours via a mode of action involving direct interaction with genetic material. Methyl eugenol is also associated with non-cancer effects in experimental animals including cytological alteration, necrosis, hyperplasia, atrophy, organ or body weight changes in rats and mice. The critical non-cancer effect was reduced body weight or body ii Screening Assessment CAS RN 93-15-2 weight gain. With respect to non-cancer effects, comparison of the critical effect level with upper-bounding estimates of exposure to the general population from the use of methyl eugenol-containing personal care products and citronella oil (containing methyl eugenol) based personal insect repellents results in margins of exposure that are considered adequate. On the basis of the carcinogenic potential of methyl eugenol, for which there may be a probability of harm at any exposure level, it is concluded that methyl eugenol is a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Based on its physical and chemical properties and available limited degradation data, methyl eugenol does not meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA 1999. In addition, both experimental and modelled toxicity data suggest that the substance is only moderately hazardous to aquatic organisms. Given the low quantity of methyl eugenol in commerce in Canada, its environmental concentration is predicted to be well below the predicted no effect concentration. On this basis it is concluded that methyl eugenol is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase. Based on the information available, it is concluded that methyl eugenol meets one or more of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999. iii Screening Assessment CAS RN 93-15-2 Introduction The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that were prioritized during the categorization of substances on the Domestic Substances List (DSL) to determine whether these substances present or may present a risk to the environment or to human health. Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that • • met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), which challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities. The substance methyl eugenol was identified as a high priority for assessment of human health risk because it was considered to present an IPE and had been classified by other agencies on the basis of carcinogenicity. The Challenge for methyl eugenol was published in the Canada Gazette on March 14, 2009 (Canada 2009). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the substance were received. Although methyl eugenol was determined to be a high priority for assessment with respect to human health, it did not meet the criteria for persistence, bioaccumulation potential or inherent toxicity to aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health. Screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of CEPA 1999. 1 Screening Assessment CAS RN 93-15-2 Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution 1 . This final screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents and stakeholder research reports and from recent literature searches, up to November 2009 for ecological effects and March 2010 for human health effects and exposure 2 . Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of (non-occupational) exposure of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The final screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the existing critical information upon which the conclusion is based. This final screening assessment was prepared by staff in the existing substances programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from Dr. Bernard Gadagbui, Toxicology Excellence for Risk Assessment; Dr. Michael Jayjock, The LifeLine Group; and Dr. Chris Bevans, CJB Consulting. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel. 1 A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge Batches 1-12 is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of regulatory framework for the Workplace Hazardous Materials Information System [WHMIS] for products intended for workplace use. 2 A publication was identified after finalizing the assessment and is added to the list of references for completeness (Smith et al, 2010) 2 Screening Assessment CAS RN 93-15-2 The critical information and considerations upon which the final assessment is based are summarized below. 3 Screening Assessment CAS RN 93-15-2 Substance Identity For the purposes of this document, benzene, 1,2-dimethoxy-4-(2-propenyl)- will be referred to as methyl eugenol, derived from the Philippine Inventory of Chemicals and Chemical Substances (PICCS). Information on the identity of methyl eugenol is summarized in Table 1. Table 1. Substance identity for methyl eugenol Chemical Abstracts Service Registry Number (CAS RN) DSL name National Chemical Inventories (NCI) names1 Other names Chemical group (DSL Stream) Major chemical class or use Major chemical sub-class Chemical formula 93-15-2 Benzene, 1,2-dimethoxy-4-(2-propenyl)4-Allylveratrole (REACH, EINECS) Benzene, 1,2-dimethoxy-4-(2-propen-1-yl)- (TSCA) Benzene, 1,2-dimethoxy-4-(2-propenyl)- (AICS, ASIAPAC, ENCS, NZIoC, PICCS, SWISS) 1,2-Dimethoxy-4-(2-propenyl)benzene (ECL) Eugenyl methyl ether extra (PICCS) Methyl eugenol (PICCS) 1,2-Dimethoxy-4-allylbenzene 1,3,4-Eugenol methyl ether 1-(3,4-Dimethoxyphenyl)-2-propene 1-Allyl-3,4-dimethoxybenzene 3,4-Dimethoxy-1-(2-propenyl)benzene 3,4-Dimethoxyallylbenzene 3-(3,4-Dimethoxyphenyl)propene 4-Allyl-1,2-dimethoxybenzene Benzene, 4-allyl-1,2-dimethoxyChavibetol methyl ether Ent 21040 Eugenyl methyl ether Methyl ether Methyl eugenyl ether Methylchavibetol Methyleugenol NSC 209528 NSC 8900 O-Methyleugenol Veratrole methyl ether Veratrole, 4-allyl-Discrete organics Aromatic ether Alkoxy allylbenzene C11H14O2 4 Screening Assessment CAS RN 93-15-2 Chemical structure SMILES2 Molecular mass O(c(c(OC)cc(c1)CC=C)c1)C 178.23 g/mol Abbreviations: AICS, Australian Inventory of Chemical Substances; ASIA-PAC, Asia-Pacific Substances Lists; CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List; ECL, Korean Existing Chemicals List; EINECS, European Inventory of Existing Commercial Chemical Substances; ENCS, Japanese Existing and New Chemical Substances; NZIoC, New Zealand Inventory of Chemicals; PICCS, Philippine Inventory of Chemicals and Chemical Substances; REACH, Registration, Evaluation, Authorisation and Restriction of Chemical substances; SWISS, Swiss Giftliste 1 and Inventory of Notified New Substances; TSCA, Toxic Substances Control Act Chemical Substance Inventory. 1 2 Source: National Chemical Inventories (NCI) 2009 SMILES: Simplified Molecular Input Line Entry Specification Physical and Chemical Properties Table 2 contains experimental and modelled data for the physical and chemical properties of methyl eugenol that are relevant to its environmental fate. Where experimental data were not available, models based on quantitative structure-activity relationships (QSARs) were used to fill data gaps. These models are mainly based on fragment addition methods, i.e. they rely on the structure of a chemical. Based on its physical and chemical properties (Table 2), methyl eugenol is characterized by moderate water solubility (500 mg/L), moderate vapour pressure (modelled 1.6 Pa), low to moderate log Kow (modelled 3.0) and log Koc (modelled 2.7), and low Henry’s Law Constant (0.567 Pa·m3/mol). 5 Screening Assessment CAS RN 93-15-2 Table 2. Physical and chemical properties for methyl eugenol Property Type Value1 Melting point (ºC) Boiling point (ºC) Experimental -4 Experimental 254.7 Experimental Experimental Modelled 249 1032-1036 (1.032-1.036 g/cm3)1 1036 (1.036 g/cm3) 1.6 (0.012 mm Hg) 0.567 (5.60 x 10-6 atm·m3/mol) 3.0 Modelled 2.7 PCKOCWIN 2008 Experimental 500 MITI 1992 Density (kg/m3) Experimental Vapour pressure (Pa) Henry’s Law constant (Pa·m3/mol) Log Kow (Octanol-water partition coefficient, Dimensionless) Log Koc (Organic carbonwater partition coefficient, Dimensionless) Water solubility (mg/L) 1 Extrapolated Experimental Temperature (°C) 25 Lide and Milne 1994 Lide and Milne 1994 MITI 1992 Lewis 2001 Merck 1997 25 Values and units in brackets represent those originally reported by the authors or estimated by the models. 6 Reference Perry and Green 1984 HENRYWIN 2008 KOWWIN 2008 Screening Assessment CAS RN 93-15-2 Sources Methyl eugenol is a naturally occurring substance found in the essential oils of several plant species. The oils are extracted from plants by steam distillation or with organic solvents, typically for use as flavour or fragrance ingredients. The amount of methyl eugenol in an essential oil extracted from a given type of plant varies with the variety, plant maturity at harvest, harvesting method, storage conditions and extraction method (Smith et al. 2002). Methyl eugenol is also manufactured synthetically in small quantities. Annual production in the United States in 1990 was estimated to be 11,400 kg (NTP 2005a); in a more recent report, annual production in the United States was reported as 77 kg (FAO/WHO 2009). There are currently four manufacturers of methyl eugenol in the United States and three manufacturers elsewhere, but none in Canada (2009 email from SRI Consulting to Risk Assessment Bureau, Health Canada; unreferenced). In response to the section 71 notice pursuant to CEPA 1999, no company reported the manufacture, import or use of methyl eugenol in 2006 above the reporting thresholds (i.e., 100 kg for manufacturing and importing, and 1000 kg for using the substance). There are no other data on industrial activity with respect to methyl eugenol in Canada. An extensive listing of the methyl eugenol content of essential oils from different botanical sources is given in Appendix 1, which summarizes data from Burfield (2004). The concentrations of methyl eugenol typically found in essential oils used in consumer products in Canada have not been quantified to date. Uses In Canada, flavouring ingredients such as methyl eugenol or essential oils containing methyl eugenol can be added to any food that does not have a standard of identity and composition in the Food and Drug Regulations and to those foods that have a standard of identity and composition that allows for the addition of flavours to the food. Plant materials such as leaves, stems, and seeds containing methyl eugenol may also be added to foods that do not have a regulatory standard, and to those that have a standard where there is provision for the addition of spices or seasoning. Some examples of common culinary herbs and spices that contain methyl eugenol are basil, tarragon, lemon grass, bay leaf, nutmeg, allspice, cloves and mace. Methyl eugenol is also reported to have been found in oranges, bananas and grapefruit juice (Johnson and Abdo 2005; Smith et al. 2002). Commercially prepared foods in which methyl eugenol may be found include ice cream; baked goods such as cookies, pies, pastries and buns; puddings and other gelatine-based desserts; condiments, soups and sauces, especially pesto; various meat products; candy and chewing gum; and beverages made with spices and herbs containing methyl eugenol (Council of Europe 2001). 7 Screening Assessment CAS RN 93-15-2 Methyl eugenol, when used as a flavouring agent, was classified as GRAS (Generally Recognized as Safe) by the Flavour and Extract Manufacturers Association (FEMA) in 1965 and that classification remained unchanged following a re-evaluation of methyl eugenol by FEMA in 2001 (Smith et al 2002). In the United States, methyl eugenol is a permitted food additive, provided that it is used in the minimum quantity required to produce its intended effect, and otherwise in accordance with all the principles of good manufacturing practice (US FDA 2001). In the European Union, EC Regulation 1334/2008, which will be effective in January 2011, prohibits the addition of methyl eugenol to foods and restricts the concentration of methyl eugenol in compound foods that have been prepared with flavourings or food ingredients with flavouring properties; however, if the only food ingredients with flavouring properties that have been added are fresh, dried or frozen herbs and spices, the maximum limits do not apply for methyl eugenol. For instance, pesto made with basil is permitted in food preparation, regardless of methyl eugenol content. The permitted maximum concentrations range from 1 mg/kg in non-alcoholic beverages up to 60 mg/kg in soups and sauces (European Commission 2008). Some essential oils including citronella (Cymbopogon spp.), basil (Ocimum spp.), bay (Laurus nobilis) and tea tree (Melaleuca spp.) that may contain a high percentage of methyl eugenol are used in fragranced consumer products such as personal care products and household cleaners. The European Union has conducted a risk assessment of methyl eugenol in cosmetic and non-food products. Based on the findings of this assessment, methyl eugenol is permitted in cosmetics as a component of plant extracts only. The permitted concentrations are as follows: 0.01% in fine fragrances, 0.004% in eau de toilette, 0.002% in a fragrance cream, 0.0002% in other leave-on products and oral hygiene products, and 0.001% in rinse-off products. Methyl eugenol may not be added as a pure chemical to cosmetics (European Commission 2000a; b). These concentration limits on the methyl eugenol content of essential oils in cosmetic products were adopted by Canada and are outlined in the Cosmetic Ingredients Hotlist (available at http://www.hc-sc.gc.ca/cpsspc/person/cosmet/info-ind-prof/_hot-list-critique/prohibited-eng.php) (Health Canada 2007). In Canada, citronella oil, which can contain methyl eugenol, is an active ingredient in some commercially available personal insect repellent lotions and sprays applied to the skin. In 2004, under the Pest Control Products Act, Health Canada conducted a reevaluation of the safety of citronella oil use in personal insect repellents. As a result of that review (PACR2004-36) (Health Canada 2004), and a review by a scientific advisory panel, Health Canada recommended adopting the methyl eugenol concentration limits proposed by the European Commission (REV2008-03) (Health Canada 2008). Health Canada has requested information on the levels of methyl eugenol in insect repellents containing citronella oil and will propose a phase out plan for personal insect repellents 8 Screening Assessment CAS RN 93-15-2 containing citronella oil as an active ingredient if additional information to confirm the safe use of these products is not provided. Methyl eugenol is also a component of certain fragrances present in 15 pest control products in Canada, with a resulting methyl eugenol concentration ranging from of 0.00233% to 0.005 %. However, none of these pesticides are registered for use on food. (2009 email from PMRA to Risk Assessment Bureau, Health Canada; unreferenced). In the United States, methyl eugenol is used as a bait attractant for insect traps and lure products for control of fruit flies in fields and orchards (US EPA 2006). In addition to its use in personal insect repellents, citronella oil is used in outdoor candles and torches as an area insect repellent. The tobacco of flavoured bidis and clove cigarettes has been analysed for a number of alkenylbenzene compounds, among them methyl eugenol. The concentration of methyl eugenol found in the cigarettes in this study ranged from not detected to 61 μg/g in strawberry-flavoured tobacco (Stanfill et al. 2003). The source of methyl eugenol in flavoured tobacco is presumed to be in the flavouring and not the cured tobacco. In May 2009, the Government of Canada introduced amendments to the Tobacco Act to prohibit the selling of cigarettes, little cigars and blunt wraps (leaf-wrapped tobacco) with flavours and additives that taste like candy (Health Canada 2009). Essential oils are sold to individuals who choose to make their own preparations. Essential oils are used in a number of specialized applications such as aromatherapy, as ingredients of massage oils and in alternative medicine practices, among others. Methyl eugenol is a component of several essential oils which may be used in these practices (see Appendix 1). Methyl eugenol is listed in the Natural Health Products Ingredients Database. Health Canada does not authorize the use of pure methyl eugenol for either medicinal or nonmedicinal purposes in oral and topical Natural Health Products. More information regarding safe use of methyl eugenol and natural products that contain methyl eugenol can be found in the Natural Health Products Ingredients Database (available at http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do). Releases to the Environment There are very limited data on which to base an estimate of releases of methyl eugenol to the environment. There are no known industrial sources of methyl eugenol releases to the Canadian environment; however, it is expected that, as in the United States (Barr et al. 2000), this substance is ubiquitous in air and water at low part per trillion levels. Environmental Fate 9 Screening Assessment CAS RN 93-15-2 Based on its physical and chemical properties (Table 2), the Level III fugacity model has been used to predict the environmental partitioning for methyl eugenol, with consideration of the half-lives in air (estimated as 5 hours, HSDB 1983-2009), water (measured as 8 days, CHRIP c2008), soil (8 days estimated as the same as half-life in water), and sediments (32 days estimated as four times the half-life in water). The results from the modelling suggest that methyl eugenol is expected to mainly reside in the environmental compartment to which it is released (Table 3). Table 3. Results of Level III fugacity modelling (EQC 2003) Substance released to: Air (100%) Water (100%) Soil (100%) Percentage of substance partitioning into each compartment Air Water Soil Sediment 80.7 9.2 10.0 0.1 <0.1 99.2 <0.1 0.7 <0.1 0.5 99.5 <0.1 Persistence and Bioaccumulation Potential Environmental Persistence Both empirical and modelled data were available and used in a weight of evidence approach to determine the environmental persistence and bioaccumulation potential of the substance. In the atmosphere, methyl eugenol is not expected to undergo photolysis due to the lack of absorption in the environmental UV spectrum (>298 nm) (Meylan and Howard 1993). If released to air, the substance will degrade by reaction with photochemicallyproduced hydroxyl radicals. The half-life for this reaction in air is estimated to be 5 hours (HSDB 1983-2009). In water, methyl eugenol is not expected to undergo hydrolysis in the environment due to the lack of hydrolysable functional groups. The empirical data from a biodegradation study (HSDB 1983-2009) show that there is approximately 90% ultimate biodegradation over 28 days using an activated sludge inoculum in a ready-biodegradation test for methyl eugenol (Table 4a). The rapid degradation observed in the test can be translated into a half-life of approximately 8 days in water, assuming first order degradation kinetics. The substance is therefore not expected to persist in water. Shaver and Bull (1980) identified a dissipation half-life of 34 hours in water and 16 hours in soil. Although they did not determine a mechanism for the dissipation, they speculated that the losses were mostly a result of evaporation. 10 Screening Assessment CAS RN 93-15-2 Table 4a. Empirical data for degradation of methyl eugenol Medium Fate Process Air Photochemical reaction Biodegradation Activated sludge inoculum Degradation Value 5 Degradation Endpoint (Units) Half-life (hour) Reference 90 Biodegradation (% over 28 days) HSDB 1983-2009 HSDB 1983-2009 In addition to experimental data on the degradation of methyl eugenol, a QSAR-based weight-of-evidence approach (Environment Canada 2007) was applied using the degradation models (see Table 4b below). Given the ecological importance of the water compartment and the fact that most of the available models apply to water (BIOWIN 2009), biodegradation in water was primarily examined. Methyl eugenol has been included in the training set in BIOWIN 2009 for developing the MITI biodegradation model. It is also within the domains of TOPKAT and CATABOL for predicting biodegradation in water. These QSARs are therefore expected to model methyl eugenol well. Table 4b summarizes the results from available QSAR models for degradation of methyl eugenol in water and air. Table 4b. Modelled data for degradation of methyl eugenol Fate Process AIR Atmospheric oxidation Ozone reaction WATER Biodegradation (aerobic) Biodegradation (aerobic) Biodegradation (aerobic) Biodegradation (aerobic) Biodegradation (aerobic) Model And Model Basis Model Result and Prediction Extrapolated Half-life (days) AOPWIN 2008 t 1/2 = 0.14 days (3.4 hours) t 1/2 = 0.96 days (23 hours) <2 2.61 “biodegrades fast” < 182 3.681 “biodegrades fast” < 182 0.562 “biodegrades fast” < 182 0.602 “biodegrades fast” < 182 1.002 “biodegrades fast” < 182 AOPWIN 2008 BIOWIN 2009 Sub-model 3: Expert Survey (ultimate biodegradation) BIOWIN 2009 Sub-model 4: Expert Survey (primary biodegradation) BIOWIN 2009 Sub-model 5: MITI linear probability BIOWIN 2009 Sub-model 6: MITI non-linear probability TOPKAT 2004 Probability 11 <2 Screening Assessment Biodegradation (aerobic) 1 2 CAS RN 93-15-2 CATABOL c2004−2008 % BOD (biological oxygen demand) % BOD = 73.1 “biodegrades fast” < 182 Output is a numerical score from 0 to 5 Output is a probability score In air, a predicted atmospheric oxidation half-life of 0.14 day (see Table 4b) demonstrates that methyl eugenol is likely to be rapidly oxidized. It is also predicted to react quickly with ozone, with a half-life value of 0.96 days. Based on experimental data (HSDB 19832009) and model predictions, the substance is considered to be not persistent in air. QSAR models were also used to predict the biodegradation potential in water. The BIOWIN (2009) aerobic biodegradation models (BIOWIN submodels 3, 4, 5 and 6) suggest that methyl eugenol biodegrades rapidly. The BIOWIN submodels 5 and 6 probability results are both greater than 0.3, the cut-off suggested by Aronson et al. (2006) to identify substances as having a half-life <60 days (based on the MITI probability models). The other two ultimate degradation models, TOPKAT and CATABOL, both predict that the substance may biodegrade rapidly in water. There is sufficient confidence, based on the experimental data together with support from aerobic models (in Table 4b), to predict that methyl eugenol biodegrades rapidly and the half-life is far below 90 days. According to experimental data (MITI 1992) and model predictions, methyl eugenol is not considered persistent in water. To extrapolate a half-life in water to half-lives in soil and sediment, Boethling’s factors t½ water:t½ soil:t½ sediment = 1:1:4 (Boethling et al. 1995) were applied. Using the half-life in water of approximately 8 days (estimated from the MITI ready biodegradation test result assuming first order kinetics) and the extrapolation factors, the half-lives in soil and sediment are estimated to be about 8 and 32 days. It is thus concluded that methyl eugenol is not persistent soil and sediment. Based on the empirical and modelled data, it is concluded that methyl eugenol does not meet the persistence criteria in air, water, soil or sediment (half-life in air ≥ 2 days, halflives in soil and water ≥182 days and half-life in sediment ≥ 365 days), as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). Potential for Bioaccumulation Since no experimental bioaccumulation factor (BAF) or bioconcentration factor (BCF) data for methyl eugenol were available, a predictive approach was applied using available BAF and BCF models to estimate the bioaccumulation potential for the substance. The modelled predictions (mid level trophic by BCFBAF 2008) are summarized in Table 5 below. Given that the log Kow is <4 as described below, the uptake of the substance is expected to be mainly via the gills and thus metabolism via the gut is not expected to be significant. 12 Screening Assessment CAS RN 93-15-2 The modified Gobas BAF middle trophic-level model (Arnot and Gobas 2003) for fish predicted a bioaccumulation factor (BAF) and a bioconcentration factor (BCF) of 72 and 69 L/kg respectively, indicating that methyl eugenol does not have the potential to appreciably bioaccumulate or to biomagnify in the environment. The result of the Gobas BCF model calculation also suggests a low bioconcentration potential for this substance. It is noted that the metabolic biotransformation potential for this substance was calculated from modelled BCF data, and was then used in calculating the QSAR-based Gobas BAF and Gobas BCF values. There is little difference, however, between the BCF and BAF estimates when metabolic biotransformation rates were included. This is because the predicted rate of biotransformation was relatively inconsequential compared to the other rates of chemical elimination most notably gill elimination (i.e., 2.6 /d). The results of other BCF model calculations (OASIS Forecast 2005, and CPOPs 2008) shown in Table 5 below, add to the weight-of-evidence supporting the low bioconcentration potential of this substance with respect to the bioaccumulation criteria of 5,000 L/kg. Table 5. Modelled data of bioaccumulation for methyl eugenol Test Organism Endpoint Fish Fish Fish BAF BCF BAF Value (wet weight, L/kg) 61 61 72 Fish BCF 69 Fish Fish BCF BCF 266 53 Reference BCFBAF 2008 (mid trophic level) BCFBAF 2008 (mid tropic level) Arnot and Gobas 2003 (Gobas BCF/BAF Middle Trophic Level) Arnot and Gobas 2003 (Gobas BCF/BAF Middle Trophic Level) OASIS Forecast 2005 CPOPs 2008 Based on the model predictions of BAF and BCF, it is concluded that methyl eugenol does not meet the bioaccumulation criteria (BAF or BCF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). Potential to Cause Ecological Harm Empirical ecotoxicity studies for methyl eugenol are available for fish, daphnia, and alga, and results are summarized in Table 6a. The EC50/LC50 values from acute studies ranged from 6 to 22 mg/L, while the chronic no-observed-effect-concentrations (NOECs) for algae and daphnia ranged from 1.1 to 4.6 mg/L. In the chronic study with daphnia, the EC50 was reported as 13 mg/L. There were no experimental toxicity data for methyl eugenol in soil. 13 Screening Assessment CAS RN 93-15-2 Table 6a. Empirical data for aquatic toxicity of methyl eugenol Test Organism Test Type Endpoint Medakafish Acute 96-hr (Oryzias latipes) Bluegill sunfish (Lepomis macrochirus) Bluegill sunfish (Lepomis macrochirus) Rainbow trout (Oncorhynchus mykiss) Rainbow trout (Oncorhynchus mykiss) Alga (Pseudokirchneriella subcapitata) Water flea (Daphnia magna) LC501 Value (mg/L) 14 Reference* CHRIP c2008 Acute 96-hr LC50 8.1 US EPA 2008 Acute 24-hr Acute 48-hr Acute 96-hr Acute 96-hr LC50 LC50 LC50 LC50 8.5 8.1 8.1 6 Beroza et al. 1975 Acute 24-hr Acute 48-hr Acute 96-hr Acute 72-hr Growth rate Acute 72-hr AUG*** Acute 48-hr Immobilization Chronic 21-day Reproduction LC50 LC50 LC50 EC502 NOEC3 EC50 NOEC EC50 5.6-10 6.9 6.0** 22 4.6 9.6 2.1 38 EC50 NOEC 13 1.1 US EPA 2008 Beroza et al. 1975 CHRIP c2008 CHRIP c2008 1 LC50 – The concentration of a substance that is estimated to be lethal to 50% of the test organisms EC50 − The concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms 3 NOEC – The No Observed Effect Concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls * Some of the original studies have not been reviewed for quality ** Critical toxicity value which is used to derive the predicted no effect concentration *** AUG – Area Under Growth curve 2 In addition to the empirical toxicity data for methyl eugenol, the predictive QSAR model ECOSAR (2009) was also used to estimate both acute and chronic effects of the substance on aquatic organisms. Predicted ecotoxicity values were summarized in Table 6b below and used in the QSAR weight-of-evidence approach for assessing aquatic toxicity (Environment Canada 2007). 14 Screening Assessment CAS RN 93-15-2 Table 6b. Modelled data for ecotoxicity of methyl eugenol (ECOSAR 2008) Test organism Fish Fish (sea water) Daphnid Green Algae Mysid Shrimp Fish Fish Fish (sea water) Daphnid Green Algae Mysid Shrimp (sea water) Earthworm 1 2 3 Type of test Acute 96-hr Acute 96-hr Acute 48-hr Acute 96-hr Acute 96-hr Chronic 14-day Chronic 30-day Chronic Chronic Chronic Chronic Chronic 14-day Endpoint LC501 LC50 LC50 EC502 LC50 LC50 ChV3 ChV ChV ChV ChV LC50 Value (mg/L) 17.01 21.86 11.17 8.30 8.13 17.48 1.90 4.48 1.53 3.77 0.52 242.25 LC50 – The concentration of a substance that is estimated to be lethal to 50% of the test organisms EC50 − The concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms ChV – Chronic toxicity value The range of empirical toxicity values and the predicted aquatic toxicity (obtained from the QSAR model considered) indicates that methyl eugenol is not likely to cause acute harm to aquatic organisms at low concentrations (< 1 mg/L). The lowest empirical acute effect value of 6 mg/L was selected as the Critical Toxicity Value (CTV) and used to derive the predicted no effect concentration (PNEC). An assessment factor of 100 was applied to account for inter- and intra-species variations in sensitivity, and to extrapolate from a laboratory-based endpoint to a chronic effect value in the field. This resulted in a conservative predicted no effect concentration (PNEC) of 0.06 mg/L (Robust Study Summary available upon request). Given the very low quantity of this substance imported in Canada, a conservative aquatic exposure scenario was developed to estimate the potential release into the aquatic environment from a hypothetical industrial operation and the resulting aquatic concentration. The 100-kg reporting threshold for the section 71 notice was used to represent the total amount used at the facility. The predicted environmental concentration (PEC) is conservatively estimated (assuming 5% of the mass in use is released over the course of one year to a small watercourse) to be 0.0006 mg/L (Environment Canada 2009). The resulting risk quotient (PEC/PNEC) is 0.01 (Environment Canada 2009), indicating that methyl eugenol is unlikely to cause harm to aquatic organisms in Canada. With the exception of a predicted LC50 for an earthworm (Table 6b), no ecological effects information were found for this substance in media other than water. Given the short halflife due to reactions with photochemically produced hydroxyl radicals, the substance will 15 Screening Assessment CAS RN 93-15-2 degrade fast and exposure in air is not expected to be significant. Based on the model predictions of environmental fate, if released to soil, there is the potential for soil-dwelling organisms to be exposed to methyl eugenol. Given that emissions to soil are expected to be very low based on the section 71 reporting information, as well as the short dissipation half-life (measured as 16 hours at 22 oC) in that compartment, the exposure to methyl eugenol in soil is anticipated to be limited. Based on this, and on the predicted LC50 for earthworms and the relatively low aquatic toxicity of this substance, significant ecological effects on soil organisms are unlikely. Uncertainties in Evaluation of Risk to Environment There is uncertainty associated with the exposure assessment. Although methyl eugenol has been identified in various natural substances and in some industrial raw and partially treated effluent, there is no quantitative study monitoring ambient environmental (nondietary) concentrations of the substance at which organisms in water and other environmental media are exposed. However, given the low use quantity based on section 71 reporting information and the conservative nature of the aquatic exposure estimate, confidence is high that the risk associated with the predicted environmental exposures is low. The bioaccumulation assessment is limited by the absence of experimental data; this necessitated that predictions using QSAR models be generated. Although the predictions using models have some degree of error, methyl eugenol is well within the domain of applicability for the QSAR models and estimates from all QSAR models indicate that methyl eugenol is expected to have a low bioaccumulative potential. The low to moderate log Kow of methyl eugenol confirms the validity of the modelled values for the substance. 16 Screening Assessment CAS RN 93-15-2 Potential to Cause Harm to Human Health Exposure Assessment Environmental Media and Diet Methyl eugenol was detected in the raw and partially treated effluent of one unbleached kraft mill at concentrations of 0.001–0.002 mg/L, but not in the final effluent (Keith 1976). Air in the vicinity of insect traps baited with methyl eugenol was analysed for the presence of the substance 0–5 days after bait stations were set out. Methyl eugenol was detected in samples on days 0 and 1, but not on day 5, at distances of 1 and 25 m from the traps (Turner et al. 1989). No other reports of methyl eugenol levels in environmental media have been located. There are insufficient data with which to prepare an estimate of exposure to methyl eugenol from environmental media (air, water and soil); however, the exposure from these media is expected to be low. Estimates of the dietary intake of methyl eugenol from foods and beverages were prepared by the UK delegation to the Council of Europe Committee of Experts in Flavouring Substances for its 47th session in October 2000. The overall average and 97.5th-percentile intakes were estimated to be 190 and 530 µg/kg body weight (kg-bw) per day, respectively, for consumers only. These estimates were calculated using dietary intake data from a British survey of daily food and beverage consumption. For each food or beverage category, the highest level of methyl eugenol in that category was used to estimate dietary intake of methyl eugenol. The delegation report stated that these numbers were likely to be overestimates (Council of Europe 2001). Smith et al. (2002) prepared estimates of daily dietary intake of methyl eugenol and estragole in one of a series of safety evaluations by the Expert Panel of FEMA. The researchers used data on the annual volumes of plant materials with methyl eugenolcontaining essential oils, methyl eugenol-containing essential oils and neat methyl eugenol imported and consumed in the United States in 1999. These data were combined with the average methyl eugenol content of essential oil derived from each plant type. Dietary intake of methyl eugenol was calculated by considering the intake of methyl eugenol from traditional food (principally spices), from essential oils added as flavour ingredients and from neat methyl eugenol as a flavouring substance. The estimated mean dietary intake of methyl eugenol for consumers only was 8 µg/kg-bw per day. The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) published an evaluation of a group of alkoxy-substituted allylbenzenes, including methyl eugenol, in which the FEMA population-based estimation methodology as well as FEMA trade data 17 Screening Assessment CAS RN 93-15-2 were used to derive an estimate of the maximum dietary intake of methyl eugenol in the United States of 424 µg/person per day or about 6–8 µg/kg-bw per day for an adult (FAO/WHO 2009). The concentration of methyl eugenol in basil is highly variable and the use of basil to make pesto may occasionally result in the ingestion of a large amount of methyl eugenol. In the estimate of daily dietary intake of methyl eugenol from basil, Smith et al (2002) used an average concentration of methyl eugenol in dried basil of 2.6% and in fresh basil of 0.11%. Miele et al (2001) reported that the concentration of methyl eugenol in essential oil extracted from Ocimum basilicum cv Genovese Gigante ranged from 5.5% to 100% and estimated that the intake from a single serving of pasta with pesto could reach 250 μg/kg-bw per meal for adults and 500 μg/kg-bw per meal for children, based on a concentration of methyl eugenol in basil oil of about 40%. Consumer Products Methyl eugenol is not permitted to be intentionally added as an ingredient in personal care products and is present only as a naturally occurring component of essential oils. Essential oils which may contain methyl eugenol are used in the formulation of thousands of personal care products in Canada (CNS, 2009). The Cosmetic Ingredient Hotlist details the maximum concentration of methyl eugenol in essential oils used in personal care products to: 0.01% in fine fragrances, 0.004% in eau de toilette, 0.002% in a fragrance cream, 0.0002% in other leave-on products and oral hygiene products, and 0.001% in rinse-off products (Health Canada 2007). The potential exposure to methyl eugenol from the use of personal care products made with essential oils containing methyl eugenol was derived using consumer exposure modelling software (ConsExpo 2006). A representative calculation is provided in Appendix 2. Based on information from notifications to Health Canada (CNS, 2009) upper-bounding estimates of systemic exposure to methyl eugenol from use of certain personal care products were derived and are shown for females in Table 7. Table 7. Estimated adult female systemic exposure of methyl eugenol from personal care products containing methyl eugenol. based on ConsExpo (ConsExpo 2006) Product category fragrance body lotion face cream skin cleanser Amount per application in grams 0.61 8 0.8 2.5 Frequency of use per day Percent methyleugenol in product for modelling* Systemic exposure μg/kg-bw/ per day 0.01 0.0002 0.0002 0.001 3 2 2 2 Total 1.0 0.2 0.0 0.3 1.5 18 Screening Assessment CAS RN 93-15-2 The upper range of methyl eugenol reported in the table represents the Cosmetic Ingredient Hotlist limit in Canada (Health Canada 2007). For adult women, the estimated daily systemic exposure to methyl eugenol resulting from dermal exposure only from the aggregate use of four types of personal care products (body lotion, face moisturizer, skin cleanser and fragrance) formulated with various essential oils containing methyl eugenol is 1.5 µg/kg-bw per day. This estimate assumes a dermal absorption of methyl eugenol of 40% for products left on the skin (European Commission 2000b) and a permeability coefficient of 0.0221 cm/hour for skin cleanser that is washed off (DERMWIN 2000). While the estimated exposure of adult women was the only scenario presented in the current screening assessment, the estimates of exposure from the use of personal care products are not expected to differ appreciably across age groups. As previously noted, the concentration of methyl eugenol in plant-derived material is quite variable, and there is significant uncertainty associated with these estimates, precluding the need to characterize exposures of other sub-populations. In an assessment of human exposure to methyl eugenol prepared by the European Cosmetics Association (COLIPA), for the European Commission, a lower estimate of the exposure to methyl eugenol from fragranced cosmetics was presented, in part based on a concentration of methyl eugenol of only 0.05% in the essential oils. Neither estimate includes exposure arising from the use of dental or oral hygiene products. Clove flower oil is licensed for sale in Canada as a non-prescription dental analgesic (LNHPD 2009). In Health Canada’s (2004) re-evaluation of the safety of citronella oil use in personal insect repellents, dermal exposure to methyl eugenol, which can occur in these products, was estimated. Based on 15% citronella in the insect repellent and that it may be applied more than once per day for a short period each year, a dermal exposure of 3.56 μg/kg-bw per day of methyl eugenol was estimated. Methyl eugenol was detected in 98% of 206 adult human serum samples analysed in the Third National Health and Nutrition Examination Survey, an indication that human exposure in the United States is ubiquitous. The 5-95% distribution was 5-78 ng/kg in serum. It can be concluded that methyl eugenol is broadly present in human serum, however, the concentrations are extremely low. During the laboratory analysis phase of this work, it was determined that methyl eugenol was present in laboratory air and both distilled and bottled water at low parts per trillion levels. The researchers concluded that methyl eugenol is ubiquitous in air and water, albeit at very low levels and that human exposure arises from multiple sources (Barr et al. 2000). Confidence is high that the predominant sources of exposure to methyl eugenol for Canadians are diet and personal care products. While the highest concentration of methyl eugenol suggested for use in cosmetics is outlined in the Cosmetic Ingredient Hotlist, there are no data to characterize actual levels of methyl eugenol typically found in essential oils used in personal care products. There are no Canadian data on dietary 19 Screening Assessment CAS RN 93-15-2 consumption of methyl eugenol. Exposure to methyl eugenol via cleaning products is likely a minimal contributor to overall exposure. Health Effects Assessment Appendix 3 contains a summary of the available health effect information for methyl eugenol. The US National Toxicology Program (NTP) classified methyl eugenol as reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (NTP 2000a, b; NTP 2005a). The International Agency for Research on Cancer (IARC), has not assessed methyl eugenol for carcinogenicity. However, the structurally related allylbenzene, safrole, was classified by the IARC as possibly carcinogenic to humans (Group 2B) and was listed as reasonably anticipated to be a human carcinogen by the US NTP (IARC 1976; NTP 2005b). In experimental animal studies, methyl eugenol induced tumours in two species, in both genders, and at multiple sites. In the standard 2-year NTP carcinogenicity studies with rats and mice, methyl eugenol induced multiple types of tumours in liver and neuroendocrine tumours in the glandular stomach in both males and females in a dose-related manner. In the NTP studies, significant dose-related increased incidences of hepatocellular carcinoma or adenoma were observed in male Fischer 344 rats (7/50, 14/50, 28/50, 43/50, 45/50, respectively); and in female Fischer 344 rats (1/50, 8/50, 14/50, 34/50, 43/50, respectively) after exposure to methyl eugenol by gavage at doses of 0, 37, 75 or 150 mg/kg-bw per day for 105 weeks or at a stop-exposure of 300 mg/kg-bw per day for 53 weeks, followed by vehicle only for the remaining 52 weeks. The incidences of benign or malignant neuroendocrine tumours in the glandular stomach were also significantly increased in both male (0/50, 0/50, 0/50, 7/50, 4/50 for 0, 37, 75, 150 or 300 mg/kg-bw per day, respectively) and female rats (0/50, 1/50, 25/50, 34/50, 41/50 for 0, 37, 75, 150 or 300 mg/kg-bw per day, respectively). In addition, methyl eugenol significantly increased the incidences of kidney neoplasms, mammary gland fibroadenoma, malignant mesothelioma, subcutaneous fibroma or fibrosarcoma in male rats; and the liver tumours, hepatocholangioma or hepatocholangiocarcinoma, in both male and female rats (NTP 2000a). In the carcinogenicity bioassay with B6C3F1 mice, the incidences of hepatocellular adenoma or carcinoma were increased significantly in male mice (31/50, 47/50, 46/50, 40/50, respectively) and female mice (25/50, 50/50, 49/49, 49/50, respectively) when exposed to methyl eugenol by gavage at doses of 0, 37, 75 or 150 mg/kg-bw per day for 104 weeks. Significantly increased incidences of hepatoblastoma were also seen in female mice in a dose-related manner (NTP 2000a). The significantly increased incidences of induced liver tumours were observed at the lowest dose tested (37 mg/kg bw per day) in both rats and mice. In addition, another study showed that methyl eugenol or its metabolite, 1’-hydroxyl methyl eugenol, significantly induced liver tumour in mice that 20 Screening Assessment CAS RN 93-15-2 had received four intraperitoneal injections before weaning (total 28.3 mg/kg bw methyl eugenol or 18.3 mg/kg bw 1’-hydroxyl methyl eugenol) and were observed for up to 18 months (Miller et al. 1983). Methyl eugenol was genotoxic in a number of in vivo assays in experimental animals, in vitro assays in mammalian cells and its metabolites were mutagenic in some Salmonella test strains (Appendix 3). In in vivo bioassays, methyl eugenol caused chemical-specific mutation of β-catenin gene in mouse liver tumours at 69% incidence at codons 32, 33, 34 or 41 compared with only 9% in spontaneous tumours. Mutations in β-catenin gene caused β-catenin accumulation and up-regulation of Wnt-signaling, subsequently stimulating cell proliferation and inhibiting apoptosis (Devereux et al. 1999; NTP 2000b). In a gene mutation assay with transgenic animals, methyl eugenol significantly increased the mutation frequency of lacI gene in the liver of female Big Blue® rats administered with methyl eugenol by gavage at 1,000 mg/kg bw per day for 90 days compared with controls (Tyrrell et al. 2000). By contrast, in a transgenic Big Blue® male mice study, the mutation frequency of lacI in liver in methyl eugenol-treated mice (by gavage at 300 mg/kg bw per day for 90 days) was not significantly different from the controls. However, the mutation spectrum (pattern) of the methyl eugenol-treated group was significant different from the control group (Tyrrell et al. 2000). DNA adducts were observed in the liver of CD-1 female mice treated with methyl eugenol by intraperitoneal injection of 100 or 500 mg/kgbw (Randerath et al. 1984); and in the liver of newborn male B6C3F1 mice treated by intraperitoneal injection with 0.25 to 3.0 µmol methyl eugenol on days 1, 8, 15, 22 after birth, respectively (Phillips et al 1984). Moreover both studies showed that methyl eugenol was more potent than the structurally related carcinogen safrole, in the formation of liver DNA adducts. Methyl eugenol did not induce micronuclei formation in the bone marrow of male or female B6C3F1 mice (NTP 2000a). In in vitro mammalian cell bioassays, methyl eugenol induced sister chromatid exchange in Chinese hamster ovary (CHO) cells in the presence of S9 (NTP 2000a); induced cell transformation in Syrian hamster embryo cells (Kerckaert et al. 1996); and formed DNA adducts in cultured human HepG2 cells and fibroblast V79 cells transfected with human sulphotransferase (Stening et al. 1997; Zhou et al. 2007). Methyl eugenol and its metabolite, 1′-hydroxymethyl eugenol, induced dose-related unscheduled DNA synthesis in cultured primary rat hepatocytes (Howes et al. 1990; Chan and Caldwell 1992). Moreover, as seen in vivo, higher DNA binding activity was observed for methyl eugenol than for the structurally related carcinogen, safrole. However, methyl eugenol did not cause chromosomal aberration in CHO cells (NTP 2000a). In bacterial bioassays, methyl eugenol was not mutagenic in several Salmonella typhimurium strains in the presence or absence of S9; nor was it mutagenic in Escherichia coli WP2uvrA in the presence of S9 (Dorange et al. 1977; Sekizawa and Shibamoto 1982; Mortelmans et al. 1986; Schiestl et al. 1989; NTP 2000a). However, its metabolite, 2′,3′epoxymethyl eugenol, induced point mutation in S. typhimurium strains TA1535 and TA100 (Dorange et al. 1977); caused DNA damage in Bacillus subtilis strains M45 Rec− and H17 Rec+ (Sekizawa and Shibamoto 1982); and caused increased frequencies of intra- 21 Screening Assessment CAS RN 93-15-2 and interchromosomal recombination in the diploid yeast Saccharomyces cerevisiae strain RS9 (Schiestl et al. 1989) and intrachromosomal recombination in yeast strain RS112 in the presence and absence of S9 (Brennan et al. 1996). The mutagenicity, cytogenicity and DNA damage data support a conclusion that methyl eugenol is genotoxic to mammalian somatic cells in vitro and in vivo. This conclusion is consistent with the opinion of the Scientific Committee on Food on the safety of the presence of methyl eugenol in food that methyl eugenol is genotoxic and carcinogenic. Therefore “the existence of a threshold can not be assumed” (European Commission 2001). A fully elucidated mode of action for tumours arising as a result of exposure to methyl eugenol has not been developed. In the literature, divergent opinions have been expressed on the mode of action for such tumours. The Expert Panel of FEMA suggested that the dose-dependent hepatotoxicity is “likely to be a necessary step in carcinogenesis” in the liver (Smith et al. 2002). The induction of benign and malignant neuroendocrine tumours of the glandular stomach in rats and mice may be due in part to induction of glandular stomach atrophy, reduced gastric acid secretion, hypergastrinemia and proliferations of enterochromaffin-like cells (NTP 2000a, 2005a). However, strong evidence of mutations in β-catenin gene in mouse liver tumours led others to suggest that mutation of β-catenin may be an early event in hepatocellular tumour formation (Devereux et al. 1999; NTP 2000b; Johnson and Abdo 2005). In addition, methyl eugenol and its metabolites form DNA adducts in in vivo and in vitro assays, which suggests that DNA-reactive intermediates may also be involved in the neoplastic transformation (Johnson and Abdo 2005; NTP 2005a; Rietjens et al. 2005a, b). Burkey et al. (2000) suggested that both cytotoxicity and genotoxicity of methyl eugenol may be involved in tumour induction. The mechanisms by which the alkoxy-substituted allylbenzenes, including methyl eugenol, induce cancer in experimental animals have not been established (FAO/WHO 2009). Based on the weight of evidence of carcinogenicity observed in both sexes of two experimental animal species in long-term studies, including hepatocellular carcinomas observed at the lowest test doses in these studies, and the evidence that methyl eugenol is genotoxic in a range of in vivo and in vitro assays, which included binding and damage to liver DNA and gene mutations in liver tumours and in transgenic animal assays, it cannot be precluded that the tumours observed in experimental animals resulted from direct interaction with genetic material. With regard to non-cancer critical effects, a significantly increased dose-related incidence of non-neoplastic lesions in the liver and glandular stomach was observed in male and female rats and mice dosed with methyl eugenol in the 2-year chronic studies. The nonneoplastic lesions in liver included eosinophilic and mixed cell foci, hepatocellular hypertrophy or hepatocyte necrosis, oval cell hyperplasia, cystic degeneration, bile duct hyperplasia, portal hypertrophy, hematopoietic cell proliferation and hemosiderin pigmentation. The non-neoplastic lesions in the glandular stomach included mucosal atrophy, neuroendocrine cell hyperplasia, glandular ectasia and chronic active inflammation. Based on the effects of the non-neoplastic lesions (hypertrophy, hyperplasia, etc.), the lowest-observed-adverse-effect level (LOAEL) was identified to be 37 mg/kg-bw per day in male and female rats and mice (NTP 2000a). In subchronic 22 Screening Assessment CAS RN 93-15-2 studies, cytological alteration, necrosis, hyperplasia, atrophy, and organ or body weight changes were observed in rats and mice dosed orally with methyl eugenol at doses of 0 to 1000 mg/kg-bw per day for 14 weeks. Among the non-cancer critical effects, the most sensitive endpoint was reduced body weight and body weight gain, with an oral lowestobserved-effect level (LOEL) of 10 mg/kg-bw per day identified in male rats in the subchronic study (NTP 2000a; Abdo et al. 2001). In addition, intrauterine growth retardation and mildly delayed skeletal ossification were observed at the highest dose in Sprague-Dawley rats administered methyl eugenol by gavage at 0 to 500 mg/kg-bw per day on gestational days 6–19. However, maternal toxicity was observed at the lowest dose of 80 mg/kg-bw per day (NTP 2004). Methyl eugenol was rapidly absorbed following oral administration to rats or mice; the plasma levels of methyl eugenol peaked within 5 minutes, and elimination of methyl eugenol from the bloodstream was rapid and multiphasic (NTP 2000a). Methyl eugenol was metabolized by the cytochrome P-450 system by three different pathways: side-chain hydroxylation, side-chain epoxidation and O-demethylation. Of the various metabolites, 1′-hydroxymethyl eugenol and methyl eugenol-2′3′-oxide were considered to contribute to the toxic effects in the liver. The metabolite, 1′-hydroxymethyl eugenol, followed by sulphation, subsequently formed electrophilic carbonium ions that could bind covalently to DNA and other cellular macromolecules, including proteins (Gardner et al. 1996, 1997; NTP 2005a). DNA-reactive metabolites of methyl eugenol may be a critical step involved in gene mutation and in liver tumour induction. The structurally related allylbenzene compounds, such as safrole, estragole and eugenol, are metabolized via similar pathways. A physiologically based pharmacokinetic model was developed by the NTP to represent the absorption, distribution, metabolism and elimination of methyl eugenol in rats and mice (NTP 2000a). The in vitro metabolism studies with various expressed recombinant human individual P-450 enzymes and with specific inhibition of enzymes showed that human cytochrome P-450 1A2 was the main enzyme involved in the bioactivation of methyl eugenol to 1′-hydroxymethyl eugenol and that cytochrome P-450 2C9 and 2C19 might contribute to the bioactivation at higher methyl eugenol substrate concentrations (Jeurissen et al. 2006). In vitro studies showed that human liver microsomes had comparable capacity (0.47 nmol/min/mg protein, average of 13 human liver microsomes) in bioactivation of methyl eugenol to 1-hydroxy methyl eugenol with microsomes from rats (0.42 nmol/min/mg protein, average of 25 rats from liver microsomes of all 5 test groups), although some variations were observed among humans. Taken together with other lines of evidence of bioactivation of methyl eugenol in the human liver (Jeurissen et al 2006), formation of DNA adducts in human hepatoma cells (HepG2) (Zhou et al. 2007) and in human sulfotransferase-transfected fibroblast V79 cells (Stening et al. 1997), all the lines of evidence suggest that methyl eugenol can be bioactivated by human liver cells and the biologically plausibility for the human cancer risk can not be discounted. The confidence in the toxicity database for methyl eugenol is considered to be moderate. Critical effects including carcinogenicity occurred at the lowest exposure levels tested. However, the modes of action for the observed carcinogenicity of methyl eugenol have not been fully elucidated. Oral dosing studies (short-term, subchronic, developmental 23 Screening Assessment CAS RN 93-15-2 toxicity, carcinogenicity and genotoxicity) are available. However, most of the animal studies used oral gavage as the route of administration. Repeated dose animal studies via diet, inhalation and dermal routes – the most relevant routes of human exposure - are limited or have not been identified. Studies have shown that the introduction of a bolus dose of test material via gavage can lead to higher peak blood plasma levels and increased metabolic demand compared with slower more steady absorption of the substance from the diet. For example, one unpublished dietary study (Jones, 2004) was cited in the recent JECFA evaluation (FAO/WHO 2009). This study was conducted in rats with microencapsulated methyl eugenol at dietary levels of 0, 5 or 50 mg/kg-bw per day over a 28 day period. No treatment-related effects were noted in the study at the highest dietary intake of 50 mg/kg bw per day. The large bolus dose delivered by oral gavage may produce metabolic and toxicological effects that are not relevant via the other routes of exposure. Characterization of Risk to Human Health Based principally on the weight of evidence–based assessments of international or other national agencies (European Commission 2001; NTP 2005a), a critical effect for the characterization of risk to human health for methyl eugenol is carcinogenicity. Methyl eugenol is a multisite carcinogen in male and female rats and mice at all doses tested in a 2-year NTP bioassays. In the carcinogenicity studies, methyl eugenol induced multiple types of tumours in the liver and in the glandular stomach in both males and females. The liver tumours were observed at the lowest dose tested (37 mg/kg-bw per day) in both rats and mice. In male rats, tumours were also observed in the kidney, mammary gland, subcutaneous tissues and mesothelium. Methyl eugenol was genotoxic in a range of in vivo and in vitro assays, although it was not mutagenic in bacterial cells. Methyl eugenol caused gene mutation in liver of transgenic animals; mutation of β-catenin gene was observed in mouse liver tumours. Modes of action have not been fully elucidated for carcinogenicity. However, based on the weight of evidence of carcinogenicity and the genotoxicity of methyl eugenol it is considered that the tumours observed in the experimental animals resulted from direct interaction with genetic material. The FAO/WHO (2009) report discussed the carcinogenic potential of methyl eugenol as a single component and general population exposure to methyl eugenol as part of a larger mixture in foods or essential oils. While there is evidence to suggest that methyl eugenol is a multi-site carcinogen, there are no available data to assess the toxicological potential of the mixtures most commonly found in foods or consumer products. The FAO/WHO (2009) report further suggested that the toxicity data may not relate to the presence of methyl eugenol in natural spices based on recent in vitro data that indicates that other components of natural spices might modulate bioactivation and/or act as detoxifying agents. In the opinion of the FAO/WHO (2009) authors, the relevance of the critical effects observed in animal studies to the exposure scenario in humans was questionable and further assessment of methyl eugenol was recommended. While structured epidemiological research exploring possible associations between spice consumption and 24 Screening Assessment CAS RN 93-15-2 hepatic cancer in humans is lacking, there is an absence of any indications of human cancer associations noted in the scientific literature. The critical non-cancer effect noted in the animal database was reductions in body weight or body weight gain noted in male rats at an oral lowest-observed-effect level (LOEL) of 10 mg/kg-bw per day following 90 days of treatment (NTP 2000a; Abdo et al. 2001). Since the predominant source of dietary exposure is from methyl eugenol’s naturallyoccurring presence in foods, derivation of a margin of exposure was not considered to be meaningful. The exposure and risk associated with the presence of methyl eugenol in environmental media and consumer products are considered to be low. The use of personal care products containing essential oils results in a potential exposure of 1.5 μg/kg-bw per day, resulting in a margin of exposure of 6670 when compared to the critical effect level (10 mg/kg-bw per day). Exposure from use of a citronella-based (containing methyl eugenol) insect repellent results in a potential exposure of 3.56 ug/kgbw per day (Health Canada, 2004), resulting in a margin of exposure of 2810, when compared to the critical effect level. With respect to non-cancer effects, these margins are considered adequate to account for uncertainty in the database on health effects and exposure. Uncertainties in Evaluation of Risk to Human Health The modes of tumour induction have not been fully elucidated for the various tumours observed in animal studies. While the evidence shows that DNA adducts, DNA damage and mutations play the primary role in the tumour initiation, cytotoxicity might also be involved in the tumour induction. Limited information indicates that there might be a marked variation in bioactivation of methyl eugenol among humans. Sufficient data are not available to show the pharmacokinetic difference between animals and humans. It is assumed that the effects observed in experimental animals are relevant to humans. Liver tumours were observed at the lowest tested dose in rats and mice. In addition, there were no adequate lifetime animal studies conducted via inhalation or dermal routes of exposure. However, there is no epidemiological evidence associating the natural presence of methyl eugenol in spices and spice oils, which are likely to be the main sources of methyl eugenol in the diet, with liver cancer in humans. There are significant uncertainties in the estimates of dietary intake of methyl eugenol as well as in the estimate of dermal exposure to methyl eugenol from the use of personal care products. The dietary intake of methyl eugenol in Canada is difficult to estimate without detailed current data on the levels of methyl eugenol in the Canadian food supply. Spices and spice-derived essential oils are probably the primary contributors to dietary exposure to methyl eugenol (based on Smith et al., 2002; FAO/WHO 2009), so the wide variation in methyl eugenol content of spices and their oils and the unknown use levels of these sources as ingredients in foods are two major factors that would lead to uncertainty in any 25 Screening Assessment CAS RN 93-15-2 dietary exposure assessment for methyleugenol. In the absence of Canadian data, this screening assessment presented the available dietary exposure estimates conducted internationally but a risk assessment was not done for dietary sources of methyl eugenol. While restrictions on levels of methyl eugenol in essential oil ingredients in personal care products are in place in Canada, there are no data on actual concentrations in these products. Modelling of dermal exposure to methyl eugenol did not account for all types of product that may be formulated with oils containing methyl eugenol, nor did the estimate account for market share of individual products within a product category. Conclusion Based on the information available with respect to the environment, it is concluded that methyl eugenol is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, the substance does not meet the criteria for persistence or the criteria for bioaccumulation as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). On the basis of the carcinogenicity of methyl eugenol, for which there may be a probability of harm at any level of exposure, it is concluded that methyl eugenol is a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Therefore, it is concluded that methyl eugenol meets one or more of the criteria under 64 of CEPA 1999. Where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase. 26 Screening Assessment CAS RN 93-15-2 References Abdo KM, Cunningham ML, Snell ML, Herbert RA, Travlos GS, Eldridge SR, Bucher JR. 2001. 14-week toxicity and cell proliferation of methyl eugenol administered by gavage to F344 rats and B6C3F1 mice. Food Chem Toxicol 39:303–316. [AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63:1953–1960. Barr DB, Barr JR, Bailey SL, Lapeza CR Jr, Beeson MD, Caudill SP, Maggio VL, Schecter A, Masten SA, Lucier GW, Needham LL, Sampson EJ. 2000. Levels of methyl eugenol in a subset of adults in the general U.S. population as determined by high resolution mass spectrometry. Environ Health Perspect 108(4):323–328. [BCFBAF] BioConcentration Factor Program for Windows [Estimation Model]. 2008. Version 3.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm Beroza M, Inscoe MN, Schwartz PH Jr, Keplinger ML, Mastri CW. 1975. Acute toxicity studies with insect attractants. Toxicol Appl Pharmacol 31(3):421–429. [cited in RTECS 2008]. [BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2009. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741–752. Brennan RJ, Kandikonda S, Khrimian AP, De Milo AB, Liquido NJ, Schiestl RH. 1996. Saturated and monofluoro analogs of the oriental fruit fly attractant methyl eugenol show reduced genotoxic activities in yeast. Mutat Res 369:175–181. Burfield T. 2004. Various references re: methyl eugenol content of essential oils. In: Blue Cypress oil [Callitris intratropica Benth. et Hook f.] etc. Cropwatch Issue 3 [Internet]. [cited 2009 Sep]. Available from: http://www.users.globalnet.co.uk/~nodice/new/magazine/cropwatch3/cropwatch3.htm Burkey JL, Sauer JM, McQueen CA, Sipes IG. 2000. Cytotoxicity and genotoxicity of methyl eugenol and related congeners—a mechanism of activation for methyl eugenol. Mutat Res 453:25–33. Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III. Vol.22, no.3. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 29 March, 2000, SOR/2000-107. Available from: http://www.gazette.gc.ca/archives/p2/2000/2000-0329/pdf/g2-13407.pdf Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109–4117. Available from: http://canadagazette.gc.ca/archives/p1/2006/2006-12-09/pdf/g1-14049.pdf 27 Screening Assessment CAS RN 93-15-2 Canada, Dept. of the Environment, Dept. of Health. 2009. Canadian Environmental Protection Act, 1999: Notice of ninth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 143, No. 11vol. 143, no. 11, p. 558-–562. Available from: http://canadagazette.gc.ca/rp-pr/p1/2009/2009-0314/pdf/g1-14311.pdf [CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. ©2004– 2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: http://oasis-lmc.org/?section=software&swid=1 Chan VSW, Caldwell J. 1992. Comparative induction of unscheduled DNA synthesis in cultured rat hepatocytes by allylbenzenes and their 1′-hydroxy metabolites. Food Chem Toxicol 30(10):831–836. [CHRIP] Chemical Risk Information Platform [database on the Internet]. ©2008. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Center. [cited 2009–2010]. Available from: http://www.safe.nite.go.jp/english/db.html [CNS] Cosmetic Notification System [Proprietary Database]. 2009. Available from Health Canada, Cosmetics Division, Consumer Product Safety Directorate, Health Environments and Consumer Safety Branch. [ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). Available from: http://www.rivm.nl/en/healthanddisease/productsafety/ConsExpo.jsp#tcm:13-42840 Council of Europe, Committee of Experts on Flavouring Substances. 2001. UK note on estimated intakes of methyl eugenol from foods and beverages. Document RD 4.9/148, submitted by the delegation of the UK for the 48th meeting in Strasbourg, April 2001. Strasbourg (FR): Council of Europe. 10 p. [CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request. [DERMWIN] Dermal Permeability Coefficient Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm Devereux TR, Colleen HA, Foley JF, White CM, Siles RG, Barrett JC. 1999. Mutation of β-catenin is an early event in chemically induced mouse hepatocellular carcinogenesis. Oncogene 18:4726–4733. Dorange JL, Delaforge M, Janiaud P, Padieu P. 1977. [Mutagenicity of the metabolites of the epoxide-diol pathway of safrole and its analogs. Study on Salmonella typhimurium. C R Seances Soc Biol Fil 171:1041–1048 (in French). [ECOSAR] Ecological Structure Activity Relationships [Internet]. 2009. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm. Ellis J, Carmichael PL, Gooderham NJ. 2005. Exposure-based safety assessment of the naturally occurring flavouring methyleugenol. Toxicologist 34: 464 (Abstract). Engelbrecht JA, Long JP, Nichols DE, Barfknecht CF. 1972. Pharmacologic evaluation of 3,4dimethoxyphenylpropenes and 3,4-dimethoxyphenylpropanediols. Arch Int Pharmacodyn Ther 199:226–244. [cited in Johnson and Abdo 2005; RTECS 2008]. 28 Screening Assessment CAS RN 93-15-2 Environment Canada. 2007. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series: draft module on QSARs. Reviewed draft working document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Environment Canada. 2009. Industrial Generic Exposure Tool – Aquatic (IGETA) report: CAS RN 93-15-2, 200911-05. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division. [EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html European Commission, Scientific Committee on Cosmetic and Non-food Products. 2000a. Minutes of the 14th Plenary Meeting, Brussels, October 24, 2000 [Internet]. Brussels (BE): European Commission. [cited 2009 Aug]. Available from: http:/ec.europa.eu/health/ph_risk/committees/sccp/docshtml/sccp_out134_en.htm European Commission, Scientific Committee on Cosmetic and Non-food Products. 2000b. Opinion of the Scientific Committee on Cosmetic and Non-food Products concerning methyleugenol. Brussels (BE): European Commission. European Commission, Scientific Committee on Food. 2001. Opinion of the Scientific Committee on Food on methyl eugenol (4-allyl-1,2-dimethoxybenzene) (adopted on 26 September 2001). SCF/CS/FLAV/FLAVOUR/4 ADD1 FINAL [Internet]. Brussels (BE): European Commission. [cited 2009 Aug]. Available from: http://www.ec.europa.eu/food/fs/sc/scf/out102_en.pdf European Commission. 2008. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Official Journal of the European Union. 31.12.2008. L354/34. Available from: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0034:0050:en:PDF [FAO/WHO] Food and Agriculture Organization of the United Nations/World Health Organization. 2009. Evaluation of certain food additives: sixty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva (CH): World Health Organization. WHO Technical Report Series No. 952. Gardner I, Bergin P, Stening P, Kenna JG, Caldwell J. 1996. Immunochemical detection of covalently modified protein adducts in livers of rats treated with methyl eugenol. Chem Res Toxicol 9(4):713–721. Gardner I, Wakazono H, Bergin P, de Waziers I, Beaune P, Kenna JG, Caldwell J. 1997. Cytochrome P450 mediated bioactivation of methyl eugenol to 1′-hydroxymethyl eugenol in Fischer rat and human liver microsomes. Carcinogenesis 18:1775–1783. Hall RL, Oser BL. 1965. Recent progress in the consideration of flavoring ingredients under the food additives amendment III. GRAS substances. Food Technol 253:151–197. Health Canada. 2004. Proposed acceptability for continuing registration: re-evaluation of citronella oil and related active compounds for use as personal insect repellents [Internet]. Ottawa (ON): Health Canada, Pest Management Regulatory Agency. [cited 2009 Aug]. Available from: http://www.hc-sc.gc.ca/cpsspc/pest/part/consultations/_pacr2004-36/index-eng.php Health Canada. 2007. The cosmetic ingredient hotlist – March 2007 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2009 Aug]. Available from: http://www.hc-sc.gc.ca/cps-spc/person/cosmet/infoind-prof/_hot-list-critique/hotlist-liste_e.html Health Canada. 2008. Review of the 2004 re-evaluation of citronella oil and related active compounds for use as personal insect repellents. Re-evaluation Note REV2008-03. 29 Screening Assessment CAS RN 93-15-2 Ottawa (ON): Health Canada, Pest Management Regulatory Agency. Available from: http://dsppsd.pwgsc.gc.ca/collection_2008/pmra-arla/H113-5-2008-3E.pdf Health Canada. 2009. An Act to amend the Tobacco Act [Internet]. Ottawa (ON): Health Canada. [cited 2009 Aug]. Available from: http://www.hc-sc.gc.ca/hc-ps/tobac-tabac/legislation/federal/amend_faq-modif-eng.php [HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm Howes AJ, Chan VS, Caldwell J. 1990. Structure-specificity of the genotoxicity of some naturally occurring alkenylbenzenes determined by the unscheduled DNA synthesis assay in rat hepatocytes. Food Chem Toxicol 28:537–542. [HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983–2009 . Bethesda (MD): US National Library of Medicine (US). [cited 2009 Oct]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1976. Some naturally occurring substances. IARC Monogr Eval Carcinog Risks Hum 10. [JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2008. In 69th Meeting, Alkoxy-Substituted Akylbenzenes present in foods and essential oils and used as flavouring agents. Available from: http://www.fao.org/ag/agn/agns/files/jecfa69_final.pdf Jenner PM, Hagan EC, Taylor JM, Cook EL, Fitzhugh OG. 1964. Food flavourings and compounds of related structure. I. Acute oral toxicity. Food Cosmet Toxicol 2:327–343. Jeurissen SMF, Bogaards JJP, Boersma MG, ter Horst JPF, Awad HM, Fiamegos YC, van Beek TA, Alink GM, Sudholter EJR, Cnubben NHP, Rietjens IMCM. 2006. Human cytochrome P450 enzymes of importance for the bioactivation of methyl eugenol to the proximate carcinogen 1′-hydroxymethyl eugenol. Chem Res Toxicol 19:111– 116. Johnson JD, Abdo KM. 2005. methyl eugenol in the diet: toxic and pathological aspects. In: Preedy VR, Watson RR, editors. Reviews in food and nutrition toxicity, vol. 3. Boca Raton (FL): CRC Press, Taylor & Francis Group, LLC. Johnson JD, Ryan MJ, Toft JD, Graves SW, Hejtmancik MR, Cunningham ML, Herbert R, Abdo KM. 2000. Twoyear toxicity and carcinogenicity study of methyl eugenol in F344/N rats and B6C3F1 mice. J Agric Food Chem 48:3620–3632. Jones LJ. 2004. Methyl eugenol: twenty-eight day repeated dose oral (dietary and gavage) toxicity study in the rat. SPL Project No. 1834/002. Unpublished report to the Flavor and Extract Manufacturers Association, Washington, DC, USA. Submitted to the World Health Organization by the International Organization of the Flavour Industry, Brussels, Belgium. [cited in FAO/WHO 2009]. Keith LH. 1976. Identification of organic compounds in unbleached treated kraft paper mill wastewaters. Environ Sci Technol 10(6):555–564. Kerckaert GA, Brauninger R, LeBoeuf RA, Isfort RJ. 1996. Use of the Syrian hamster embryo cell transformation assay for carcinogenicity prediction of chemicals currently being tested by the National Toxicology Program in rodent bioassays. Environ Health Perspect 104:1075–1084. [KOWWIN] Octanol–Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 Oct]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm 30 Screening Assessment CAS RN 93-15-2 Larsen W, Nakayama H, Fischer T, Elsner P, Frosch P, Burrows D, Jordan W, Shaw S, Wilkinson J, Marks J, Sugawara M, Nethercott M, Nethercott J. 2002. Fragrance contact dermatitis—a worldwide multicenter investigation (Part III). Contact Dermatitis 46:141–144. Lewis RJ Sr. 2001. Hawley’s condensed chemical dictionary. 14th ed. New York (NY): John Wiley & Sons. p. 735. Lide DR, Milne GWA, editors. 1994. Handbook of data on organic compounds, vol. I. 3rd ed. Boca Raton (FL): CRC Press. p. 867. [LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2009. Ottawa (ON): Health Canada. [cited 2009 Oct]. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/lnhpdbdpsnh-eng.php Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1–2):103–133. Merck. 1997. Safety data sheet. The Merck Chemical Database [online]. Available from: http://chemdat.merck.de Meylan WM, Howard PH. 1993. Computer estimation of the atmospheric gas-phase reaction rate of organic compounds with hydroxyl radicals and ozone. Chemosphere 26(12):2293–2299. Miele M, Dondero R, Ciarallo G, Mazzei M. 2001. Methyl eugenol in Ocimum basilicum L. cv. Genovese Gigante. J Agric Food Chem 49(1):517–521. Miller EC, Swanson DH, Phillips DH, Fletcher TL, Liem A, Miller JA. 1983. Structure–activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res 43:1124–1134. [MITI] Ministry of International Trade & Industry (JP), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (JP): Japan Chemical Industry Ecology-Toxicology & Information Centre. Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests. II. Results from the testing of 270 chemicals. Environ Mutagen 8:1–119. [NCI] National Chemical Inventories [database on a CD-ROM]. 2009. Columbus (OH): American Chemical Society. [cited 2009 Oct]. Available from: http://www.cas.org/products/cd/nci/index.html [NTP] National Toxicology Program (US). 2000a. Toxicology and carcinogenesis studies of methyl eugenol (CAS No. 93-15-2) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Technical Report 491. NIH Publication No. 003950. Available from: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr491.pdf [NTP] National Toxicology Program (US). 2000b. Final report on carcinogens. Background document for methyleugenol (CAS No. 93-15-2). December 13–14, 2000. Meeting of the NTP Board of Scientific Counselors, Report on Carcinogens Subcommittee. Available from: http://ntp.niehs.nih.gov/ntp/newhomeroc/roc10/ME.pdf [NTP] National Toxicology Program (US). 2004. Final study report of the developmental toxicity evaluation for methyl eugenol (CAS No. 93-15-2) administered by gavage to Sprague-Dawley (CD) rats on gestational days 6 through 19. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Study TER97007. [Full report available from National Technical Information Service as NTIS No. PB2004-104348]. [NTP] National Toxicology Program (US). 2005a. Substance profile: methyl eugenol (CAS No. 93-15-2). In: Report on carcinogens. 11th ed. Research Triangle Park (NC): US Department of Health and Human Services, National 31 Screening Assessment CAS RN 93-15-2 Toxicology Program. [cited 2009 Sep 28]. Available from: http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s109meth.pdf [NTP] National Toxicology Program (US). 2005b. Substance profile: safrole (CAS No. 94-59-7). In: Report on carcinogens. 11th ed. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. [cited 2009 Sep 28]. Available from: http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s159safa.pdf [OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [cited 2009 Oct]. Available from: http://oasis-lmc.org/?section=software Osborne BE, Plawiuk M, Graham C, Bier C, Losos G, Broxup B, Procter BG. 1981. A 91-day single dose level dietary study of eugenyl methyl ether and isoeugenyl methyl ether in the albino rat. Bio-Research Laboratories Ltd. Confidential Research Report No. 9203, submitted to the Flavor and Extract Manufacturers Association. [cited in Smith et al. 2002]. [PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 Oct]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm Perry RH, Green D. 1984. Perry’s chemical engineers’ handbook. 6th ed. New York (NY): McGraw-Hill. Phillips DH, Reddy MV, Randerath K. 1984. 32P-Postlabelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally occurring alkenylbenzenes. II. Newborn male B6C3F1 mice. Carcinogenesis 5:1623–1628. Randerath K, Haglund RE, Phillips DH, Reddy MV. 1984. 32P-Postlabelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis 5:1613–1622. Rietjens IM, Boersma MG, van der Woude H, Jeurissen SM, Schutte ME, Alink GM. 2005a. Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat Res 574(1–2):124–138. Rietjens IM, Martena MJ, Boersma MG, Spiegelenberg W, Alink GM. 2005b. Molecular mechanisms of toxicity of important food-borne phytotoxins. Mol Nutr Food Res 49(2):131–158. [RTECS] Registry of Toxic Effects of Chemical Substances. 2008. Record for benzene, 4-allyl-1,2-dimethyloxyl(CAS Registry No. 93-15-2). [updated 2008 Aug; cited 2009 Sep 28]. Hamilton (ON): Canadian Centre for Occupational Health and Safety. Schecter A, Lucier GW, Cunningham ML, Abdo KM, Blumenthal G, Silver AG, Melnick R, Portier C, Barr DB, Barr JR, Stanfill SB, Patterson DG Jr, Needham LL, Stopford W, Masten S, Mignogna J, Tung KC. 2004. Human consumption of methyl eugenol and its elimination from serum. Environ Health Perspect 112(6):678–680. Schiestl RH, Chan WS, Gietz RD, Mehta RD, Hastings PJ. 1989. Safrole, eugenol and methyl eugenol induce intrachromosomal recombination in yeast. Mutat Res 224(4):427–436. Sekizawa J, Shibamoto T. 1982. Genotoxicity of safrole-related chemicals in microbial test systems. Mutat Res 101:127–140. Shaver TN, Bull DL. 1980. Environmental fate of methyl eugenol. Bull Environ Contam Toxicol 24:619–626. 32 Screening Assessment CAS RN 93-15-2 Smith B, Cadby P, Leblanc JC, Setzer RW. 2010. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic. Example: Methyleugenol, CASRN: 93-15-2. Food Chem Toxicol 48:S89-S97. Smith RL, Adams TB, Doull J, Feron VJ, Goodman JI, Marnett LJ, Portoghese PS, Waddell WJ, Wagner BM, Rogers AE, Caldwell J, Sipes IG. 2002. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances—methyl eugenol and estragole. Food Chem Toxicol 40:851–870. Snell ML, Abdo KM, Herbert RA, Eldridge S, Cummingham ML. 2000. Subchronic toxicity of methyl eugenol administered by gavage to F-344 rats and B6C3F1 mice. Toxicologist 54:267 (abstract). Stanfill SB, Calafat AM, Brown CR, Polzin GM, Chiang JM, Watson CH, Ashley DL. 2003. Concentrations of nine alkenylbenzenes, coumarin, piperonal and pulegone in Indian bidi cigarette tobacco. Food Chem Toxicol 41(2):303– 317. Stening P, Gardner I, Kenna JE, Coughtrie MWH, Caldwell J. 1997. Formation of alkenylbenzene macromolecular adducts in human fibroblast V79 cells transfected with human sulfotransferases. Human Exp Toxicol 16:62 (abstract). [TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. [cited 2009 Oct]. Available from: http://accelrys.com/products/discovery-studio/predictivetoxicology.html Turner B, Miller N, Tran D, Powell S. 1989. The environmental monitoring of methyl eugenol, naled and dichlorvos during a pest trapping and eradication program. Sacramento (CA): California Department of Food and Agriculture. 48 p. Tyrrell SP, Zhang X, Cunningham ML, Shane BS. 2000. Comparison of the mutagenicity of methyl eugenol (ME) in vivo in the liver of Big Blue® transgenic rats and mice. Toxicologist 54:229 (abstract). [US EPA] US Environmental Protection Agency. 2006. Methyl eugenol (ME) (203900) fact sheet [Internet]. Washington (DC): US EPA. [cited 2009 Aug]. Available from: http://www.epa.gov/pesticides/biopesticides/ingredients/factsheets/factsheet_203900.htm [US EPA] US Environmental Protection Agency. 2008. Screening-level hazard characterization of high production volume chemicals. Sponsored chemical: estragole (CAS No. 140-67-0). Prepared by: US EPA, Risk Assessment Division, High Production Volume Chemicals Branch, Risk Assessment Division, United States Environmental Protection Agency [US FDA] US Food and Drug Administration. 2001. 21 CFR §172.515. Synthetic flavoring substances and adjuvants [Internet]. Washington (DC): US Department of Health and Human Services. [cited 2009 Aug]. Available from: http://edocket.access.gpo.gov/cfr_2001/aprqtr/pdf/21cfr172.515.pdf Zhou GD, Moorthy B, Bi J, Donnelly KC, Randerath K. 2007. DNA adducts from alkoxyallylbenzene herb and spice constituents in cultured human (HepG2) cells. Environ Mol Mutagen 48(9):715–721. 33 Screening Assessment CAS RN 93-15-2 Appendix 1. Various references re: Naturally occurring methyl eugenol content of essential oils. (adapted from Burfield 2004)) Essential oil Remarks Acorus calamus Acorus calamus Acorus calamus (?) Calamus Indian Calamus Mediterranean Calamus oil Anasarum canadense Aniba rosaedora Artemisia dracunculus Snakeroot oil Rosewood oil Tarragon oil Russian type Tarragon oil Russian type Tarragon oil French Artemisia dracunculus Artemisia dracunculus methyl eugenol content 1.0% 0.9% max <1.0% 36.0- 45.0% 0.11% 11.5% Reference key (see below) Shiva et al. BEOA IFRA website IFRA 06.04.04 EOS TQ TB 5 – 29% EOS 0.8% TB 0.1 to 1.5% EOS type Artemisia dracunculus Tarragon oil French type Artemisia dracunculus (?) Estragon oil <1.5% IFRA website IFRA 06.04.04 Canarium indicum Canarium lucozonium Essential oil Elemi oil Philipines Cananga oil 300-750 ppm 0.44% 0.17% max Duke 2 TQ BEOA Cananga odorata subsp. macrophylla (?) Cananga oil <0.5% IFRA website IFRA 06.04.04 Cananga odorata subsp. genuina Ylang ylang IInd quality Ylang ylang. No details. 0.15% TB 0.154% TQ Croton elutaria Croton elutaria (?) Cascarilla oil W.I. Cascarilla oil W.I. 0.2% max <1.0% BEOA Cinnamomum camphora Not detected BEOA Cinnamomum cassia Cinnamomum cassia (?) Camphor oil white, China Cassia bark oil China Cassia oil 0.03% max. <0.1% Cinnamomum tamala Citrus paradisi Citrus sinensis Cymbopogon citratus Tejpat oil Grapefruit oil Sweet? orange oil geraniol chemotype 0.5% 0.0002% 0.0004% to 18.0% BEOA IFRA website IFRA 06.04.04 Lawr TQ TQ TB Cananga odorata subsp. macrophylla Cananga odorata subsp. genuina 34 IFRA website IFRA 06.04.04 Screening Assessment CAS RN 93-15-2 Cymbopogon nardus Cympopogon nardus Cymbopogon nardus (?) Sri Lanka Sri Lanka Citronella oil Sri Lanka 1.8% max. 3.0% <0.2% Cymbopogon winterianus 0.2% max. Cymbopogon sp. Cymbopogon winterianus (?) Citronella oil, China (Java type) Citronella oil Citronella oil Java <2.0% <2.0% Dacrydium franklinii Daucus carota Daucus carota Daucus carota Huon Pine Oil Carrot seed oil Carrot seed oil Chinese Carrot oil to 98.0% 0.165% 1.23% <0.5% Daucus carota Echinophora tenuifolia Elettaria cardamomum Eucalyptus (globulus?) Hyssop Hyssopus officinalis (?) Carrot oil CO2 extract Turkey Cardamom oil, India sp. name not indicated sp. name not indicated Hyssop oil 0.1% 17.5 – 50.0% tr. to 0.1% 1.07% 0.55% <1.0% Illicium verum Laurus nobilis Laurus nobilis Laurus nobilis Levisticum officianale Levisticum officianale (?) Star Anise oil Bay Laurel oil Bay Laurel oil Bay Laurel oil Lovage Leaf Lovage leaf oil 0.11% 2.8% max. 4.0% 4.62% 1.3% max. <1.5% Lippia citriodora “Magnolia” Verbena oil Michaelia or Magnolia spp. ?? Tea tree oil (chemotypes II, III, IV) (chemotypes I,II,III, IV) 2.3% 2.64% TB TQ trace to >40% IS TB Melaleuca alternifolia Melaleuca bracteata Melaleuca bracteata Melaleuca leucadendron Melaleuca leucadendron (chemotype II, methyl eugenol form) (chemotype I, Ila and llb) Michelia alba Flower and leaf oils Myrstica fragrans Nutmeg Oil Sri Lanka East Indian Nutmeg oil West Indian Nutmeg oil Myrstica fragrans Myrstica fragrans 35 trace; 1.5%; 8.7% and 50% respectively 95-97% 1.6, 94.6 and 6.7% respectively 0.38 & 0.22% respectively 0.8% tr – 1.2% 0.1- 0.2% BEOA FEMA IFRA 06.04.04 BEOA IFRA website IFRA 06.04.04 TB TQ Kam IFRA website IFRA 06.04.04 IFRA TB TB TQ TQ IFRA website IFRA 06.04.04 TQ BEOA TB TQ BEOA IFRA website IFRA 06.04.04 Brophy et al. TB Brophy JJ Kam. TB EOS EOS Screening Assessment CAS RN 93-15-2 Myrstica fragrans (?) Nutmeg oil < 1.0% IFRA website IFRA 06.04.04 Myrstica fragrans (?) Mace oil < 0.5% IFRA website IFRA 06.04.04 Myrtus communis Myrtus communis Ocimum basilicum Myrtle oil Myrtle berry oil Sweet basil oil Ocimum basilicum Ocimum spp. Oil of Egyptian origin Basil oil 5.6% max < 6.0% Ocimum basilicum Ocimum basilicum var. basilicum Basil Oil Described by F & P as Exotic type Basil oil Described by F & P. as European type Basil oil Oil 2.6% 1.6% Described by F & P. as “Small Basil” Described by F & P. as “Basil oil thymol type” (Brazilian Sassafras oilmethyl eugenol type) Geranium oil China Geranium oil Bourbon Geranium oil Egypt Leaf Pimento leaf oil Pimento leaf oil Pimento leaf oil Pimento leaf oil Pimento berry oil Pimento berry oil Pimento berry oil Pimento leaf oil Ocimum basilicum var. “feuilles de laitre” Ocimum basilicum var. “grand vert” Ocimum basilicum var. minimum Ocimum gratissimum var. thymoliferum Ocotea pretiosa Pelargonium graveolens Pelargonium odoratissum Peumus boldus Pimenta dioica Pimenta dioica Pimenta dioica Pimenta dioica Pimenta dioica Pimenta dioica Pimenta dioica (?) Pimenta dioica Pimenta racemosa var. racemosa Pimenta racemosa Pimenta racemosa Pimenta racemosa (?) 1.21% 2.3% Often below 0.2%, Comores (exotic type) to 1.6% BEOA IFRA website IFRA 06.04.04 FEMA F & P. 2.5 to 7% F & P. 55-65% F & P. 55-65% F & P. 1.7% F & P. > 50.0% TB Not detected in either oil Not detected 100-125 ppm to 2% 2% 15.4% 3.9% to 8% 15.0% < 15.0% <15.0% Plant part to produce oil not stated 1.2 – 4.4% Methyl chavicol/methyl eugenol chemotype Bay leaf oil Bay leaf oil Bay oil 48.1% 36 TQ Mazza 4.6% 0.4 to 12.6% < 4.0% BEOA BEOA Duke TB FEMA TQ BEOA TB BEOA IFRA website IFRA 06.04.04 F & P. Aurore et al. TQ TB IFRA website IFRA 06.04.04 Screening Assessment Pimpinella anisum Piper cubeba Ravensara aromatica CAS RN 93-15-2 Rosa sp. Anise oil Cubeb oil Ravensara oil Madagascar Rose absolute Rose otto Rose otto Rose otto Bulgaria Rose oil Bulgaria “different types” Rose oil China Rosa damascena Rosa sp. Rose otto Morocco Rose oil Morocco 0.5% max <2.6% Rosa damascena Rosa sp. Rose otto Turkey Rose oil Turkey 0.5% max <3.0% Rosa sp. Rosa damascena Rosa damascena Rosa spp. Rosa rugosa Rosmarinus officinalis Rosmarinus officinalis Satureia hortensis Satureia montana Satureia montana Rose oil Absolute Rose otto India Rose bud oil Georgia Rose otto, China Rosemary oil Rosemary oil Tunis Summer savoury oil Winter savoury oil Winter savoury oil Balkans Winter savoury oil <3.5% 0.8 to 1.6% 2.0-2.5% <0.1% 0.10% 0.011% >0.01% 0.88% 0.11% 0.7% TB TB TB BEOA IFRA 06.04.04 IFRA 06.04.04 BEOA IFRA 06.04.04 BEOA IFRA 06.04.04 IFRA website TB Shiva et al. TBb SCIB TQ TBa TQ TQ BEOA <1.0% IFRA website Rosa centifolia Rosa centifolia Rosa damascena Rosa damascena Rosa spp. Satureia montana (?) 0.11% Not detected 0.10% 0.6% to 1.9% 1.1 to 3.0% 1.1 to 3.0% 1.6% max < 2.5% < 3.5% TQ BEOA F. & P. IFRA 06.04.04 Syzygium aromaticum Syzygium aromaticum Syzygium aromaticum Syzygium aromaticum Clove bud oil Clove bud oil Clove leaf oil Indonesia Clove oil to 0.15% 0.2% 0.5% <0.5% Tagetes minuta Tagete oil Ajowan oil, India 0.03% 0.03% Trachyspermum ammi TB Shiva et al. TB IFRA website IFRA 06.04.04 Lawr. a TBb Aurore et al.: Aurore GS, Abaul J, Bourgeois P, Luc J. 1998. Antibacterial and antifungal activities of the essential oils of Pimenta racemosa var. racemosa P. Miller (J.W. Moore) (Myrtaceae). J Essential Oil Res 10(2):161–164. BEOA: Brophy JJ: British Essential Oils Association, November 9, 2001; data reproduced by kind permission. Brophy JJ. 1999. Potentially commercial melaleucas. In: Southwell I, Lowe R, editors. Tea tree— the genus Melaleuca. Harwood Academic Publishers. Brophy et al: Brophy et al. 1999. J Essential Oil Res 11:327–332. Duke: Duke J. Chemicals and their biological activities in: Peumus boldus MOLINA (Monimiaceae) – Boldo. See http://www.rain-tree.com/db/Peumus-boldus-phytochem.htm 37 Screening Assessment CAS RN 93-15-2 Duke 2: See http://www.ars-grin.gov:8080/npgspub/xsql/duke/chemdisp.xsql?chemical=METHYLEUGENO EOS: Essential oil safety. Tisserand R, Balacs T. Churchill-Livingstone. 1996. F & P: Franchomme P, Peneol D. 1995. L’Aromatherapie. FEMA: No reference provided in Burfield (2004). IFRA: Annex 1 International Fragrance Association (IFRA) Standards.doc, April 6, 2004. IFRA website: http://www.ifraorg.org/; information as at 01.05.2004. IS: Southwell I. 1999. Tea tree constituents. In: Southwell I, Lowe R. Tea tree—the genus Melaleuca. Harwood Academic Publishers Kam: Kameoka H. 1993. The essential oil constituents of some useful plants from China. In: Recent developments in flavour & fragrance chemistry—Proceedings of the 3rd International Haarman & Reimer Symposium. Publ. VCH NY 1993. Lawr.: Lawrence BW. 1989. Essential oils 1981-7 Allured Publ. Lawr. a: Lawrence BM et al. 1985. Perf Flav 10(6):56–58. December 1985–January 1986. Mazza: Mazza G. 1983. GCMS investigation of volatile components of myrtle berries. J Chromatogr 264:304–311. SCIB: Zhu Lianfeng et al. 1993. Aromatic plants & essential constituents. South China Institute of Botany. Hai Feng Publishing Co. Shiva et al: Shiva MP, Lehri A, Shiva A. 2000. Aromatic & medicinal plants. Publ. IBD 2000. TB: Burfield T. 2000. Natural aromatic materials: odours and origins. Publ. AIA Tampa. TBa: See: http://www.users.globalnet.co.uk/~nodice/new/magazine/odprofile.htm TBb: T. Burfield (unpublished data). TQ: Trade suppliers questionnaire (IFF 2003). 38 Screening Assessment CAS RN 93-15-2 Appendix 2 ConsExpo 4.1 report Product Body Lotion 0.005% methyl eugenol Compound Compound name : CAS number : molecular weight vapour pressure KOW General Exposure Data exposure frequency body weight methyl eugenol 93-15-2 178 0.01 3 g/mol mmHg 3.03 10Log 730 70.9 1/year kilogram Inhalation model: Exposure to vapour : instantaneous release weight fraction compound 5E-5 exposure duration 12 room volume 80 ventilation rate 1 applied amount 8 fraction hour m3 1/hr gram Uptake model: Fraction uptake fraction inhalation rate fraction m3/day 0.7 22 Dermal model: Direct dermal contact with product : instant application weight fraction compound 5E-5 exposed area 1.57E4 applied amount 8 fraction cm2 gram Uptake model: fraction uptake fraction fraction 0.4 Output Inhalation (point estimates) inhalation mean event concentration : inhalation mean concentration on day of exposure: inhalation air concentration year average : inhalation acute (internal) dose : inhalation chronic (internal) dose : 0.000417 0.000416 0.000416 4.53E-5 9.04E-5 mg/m3 mg/m3 mg/m3/day mg/kg mg/kg/day Dermal : point estimates dermal load : dermal external dose : dermal acute (internal) dose : dermal chronic (internal) dose : 2.55E-5 0.00564 0.00226 0.00451 mg/cm2 mg/kg mg/kg mg/kg/day Integrated (point estimates) total external dose: total acute dose (internal): total chronic dose (internal): 0.00571 0.0023 0.0046 mg/kg mg/kg mg/kg/day 39 Screening Assessment CAS RN 93-15-2 Appendix 3. Summary of health effects information for methyl eugenol Endpoint Lowest effect levels1/Results Experimental animals and in vitro Acute toxicity Oral LD50 (mouse) = 540 mg/kg-bw (NTP 2000a). Oral LD50 (rat) = 810 mg/kg-bw (RTECS 2008). Other oral LD50 (rat) = 1179 mg/kg-bw (Beroza et al 1975); 810-1560 mg/kg-bw (Jenner et al. 1964; NTP 2000a; European Commission 2001). Inhalation LD50 (rat) >4800 mg/m3 (Beroza et al. 1975). Dermal LD50 (rabbit) > 2025 mg/kg-bw (Beroza et al. 1975). Intraperitoneal LD50 (mouse) = 540 mg/kg-bw (Engelbrecht et al. 1972, cited in Johnson and Abdo 2005; RTECS 2008). Intravenous LD50 (mouse) = 112 mg/kg-bw (Engelbrecht et al. 1972, cited in Johnson and Abdo 2005; RTECS 2008). Short-term repeated- Lowest oral LOAEL: The LOAEL for maternal toxicity was estimated to be 80 mg/kgdose toxicity bw based on increased liver weight and aversion to dosing in a developmental toxicity evaluation study, in which timed-mated Sprague-Dawley rats (25 per group) were administered by gavage with methyl eugenol at 0, 80, 200, or 500 mg/kg-bw per day on gestational days 6–19 (NTP 2004). Other oral LOAEL: 150 mg/kg-bw based on significantly increased serum gastrin levels in female F-344 rats administered by gavage with methyl eugenol at 0, 9,18.5, 37, 75, 150, or 300 mg/kg-bw per day for 30 and 90 days (Snell et al. 2000). No inhalation or dermal studies were identified. 40 Screening Assessment Endpoint Subchronic toxicity CAS RN 93-15-2 Lowest effect levels1/Results Lowest oral LOAEL: Groups of F344/N rats (10 per sex) were administered methyl eugenol by gavage at doses of 0, 10, 30, 100, 300 or 1000 mg/kg-bw per day, 5 days/week for 14 weeks. At the two highest doses (300 and 1000 mg/kgbw per day), significant increases in the incidence of cytological alteration, cytomegaly, Kupffer cell pigmentation, mixed foci of cellular alteration and bile duct hyperplasia of the liver and atrophy and chronic inflammation of the mucosa of the glandular stomach were observed. At the middle doses (30 and 100 mg/kgbw per day), some changes in hematology, clinical chemistry and relative organ weights were observed. A LOEL of 10 mg/kg-bw per day was identified based on significant (p < 0.05) decreases in body weight or body weight gain (10%), an increase in relative kidney weight in female rats and a decrease in relative thymus weight in male rats (NTP 2000a; Abdo et al. 2001). Other oral LOAEL: 18 mg/kg-bw per day based on significant (p<0.05) increase in relative liver weight in male rats (24 per sex) exposed to methyl eugenol in diets for 91 days (Osborne et al. 1981). Oral LOAEL in mice: Groups of B6C3F1 mice (10 per sex) were administered with methyl eugenol by gavage at doses of 0, 10, 30, 100, 300 or 1000 mg/kg-bw per day, five days per week for 14 weeks. A significant increase in the incidence of cytological alteration, necrosis, bile duct hyperplasia and subacute inflammation of the liver; and atrophy, degeneration, necrosis, edema, mitotic alteration, and cystic glands of the fundic region of the glandular stomach were observed. A LOEL of 30 mg/kg-bw per day was determined based on a significantly (p<0.05) increased incidence of lesions. The NOEL was estimated to be 10 mg mg/kg-bw per day based on mortality, body weight gain, gross and microscopic results (NTP 2000a; Abdo et al 2001). Chronic toxicity/ carcinogenicity No inhalation or dermal studies were identified. Oral carcinogenicity in rats Groups of F344/N rats (50 per sex) were administered methyl eugenol (0.5% methylcellulose as vehicle) by gavage at doses of 0, 37, 75 or 150 mg/kg-bw per day, 5 days/week for 105 weeks. A stop-exposure group of 60 male or female F344/N rats received methyl eugenol by gavage at 300 mg/kgbw per day, 5 days/week for 53 weeks, followed by vehicle only for the remaining 52 weeks. In male rats, significantly increased incidences of hepatocellular adenoma or carcinoma were observed in a dose-related manner: 7/50, 14/50 (p < 0.05), 28/50 (p < 0.01), 43/50 (p < 0.01) and 45/50 (p < 0.01) for 0, 37, 75, 150 and 300 mg/kg-bw per day, respectively. Significantly increased incidences of adenoma were also observed in kidney: 4/50, 6/50, 17/50 (p < 0.01), 13/50 (p < 0.01) and 20/50 (p < 0.01), respectively; fibroadenoma in mammary gland: 5/50, 5/50, 15/50 (p < 0.01), 13/50 (p < 0.01) and 6/50, respectively; and fibroma or fibrosarcoma in skin: 1/50, 12/50 (p < 0.01), 8/50 (p < 0.05), 8/50 (p < 0.01) and 4/50, respectively. In addition, other significantly increased incidences of tumours included hepatocholangioma or hepatocholangiocarcinoma in liver: 13/50 (p < 0.01) at highest dose vs. 0/50 in control; benign or malignant neuroendocrine tumour (cancers of the interface between the endocrine [hormonal] system and the nervous system) in stomach: 7/50 (p < 0.01) at 150 mg/kg-bw vs. 0/50 in control; and mesothelioma in all organs examined: 12/50 (p < 0.01) at 150 mg/kg-bw vs. 1/50 in control. In female rats, significantly increased incidences of hepatocellular adenoma or carcinoma were observed in a dose-related manner: 1/50, 8/50 (p < 0.05), 14/50 (p < 0.01), 34/50 (p < 0.01) and 43/50 (p < 0.01) for 0, 37, 75, 150 and 300 mg/kg-bw per day, respectively. In addition, significantly increased 41 Screening Assessment Endpoint CAS RN 93-15-2 Lowest effect levels1/Results incidences of hepatocholangioma or hepatocholangiocarcinoma were seen in liver at the highest dose: 17/50 (p < 0.01) vs. 0/50 in control. Non-neoplastic LOAEL = 37 mg/kg-bw per day based on a significantly increased incidence of non-neoplastic lesions in the liver and glandular stomach in both sexes in a dose-related manner. The non-neoplastic lesions in liver included eosinophilic and mixed cell foci, hepatocellular hypertrophy, oval cell hyperplasia, cystic degeneration and bile duct hyperplasia (females); while the non-neoplastic lesions in the glandular stomach included mucosal atrophy and neuroendocrine cell hyperplasia (Johnson et al. 2000; NTP 2000a). Oral carcinogenicity in mice: Groups of B6C3F1 mice (50 per sex) were administered methyl eugenol (0.5% methylcellulose as vehicle) by gavage at doses of 0, 37, 75 or 150 mg/kg-bw per day, 5 days/week for 104 weeks. In male mice, significantly increased incidences of hepatocellular adenoma or carcinoma were observed in liver in all methyl eugenol-treated groups: 31/50, 47/50 (p < 0.01), 46/50 (p < 0.01) and 40/50 (p < 0.01) for 0, 37, 75 and 150 mg/kg-bw per day, respectively. In female mice, significantly increased incidences of hepatocellular adenoma or carcinoma were also observed in liver in all methyl eugenol-treated groups: 25/50, 50/50 (p < 0.01), 49/49 (p < 0.01) and 49/50 (p < 0.01) for 0, 37, 75 and 150 mg/kg-bw per day, respectively. In addition, significantly increased incidences of hepatoblastoma were seen in a dose-related manner: 0/50, 6/50 (p < 0.01), 11/50 (p < 0.01) and 15/49 (p < 0.01) for 0, 37, 75 and 150 mg/kg-bw per day, respectively. Non-neoplastic LOAEL = 37 mg/kg-bw per day based on a significantly increased incidence of non-neoplastic lesions in liver and glandular stomach in both sexes in a dose-related manner. The non-neoplastic lesions in liver included eosinophilic foci, oval cell hyperplasia, hepatocyte necrosis, portal hypertrophy, hematopoietic cell proliferation, bile duct hyperplasia and hemosiderin pigmentation; while the non-neoplastic lesions in the glandular stomach included glandular ectasia, mucosal atrophy, chronic active inflammation, epithelial hyperplasia and neuroendocrine cell hyperplasia (Johnson et al. 2000; NTP 2000a). Carcinogenicity in mice by intraperitoneal injection: Groups of young mice (44–56) were treated by intraperitoneal injection to methyl eugenol or its metabolite 1′-hydroxymethyl eugenol on days 1, 8, 15 and 22 of age. The total administered dose per mouse was 0.85 mg methyl eugenol or 0.55 mg 1′hydroxymethyl eugenol (equivalent to 28.3 and 18.3 mg/kg-bw, respectively). Significantly increased incidences of hepatomas were observed during 13 and 18 months (96% for methyl eugenol, p < 0.001, and 93% for 1′-hydroxymethyl eugenol, p < 0.001) compared with vehicle trioctanoin control (41%) (Miller et al. 1983). No inhalation or dermal studies were identified. 42 Screening Assessment Endpoint Reproductive / developmental toxicity CAS RN 93-15-2 Lowest effect levels1/Results Lowest oral LOAEL: 500 mg/kg-bw per day for developmental toxicity based on intrauterine growth retardation and mildly delayed skeletal ossification in timedmated Sprague-Dawley rats (25 per group) administered methyl eugenol by gavage at 0, 80, 200 or 500 mg/kg-bw per day on gestational days 6–19. The NOAEL for developmental toxicity was estimated to be 200 mg/kg-bw per day. The LOAEL for maternal toxicity was determined to be 80 mg/kg-bw per day based on increased liver weight (NTP 2004). No inhalation or dermal studies were identified. Genotoxicity and related endpoints: in vitro microorganisms Mutagenicity Negative In Salmonella typhimurium TA97, TA98, TA100, TA102, TA1535, TA1537 and TA1538 in the presence or absence of metabolic activation by induced liver S9 (Dorange et al. 1977; Sekizawa and Shibamoto 1982; Mortelmans et al. 1986; Schiestl et al. 1989; NTP 2000a). Negative: In Escherichia coli strain WP2 uvrA with liver S9 metabolic activation (Sekizawa and Shibamoto 1982). Positive: methyl eugenol metabolite, 2′,3′-epoxymethyl eugenol, induced point mutation in TA1535 and TA100 (Dorange et al. 1977). DNA damage Positive: Growth inhibitions were observed in Rec assay in Bacillus subtilis strains M45 Rec− and H17 Rec+ (Sekizawa and Shibamoto 1982). Genotoxicity and related endpoints: in vitro mammalian cells Genome rearrangement Positive: Dose-related responses were observed in increased frequency of intraand interchromosomal recombination in the diploid yeast Saccharomyces cerevisiae strain RS9 (Schiestl et al. 1989). Positive: Caused increased frequency of intrachromosomal recombination (deletion) in yeast strain RS112 in the presence and absence of S9 (Brennan et al. 1996). Chromosomal aberration Negative: In Chinese hamster ovary (CHO) cells exposed to methyl eugenol in the presence or absence of S9 (NTP 2000a). Sister chromatid exchange Positive: In CHO cells exposed to methyl eugenol in the presence of S9 (NTP 2000a). Negative: In CHO cells exposed to methyl eugenol in the absence of S9 (NTP 2000a). Unscheduled DNA synthesis (UDS) Positive: Methyl eugenol and metabolite, 1′-hydroxymethyl eugenol, induced dose-related UDS in cultured primary rat hepatocytes (Howes et al. 1990; Chan and Caldwell 1992). The metabolite 1′-hydroxymethyl eugenol showed a stronger induction than the parent substance. Morphological transformation Positive: In Syrian hamster embyro (SHE) cell transformation assay without S9 (Kerckaert et al. 1996). 43 Screening Assessment Endpoint Genotoxicity and related endpoints: in vivo CAS RN 93-15-2 Lowest effect levels1/Results Macromolecular adduct formation Positive: 1′-Hydroxymethyl eugenol formed adducts with DNA and proteins in fibroblast V79 cells transfected with human sulphotransferase in a dose-related manner (Stening et al. 1997). Positive: methyl eugenol formed DNA adducts in cultured human HepG2 cells; methyl eugenol showed higher DNA binding activity than the structurally related carcinogen safrole (Zhou et al. 2007). Gene mutation in methyl eugenol-induced tumours Positive: Methyl eugenol caused chemical-specific mutation of β-catenin gene in mouse liver tumours at 69% incidence (20/29 hepatocellular neoplasms) at codons 32, 33, 34 or 41 compared with only 9% (2/22) in spontaneous tumours or 6% (1/18) in non-genotoxic carcinogen dioxin-induced tumours. However, no dose– response relationship was observed for the gene mutation. Mutations in β-catenin gene caused β-catenin accumulation and upregulation of Wnt signalling, subsequently stimulating cell proliferation and inhibiting apoptosis (Devereux et al. 1999). Positive: Big Blue® female rats were administered methyl eugenol by gavage at a dose of 0 or 1000 mg/kg-bw per day, 5 days/week for 90 days. The mutation frequency of lacI in liver was significantly (p < 0.05) higher in methyl eugenoltreated female rats ([8.69 ± 3.09] × 10−5) compared with controls ([1.20 ± 0.72] × 10−5) (Tyrrell et al. 2000). Positive: Big Blue® male mice were administered methyl eugenol by gavage at a dose of 0 or 300 mg/kg-bw per day, 5 days/week for 90 days. The mutation frequency of lacI in liver in methyl eugenol-treated mice ([4.27 ± 1.09] × 10−5) was not significantly different from that of controls ([4.20 ± 2.15] × 10−5), but the mutation spectrum from the methyl eugenol-treated group was significantly different from that in controls (p < 0.034) (Tyrrell et al. 2000). Micronuclei formation Negative: Methyl eugenol did not increase the frequency of micronucleated normochromatic erythrocytes in peripheral blood and did not alter the percentage of polychromatic erythrocytes among total erythrocytes in bone marrow of male or female B6C3F1 mice administered doses of 10–1000 mg/kg-bw by gavage for 14 weeks (NTP 2000a). DNA binding Positive: methyl eugenol formed DNA adducts in liver of CD-1 female mice administered methyl eugenol by intraperitoneal injection at 100 or 500 mg/kg-bw (Randerath et al. 1984). The major DNA adducts of alkenylbenzenes appeared to be guanine derivatives, and methyl eugenol showed a higher covalent binding index compared with safrole (Randerath et al. 1984). Positive: DNA adducts were observed in the liver of newborn male B6C3F1 mice treated with 0.25, 0.5, 1.0 or 3.0 µmol methyl eugenol on days 1, 8, 15 or 22 after birth, respectively, by intraperitoneal injection. Liver DNA was isolated on days 12, 29 and 43 and analysed by 32P post-labelling procedure. methyl eugenol produced higher levels of DNA adducts (72.7 pmol/mg DNA) compared with estragole (30 pmol/mg DNA) or safrole (14.7 pmol/mg DNA). The liver DNA adducts were prevalently on the N2 of guanine rather than the N6 of adenine (Phillips et al. 1984). 44 Screening Assessment Endpoint CAS RN 93-15-2 Lowest effect levels1/Results Protein adducts Positive: methyl eugenol formed a 44 kDa protein adduct in livers of rats by intraperitoneal injection at doses of 10, 30, 100 or 300 mg/kg-bw (Gardner et al. 1997). The protein has not been characterized. Humans Contact dermatitis was observed in 1.8% of 218 fragrance-sensitive volunteers in a patch test study with 5% methyl eugenol, when the patch test sites were evaluated initially at 2–3 days and at a further 2–5 days after the first reading (Larsen et al. 2002). 1 Each of nine healthy volunteers was given 12 gingersnaps (containing a total of 216 µg methyl eugenol) for breakfast. The background serum level of methyl eugenol was 16.2 pg/g, and the peak serum level of methyl eugenol was 53.9 pg/g in 15 min after consumption of the gingersnaps. The half-life of elimination in humans was about 90 min (Schecter et al. 2004). LC50, median lethal concentration; LD50, median lethal dose; LOAEL, lowest-observed-adverse-effect level; LOEL, lowest-observed-effect level; NOEL, no-observed-effect level. 45