Geology 311 Earth Materials II: Geochemistry
advertisement
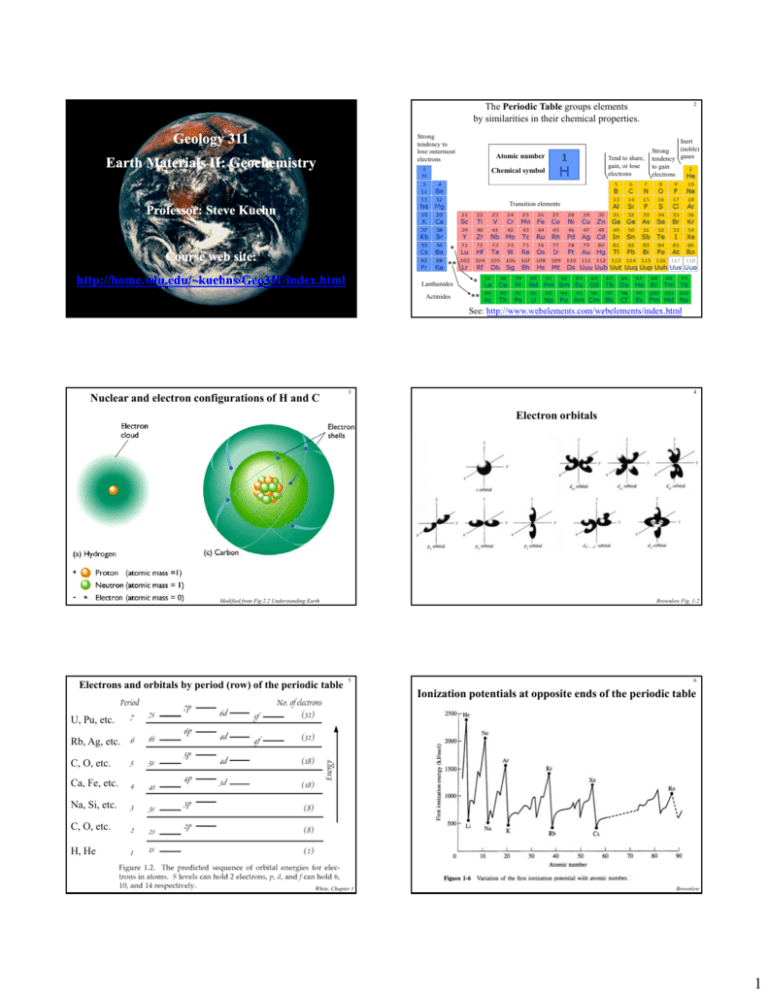
1 Geology 311 Strong tendency to lose outermost electrons Earth Materials II: Geochemistry 2 The Periodic Table groups elements by similarities in their chemical properties. Atomic number Chemical symbol Tend to share, gain, or lose electrons Inert (noble) Strong tendency gases to gain electrons Transition elements P f Professor: Steve St K Kuehn h Course web site: http://home.wlu.edu/~kuehns/Geo311/index.html Lanthanides Actinides See: http://www.webelements.com/webelements/index.html Nuclear and electron configurations of H and C 3 4 Electron orbitals Modified from Fig 2.2 Understanding Earth Electrons and orbitals by period (row) of the periodic table Brownlow Fig. 1-2 5 6 Ionization potentials at opposite ends of the periodic table U, Pu, etc. Rb, Ag, etc. C, O, etc. Ca Fe, Ca, Fe etc. etc Na, Si, etc. C, O, etc. H, He White, Chapter 1 Brownlow 1 7 Overall distribution of 1st ionization potentials 8 Ionic radii – vary by charge and period What will happen if we try and take away 2 e-? White, Chapter 1 9 Solar system elemental abundances White, Chapter 1 10 Types of meteorites, a generalized overview Carbonaceous chondrites (a subcategory of stony) appear to reflect undifferentiated, average solar system material Stony meteorite with chondrules Iron meteorite with crystalline texture Stony-iron meteorite How do we know this? Brownlow The sun’s composition is known from spectroscopy The absorption and emission lines are characteristic of specific elements and can be used to determine the composition of distant gases. 11 12 Chondrite vs. solar abundances – What does this mean? Brownlow 2 13 14 In nuclear reactions that form heavier elements, isotopes matter: Start with different isotopes = get different results Why this pattern? Atomic number = 1 Atomic weight = 2 Atomic number = 1 Atomic weight = 1 Atomic number = 1 Atomic weight = 3 Brownlow Elemental distribution in the solar system Modified from Fig 2.2 Understanding Earth 15 16 The layered, differentiated Earth White, Chapter 1 17 Composition of the atmosphere 18 Major components of clean, dry air include: • Nitrogen (N) – 78% • Oxygen (O2) – 21% • Argon – 0.93 % • Carbon dioxide (CO2) – 0.036% 0 036% Relative abundances of the top 8 elements Figure 1-6 Understanding Earth • All other gases – less than 0.1 % • Plus variable amounts of water Earth Science - Tarbuck & Lutgens 3