P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
Annu. Rev. Cell Dev. Biol. 1999. 15:393–410
c 1999 by Annual Reviews. All rights reserved
Copyright °
VERTEBRATE ENDODERM DEVELOPMENT
James M. Wells and Douglas A. Melton
Department of Molecular and Cellular Biology, and Howard Hughes Medical Institute,
Harvard University, 7 Divinity Avenue, Cambridge, Massachusetts 02138;
e-mail: wells@biohp.harvard.edu; dmelton@biohp.harvard.edu
?
Key Words mouse, chick, frog, gastrulation, gastrointestinal, respiratory,
growth factor signaling, pancreas, organ formation
■ Abstract Endoderm, one of the three principal germ layers, contributes to all organs of the alimentary tract. For simplicity, this review divides formation of endodermal
organs into four fundamental steps: (a) formation of endoderm during gastrulation, (b)
morphogenesis of a gut tube from a sheet of cells, (c) budding of organ domains from
the tube, and (d ) differentiation of organ-specific cell types within the growing buds.
We discuss possible mechanisms that regulate how undifferentiated endoderm becomes
specified into a myriad of cell types that populate the respiratory and gastrointestinal
tracts.
CONTENTS
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Endoderm Formation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Gastrulation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Regulation of Endoderm Cell Fate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A-P Patterning of Early Endoderm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Gut Tube Formation and Patterning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Endoderm Fate Map and Morphogenetic Movements . . . . . . . . . . . . . . . . . . . . .
Signals that Pattern the Forming Gut Tube . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Transcription Factors that Mark Gut Domains . . . . . . . . . . . . . . . . . . . . . . . . . .
Budding of Organs and Cell Differentiation . . . . . . . . . . . . . . . . . . . . . . . . . .
Mesenchymal/Epithelial Interactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Perspectives and Objectives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
393
394
394
395
396
397
397
398
401
403
403
405
INTRODUCTION
How does a single fertilized egg gives rise to an entire organism? The fundamental processes involved in making a worm or a human are much the same. For
example, early in development, an embryo becomes oriented from top-to-bottom
1081-0706/99/1115-0393$08.00
393
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
394
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
(anterior-posterior; A-P) and from front-to-back (dorsal-ventral; D-V). Cells of the
embryo are then partitioned into three groups: the ectoderm, which forms skin and
the central nervous system; the mesoderm, which forms blood, bone, and muscle;
and the endoderm, which forms the respiratory and digestive tracts. Parititioning
of cells occurs via a process called gastrulation, whereby totipotent cells of the epiblast divide, differentiate, and rearrange into three distinct germ layers: ectoderm,
mesoderm, and endoderm. After gastrulation, the endoderm is a one cell-layer
thick sheet of approximately 500 cells (mouse) that will form the epithelial lining of the esophagus, lungs, stomach, and intestines, and is a major component
of many glands including the thyroid, thymus, pancreas, and liver. Subsequent
morphogenetic movements result in the sheet of endoderm being pushed into the
inside of the developing embryo, eventually forming a primitive tube. The tube
will become the gut, and evaginations (buds) from this tube will grow, branch, and
eventually form differentiated functioning organs (Figure 1; see color insert). Some
endodermal functions include taste, gas exchange, digestion, nutrient absorption,
glucose homeostasis, detoxification, blood clotting, and hematopoiesis.
It is not known how endoderm gives rise to the variety of cell types of the
digestive and respiratory tracts. Presumably, a pluripotent endoderm cell (stem
cell) grown in vitro in a regulated environment could generate all endodermal
lineages. Although endoderm stem cells have not been well characterized, exciting
work has been done on mesodermally derived stem cells. Blood (hematopoietic)
stem cells and neural stem cells have been grown in vitro and can be manipulated
to give rise to their respective cell lineages (Johansson et al 1999, Ogawa 1993). In
this review, we discuss progress made in understanding early vertebrate endoderm
development and illuminate some areas that are still relatively unexplored. A longterm goal of this research is to understand how endoderm progenitor cells (stem
cells) generate all differentiated endoderm derivatives. Such endodermal stem
research has implications for the treatment of diseases of the endoderm including
diabetes, cystic fibrosis, and cancer.
?
ENDODERM FORMATION
Gastrulation
Early in vertebrate development, gastrulation results in a group of undifferentiated
cells (the epiblast) forming the three principal germ layers the ectoderm, mesoderm, and endoderm (Figure 2; see color insert. Endoderm is yellow). The start
of this process is evidenced by formation of a structure called the primitive streak
at the posterior of the epiblast. The primitive streak (PS) is marked by expression of several genes and seems to be involved in cell fate specification, whereby
endoderm and mesoderm precursors migrate through the PS in the process of differentiating. Furthermore, the PS is necessary for gastrulation to occur properly,
and embryos that do not form a PS fail to gastrulate (Conlon et al 1994, Waldrip
et al 1998). A fate map of the mouse epiblast was generated using an intracellular
P1: FDS
October 22, 1999
10:2
Annual Reviews
AR094-11
?
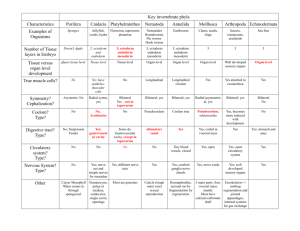
Figure 1 Stages of endoderm development. The top panels show four stages of development of the gastrointestinal tract in mouse embryos (E7.5–E14.5). These stages of gut
morphogenesis are illustrated in the lower panels. Embryonic endoderm is in yellow, and
the visceral (yolk sac) endoderm in light green. The line dividing the E7.5 embryo separates
the lower part of the conceptus, which forms the embryo proper, from the upper part from
which derives extra embryonic structures such as yolk sac. The later stage embryos do not
show the extraembryonic structures. At the end of gastrulation (E7.5), the endoderm is a one
cell-layer thick cup of approximately 500 cells, which covers the mesoderm and ectoderm
of the embryo. Within 24 h (E8.5), a series of morphogenetic processes transforms the cup
into a tube (for more detail, see Figure 4). The dotted yellow line outlines the forming gut
tube of the E8.5 embryo, which shows albumin expression in the ventral foregut. The next
step, the formation of organ buds, is seen in a E10.5 embryo, which has been stained for
the pancreatic/duodenal marker Pdx1. In the lower panel, the schematized E10.5 gut tube
shows the relative positions of organ buds (lung-Lu; liver-Li; stomach-St; dorsal pancreatic
bud-d.Panc; ventral pancreatic bud-v.Panc; and duodenum/intestine-int). The E14.5 upper
panel shows a dissected stomach, pancreas, and duodenum that have been stained for Pdx1
expression. Significant branching and differentiation of organ cell types has occurred [the
pancreas contains all major endocrine (blue) and exocrine cell types by this time].
P1: FDS
October 22, 1999
10:2
Annual Reviews
AR094-11
?
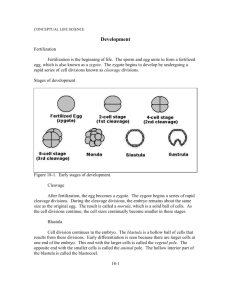
Figure 2 Gastrulation and endoderm formation. The top panel shows relative positions of the embryonic ectoderm (blue), mesoderm (red), and endoderm (yellow)
during gastrulation of the mouse embryo in a partially cut-away view (adapted from
Hogan et al 1994). All embryonic tissues derive from the blue region of the E6 embryo, which is called the primitive ectoderm or epiblast at this stage. The layer of cells
surrounding the E6 epiblast, the visceral endoderm (light green), does not contribute
to the embryonic gut, but does contribute to extraembryonic structures such as the
yolk sac. Gastrulation begins at E6 when cells migrate out of the epiblast through the
primitive streak (PS), at a site that marks the future posterior of the embryo. Within
24 h (early to mid PS), the embryo has a well-formed primitive streak and mesoderm
(red) has begun to ingress between the endoderm and ectoderm in a medial and lateral direction. Embryonic endoderm (yellow), which is first detected over the anterior
primitive streak, migrates along the mid-line in an anterior direction. Migration of
endoderm and mesoderm continues throughout gastrulation (E7-7.5) and is shown in
more detail in the bottom panel (adapted from Beddington & Smith 1993). The arrows
show the relative paths of migration of cells during mouse gastrulation. Yellow arrows
demarcate endoderm migration from the anterior part of the primitive streak. Notochord (pink), endoderm, and mesoderm (red) all derive from the epiblast/primitive
streak. Endoderm follows an anterior path of migration similar to that of the notochord
precursor cells, in contrast to the migration of lateral mesoderm (red).
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
395
tracer and it shows that most definitive (embryonic) endoderm cells originate from
the anterior primitive streak (Lawson et al 1991, Rosenquist 1971). These endoderm precursor cells are thought to migrate though the PS and intercalate into the
overlying visceral endoderm layer (Figure 3; see color insert), eventually displacing these cells (Lin et al 1994). The visceral endoderm does not contribute to the
embryonic gut, but to extraembryonic endoderm, namely, the yolk sac. We do not
discuss its development further here. Consistent with these findings, embryonic
endoderm is first detected over the anterior PS between E6 and E6.5 (Lawson
et al 1986), where it migrates medially in an anterior direction and contributes to
anterior endoderm. Endoderm that exits the PS later contributes to more posterior
endoderm.
Although mesoderm and endoderm cells both arise from the epiblast and migrate through the primitive streak (Lawson et al 1991), it is unclear when or how
cells decide between these two fates (Figure 3; see color insert). Cells may be
instructed to be mesoderm or endoderm prior to migration through the primitive
streak. It is just as likely that cells acquire a fate while migrating through the primitive streak. This possibility is strengthened by the finding that some mesoderm
begins to express Fgf3 upon exiting the streak (Niswander & Martin 1992). Another more stochastic possibility is that cells migrate out of the primitive streak and
remain multipotent until entering an environment that promotes one cell fate over
another. The molecules that regulate endoderm cell fate in chick and mouse are undetermined. In frog, however, several transcription factors (mixer and sox 17α/β)
are able to dictate endodermal cell fate in a cell-autonomous fashion (Henry &
Melton 1998, Hudson et al 1997). It is unclear whether the functionally equivalent
genes exist in higher vertebrates.
?
Regulation of Endoderm Cell Fate
The choice between a mesodermal or endodermal fate may be regulated by soluble
factors. The primitive streak and node (anterior region of the PS) produce numerous
growth factors, including members of the fibroblast growth factor (FGF), transforming growth factor beta (TGFβ), and Wnt growth factor families (Beddington
& Smith 1993, Conlon et al 1994, Faust & Magnuson 1993, Tam & Behringer 1997,
Yamaguchi & Rossant 1995), as well as the morphogen, retinoic acid (Hogan et al
1992). These soluble factors are widely known to influence cell fate and may act
in various combinations to induce either mesoderm or endoderm. Although a direct role for growth factors in mammalian endoderm differentiation is not known,
gene targeting experiments in mouse have demonstrated that growth factors are
involved in many phases of gastrulation. For example, FGF4 is necessary for initial
outgrowth of the epiblast (Feldman et al 1995), whereas the TGFβ family member
nodal, as well as SMAD2, which transmits TGFβ signals from the cell surface to
the nucleus, are necessary for epiblast patterning and formation of the primitive
streak (Conlon et al 1994, Waldrip et al 1998). Later during gastrulation, the bone
morphogenetic protein BMP4 is required for mesoderm differentiation (Winnier
et al 1995).
P1: FDS
October 22, 1999
10:2
Annual Reviews
AR094-11
?
Figure 3 Endoderm of the E7.5 embryo. Gastrulation has formed the basic building
blocks of the embryo by E7.5. (A) The embryonic region has been separated from the
extraembryonic region to show the three germ layers of the postgastrulation embryo
(a cup within a cup); endoderm, yellow; mesoderm, red; ectoderm, blue. The notochord plate (pink) is mesodermally derived, and the primitive streak is derived from the
ectoderm, but contains mesoderm and endoderm precursors (possibly until regression
of the primitive streak after gastrulation). Endoderm is a sheet of cells covering the
outside of the embryo at this stage. Gut (embryonic) endoderm is yellow; visceral (yolk
sac endoderm), which is continuous with the gut endoderm, light green. (B) A late
PS embryo (top) and a schematic representation of a section of this embryo (bottom).
Cells migrating out of the PS that are mesoderm migrate laterally between the endoderm and ectoderm. Cells migrating out of the PS that are gut (embryonic) endoderm
intercalate into the visceral endoderm layer, eventually displacing the visceral (light
green) endoderm cells into the yolk sac. At this stage, the endoderm extends from
the anterion neural ectoderm to the primitive streak. The endoderm is in contact with
different structures along the A-P axis, including ectoderm, notochord plate, node, and
primitive streak.
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
396
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
Regulation of endoderm differentiation by growth factors remains largely unstudied. Perhaps this is due to the absence of early, definitive endoderm-specific
markers in higher vertebrates. One study that circumvented the need for such a
marker used lacZ marked embryonic stem (ES) cells to study the involvement of fibroblast growth factor receptor-1 (FGFR1) during gastrulation (Ciruna et al 1997).
LacZ expressing ES cells that lacked FGFR1 were injected into pre-gastrulation
stage embryos, and the fate of the cells was followed by staining for lacZ. Fgfr1 −/−
ES cells failed to populate anterior mesoderm and endoderm. Instead, these cells
accumulate in the primitive streak and give rise to ectopic neural structures. Ciruna
et al (1997) suggest that cells lacking FGFR1 are unable to migrate properly and
fail to traverse the primitive streak. Another possibility is that these cells are unable to respond to an FGF signal, fail to differentiate into mesoderm or endoderm,
and inappropriately become neural. Neural induction in this manner is not unprecedented since blocking mesoderm/endoderm-inducing signals in frog results
in neural differentiation (Hemmati-Brivanlou & Melton 1997).
?
A-P Patterning of Early Endoderm
At the end of gastrulation (E7.5+ in mouse), gut endoderm is a sheet of approximately 500 cells that extends from the anterior headfold to the PS (Figures 3 and 4;
see color insert). Although the endoderm appears morphologically homogeneous
(Figure 4), A-P differences now exist. For example, the first endoderm to exit
the PS during gastrulation migrates in an anterior direction to overlie the forming
headfold and is by definition older than posterior endoderm, which exits the PS
later in gastrulation (Lawson & Pedersen 1987, Thomas et al 1998). Furthermore,
anterior endoderm expresses several markers not expressed in posterior endoderm,
including cerberus (Bouwmeester et al 1996), Otx1(Rhinn et al 1998), and Hesx1
(Thomas & Beddington 1996). In contrast, posterior endoderm has a higher cell
division rate and expresses intestinal fatty acid binding protein (IFABP; Green et al
1992) and Cdx2 (Beck et al 1995). In frog, Otx and Cdx homologues not only mark
anterior and posterior boundaries, but are necessary for establishing early pattern
(Epstein et al 1997). In mouse, however, it is not clear what role these genes play
in determining early A-P specification of the endoderm.
How does endoderm obtain its A-P identity? The mesoderm and ectoderm have
A-P pattern by the end of gastrulation, and it is possible that endoderm obtains
regional identity through communication with these adjacent structures. For example, anterior endoderm contacts notochord precursor cells (Figure 3; notochord
plate) and ectoderm fated to become head (Lawson & Pedersen 1987). Posterior endoderm, however, is in close association with the node, lateral mesoderm,
and primitive streak. Several experiments demonstrate that a vital interaction between germ layers establishes initial A-P polarity of the embryo. Signals from
early anterior visceral endoderm (Figure 2), which includes the secreted factor
Nodal, provide anterior patterning information to adjacent ectoderm and are necessary for subsequent head formation (Ang & Rossant 1993, Biben et al 1998b,
P1: FDS
October 22, 1999
10:2
Annual Reviews
AR094-11
?
Figure 4 Formation of the gut tube. The top left panel shows an E7.5 embryo with
the endoderm layer peeled off the underlying mesoderm and ectoderm but still attached
at the node. The bottom left panel shows an E8.5 embryo with a well-formed foregut.
The endoderm of the E7.5 embryo (yellow and green, top right) was fate mapped to
the forming E8.5 gut tube (yellow, lower right). The right panels show the endoderm
separated from the mesoderm and ectoderm. The original fate map, of which this is a
schematic, was generated by injection of single endoderm cells with a tracer (HRP),
followed by 24 to 48 h culture (Lawson & Pedersen 1986). Roman numerals I–IV
represent regions of E7.5 endoderm that fate map to regions I–IV of the E8.5 gut.
The anterior-most endoderm (region I) of the E7.5 embryo (upper right) contributes
to the ventral foregut adjacent to the developing heart (pink) and derives organs such
as the liver and ventral pancreas (lower right). Regions II and III (upper right) give
rise to dorsal foregut and midgut (lower right) that are adjacent to the notochord (red
dotted line) and somites (red boxes). These regions ultimately contribute to stomach,
pancreas, duodenum, and part of the intestine. Region IV (upper right) forms the
hindgut, which contributes to the large intestine and colon (lower right). The foregut
tube forms as region I folds over region II (arrow) and migrates in a posterior direction,
whereas the hindgut tube forms when region IV folds over and migrates in an anterior
direction (arrow). The posterior migration of the anterior intestinal portal (AIP) and the
anterior migration of the caudal intestinal portal (CIP), in combination with embryonic
turning, close the midgut and form a primitive gut tube by E9.
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
397
Thomas & Beddington 1996, Varlet et al 1997). There is evidence that anterior
ectoderm pattern is relayed back to gut endoderm adjacent to head ectoderm a day
later. Specifically, pre-gastrulation expression of Hesx1 is first in a small anterior
domain of visceral endoderm, and then in underlying ectoderm. At the end of
gastrulation, Hesx1 expression is both maintained in anterior head ectoderm and
induced in adjacent gut endoderm as it displaces visceral endoderm (Thomas &
Beddington 1996).
Although the molecules that pattern endoderm are undetermined, anterior ectoderm, node and PS express a variety of signaling molecules (Beddington & Smith
1993, Burdsal et al 1998, Conlon et al 1994, Feldman et al 1995, Niswander &
Martin 1992, Stepp et al 1994, Winnier et al 1995, Yamaguchi & Rossant 1995,
Zhao et al 1998). Recently, preliminary evidence implicates the involvement of
early mesoderm and ectoderm in patterning E7.5 mouse endoderm. These studies
have identified that the adjacent germ layers provide soluble, temporally specific
inductive signals that pattern endoderm (JM Wells & DA Melton, in preparation).
These signals are inductive rather than permissive because anterior endoderm
can be respecified when placed more posteriorly. Moreover, posterior endoderm
pattern may be established by the growth factor FGF4, which is expressed in posterior mesoderm and induces those posterior endoderm markers in a concentrationdependant manner. These facts suggest that FGF4 may be a posterior morphogen
for endoderm.
?
GUT TUBE FORMATION AND PATTERNING
The metamorphosis of endoderm from a two-dimensional sheet into a threedimensional tube occurs after gastrulation, and a comparison between fate mapping
studies in frog (Keller 1976), chick (Rosenquist 1971), and mouse (Lawson et al
1986) suggests that formation of a gut tube in many vertebrates is evolutionarily
conserved.
Endoderm Fate Map and Morphogenetic Movements
In mouse, the E7.5 endoderm is a sheet of cells with domains that have been
mapped to gut tube regions of the 8–10 somite (E8.5) embryo (Figure 4) (Lawson
et al 1986). Single endoderm cells of the late gastrula mouse embryo were injected
with an intracellular tracer, and embryos were cultured until the fore and hindgut
formed. The anterior endoderm of the E7.5 embryo (region I) maps to yolk sac and
ventral foregut, the latter of which gives rise to liver, ventral pancreas, lungs, and
stomach. Region II maps to dorsal foregut endoderm, which contributes to esophagus, stomach, dorsal pancreas, and duodenum. Region III maps to midgut/trunk
endoderm, which forms the small intestine. Region IV maps to posterior trunk
endoderm and hindgut, which forms the large intestine. The foregut and hindgut
cavities of the E8.5 embryo now define the D-V axis of the forming gut tube.
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
398
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
These studies have helped clarify the morphogenetic movements that form the
gut tube. For example, the ventral foregut is likely derived by folding over of
region I, in combination with lateral contribution from region II. Similarly, the
ventral hindgut derives from the looping over of region IV. Over the next day of
development, the opening to the foregut, the anterior intestinal portal (AIP), is
pushed caudally, as the opening to the hindgut, the caudal intestinal portal (CIP),
is pushed rostrally (Figure 4). These morphogenetic movements, in combination
with turning of the embryo, close up the midgut and form the primitive gut tube (E9
in mouse). Coincident with gut tube morphogenesis is the change of endoderm
cells from flat squamous cells (Figure 3) to a thickened columnar epithelium.
Also, regions of the tube begin to express different sets of genes. The signals that
establish these gene expression patterns are an area of intense study.
?
Signals that Pattern the Forming Gut Tube
Transcriptional control of cell fate within the gut tube has been well studied,
which is in contrast to our sparse knowledge of the extracellular control of these
genes. As mentioned above, a fundamental change occurs in endoderm between
gastrulation and early somite formation. Specifically, presomite endoderm is not
yet irreversibly determined (JM Wells & DA Melton, in preparation), whereas
endoderm, a day later, has received patterning instructions that render it more determined (Kim et al 1997). Determination is measured by the ability to respecify
endoderm by changing its relative position in the embryo. Unlike presomitic endoderm, early somite stage endoderm (posterior) is no longer competent to express
more anterior (pancreatic) markers, suggesting a determination event has occurred.
The result of these patterning events is a gut tube now loosely divided into
organ domains that can be defined by regions of gut endoderm predetermined
to contribute to certain organs, but still in need of further instruction to express
organ-specific genes. Coincident with early gut tube patterning is a morphological
differentiation of endoderm, where the cuboidal-type endoderm of the E7.5 embryo
begins to morphologically differentiate into a columnar epithelium, which will
eventually line the respiratory and digestive tracts. Although endoderm needs
further instruction throughout the ontogeny of gut organs (see below), the basic
regional pattern of endoderm seems to be established during these early stages of
development.
As discussed below, the signals that determine and pattern endoderm derive
from adjacent structures of both mesodermal, and ectodermal origin, and these
signals seem to be both inductive and permissive in nature. We describe some of
the better-studied examples of early endoderm patterning involved in liver, pancreas, and hindgut specification. Although these studies focus on individual organs,
the conclusions could be extended to specification of other organs that derive from
these domains of the gut tube (e.g. thymus, thyroid, parathyroid, lung, esophagus,
stomach, duodenum, small intestines).
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
399
The Liver The foregut is an epithelial tube ventrally adjacent to cardiac mesoderm and dorsally adjacent to the notochord (Figure 5; see color insert). The liver,
lungs, and ventral pancreas derive from the ventral foregut. In the case of the liver,
specification of ventral foregut by adjacent cardiac mesoderm has been shown in
chick (Le Douarin 1968) and in mouse (Gualdi et al 1996). In mouse, both positive and negative signals restrict liver albumin gene expression to ventral foregut
endoderm (Figure 5, lower left panel). For example, at the 6 somite stage, ventral
foregut endoderm requires a positive signal from cardiac mesoderm to express liver
albumin. At the same time, albumin expression in non-hepatic (liver) endoderm
is inhibited by dorsal mesoderm and ectoderm. Surprisingly, when non-hepatic
trunk endoderm is separated from adjacent dorsal mesoderm, it begins to express
albumin even without a positive signal from cardiac mesoderm. These data suggest
that differences exist between ventral foregut endoderm and trunk endoderm prior
to albumin expression. Transcription factors expressed in pre-hepatic endoderm
(endoderm fated to become liver) may predispose ventral foregut endoderm to
respond to permissive signals from cardiac mesoderm. One candidate is the transcription factor Hex1, which is expressed in pre-hepatic endoderm before cardiac
mesoderm signaling, and later in the liver (Thomas et al 1998). GATA and hepatocyte nuclear transcription factors are also expressed in early endoderm and have
been shown to directly regulate transcription of liver genes (Bossard & Zaret 1998,
Lai & Darnell 1991).
The cardiac mesoderm signals that pattern ventral endoderm are unidentified.
However, there are reciprocal interactions in which endoderm induces cardiac
myogenesis, and this is mediated in part by BMPs. Anterior (pre-hepatic) endoderm is in contact with and able to induce differentiation of cardiac mesoderm
precursor cells (Narita et al 1997, Schultheiss et al 1995). Cardiac induction is
at least partially mediated by BMPs and can be blocked by the BMP antagonist
noggin (Schultheiss et al 1997). Strangely enough, cerberus, another BMP antagonist, which is also expressed in anterior endoderm, has the opposite affect
of noggin. Injection of cerberus RNA into a frog embryo results in induction of
ectopic head, heart, and liver (Bouwmeester et al 1996). Although the mechanism
of cerberus induction of hearts and livers could be a secondary affect, these experiments implicate the involvement of TGFβ signaling in anterior/ventral endoderm
patterning and elucidate a reciprocal inductive event that is pivotal for patterning
of anterior/ventral structures.
?
The Pancreas The dorsal endoderm of the foregut and midgut gives rise to the
dorsal components of the esophagus, stomach, pancreas, and duodenum. Dorsal
endoderm is in contact with the notochord until the dorsal aorta fuse, which results
in separation of endoderm from notochord (E9.5 in mouse). This interaction of
endoderm with notochord is necessary for proper dorsal pancreas formation and
for expression of pancreatic genes (Figure 5, lower middle panel). Deletion of
the notochord in cultured chick embryos results in loss of dorsal pancreatic gene
expression. Co-culture of notochord with endoderm fated to become pancreas
October 22, 1999
10:2
Annual Reviews
?
Figure 5 Signals that establish pattern in the forming gut tube. The top panel represents an embryo that has a notochord, somites, a foregut and
a hindgut, but not a fully formed gut tube (early somite stage chick/mouse embryo). Endoderm, yellow; notochord and somites, red; and cardiac
mesoderm and posterior mesoderm, pink. The boxed regions are shown schematically enlarged below. The lower left panel is a schematic
of early hepatogenesis (liver formation) in mouse. As the anterior endoderm of the embryo folds over to form the foregut pocket, cardiac
mesoderm begins to condense next to the ventral foregut endoderm. Signals arising from the cardiac mesenchyme act positively on adjacent
endoderm and result in expression of the liver marker albumin. Negative signals from dorsal mesoderm and/or ectoderm act concurrently
to repress albumin expression outside of the ventral foregut (liver) domain. Interestingly, ventral foregut endoderm is necessary for cardiac
induction as well (arrow from endoderm to cardiac mesoderm). The lower middle panel depicts signals involved in pancreas formation in chick.
Prepancreatic endoderm (endoderm fated to become pancreatic) responds to permissive factors from the adjacent notochord by expressing
pancreatic markers. Deletion of the notochord results in loss of pancreas formation. Specifically, FGF2 and activin secreted by the notochord
act to repress expression of shh in pancreatic endoderm, which results in pancreas marker expression. The lower right panel illustrates signals
that pattern the hindgut in chick. shh expression in hindgut endoderm induces bmp4 expression in adjacent mesoderm,which induces posterior
Hoxd13 expression in mesoderm, thus posteriorizing adjacent endoderm. At this time mesoderm is condensing into mesenchyme around the
gut, which acts later to further pattern the gut tube.
P1: FDS
AR094-11
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
400
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
(pre-pancreatic endoderm) restores expression of pancreatic genes (Kim et al
1997). These experiments demonstrate that notochord signals are necessary for
dorsal pancreas development. However, notochord cannot induce more posterior
(non-pancreatic) endoderm to express pancreatic genes, thus demonstrating that
these signals are permissive rather than instructive.
How does the notochord induce pancreas development? A noteworthy observation indicated that the growth factor sonic hedgehog (shh) is expressed along
the gut tube, except for the early pancreas buds. Subsequently, it was shown that
deletion of notochord results in an induction of shh expression in the pancreas
and loss of pancreatic gene expression. These data suggest a model in which the
notochord represses shh in pre-pancreatic endoderm, thus facilitating pancreas
formation (Figure 5, lower middle panel). Analysis of specific growth factors secreted by the notochord determined that FGF2 and activin-betaB can repress endodermal expression of shh and that this repression is sufficient for expression of
pancreatic genes such as Pdx1 (Hebrok et al 1998). Moreover, inhibition of Shh
signaling outside of the pancreatic domain of the gut tube with cyclopamine,
a steroid alkaloid that inhibits membrane localization of Shh, results in ectopic
expression of insulin in the stomach and duodenum (Kim & Melton 1998). As
mentioned below, the gene Pdx1 is also expressed in the rostral stomach and duodenum and is necessary for pancreatic development. The ability of cyclopamine to
induce insulin in rostral stomach and duodenum raises the interesting possibility
that Shh is necessary and sufficient to repress a pancreatic phenotype in these adjacent structures. Analysis of Shh mutant mice is under way, and preliminary results
are consistent with a role for Shh in pancreas development (M Hebrok, personal
communication).
The role of other soluble factors in patterning the pancreatic gut domain has
been explored. For example, the teratogen retinoic acid (RA) has been implicated
in foregut A-P patterning. The A-P expression of hox genes in the gut is often regulated by RA, as is seen by deletion of a RA-responsive element from the promoter
of hoxa-4, which leads to loss of expression in lung, stomach, and duodenum
(Packer et al 1998). Furthermore, addition of exogenous RA to embryos in utero
causes an anterior shift in hoxb-1 expression in the developing foregut (Huang et al
1998). As mentioned above, organs such as the liver, lungs, stomach, and pancreas
all derive from the foregut, so regulation of homeobox gene expression in the gut
may play a role development of these organs.
The pancreas develops from a dorsal and ventral domain of the foregut (reviewed in Slack 1995), and preliminary evidence suggests that although RA does
not affect A-P pancreas development (Zeynali & Dixon 1998), RA may play a
role ventral pancreatic development (JM Wells & DA Melton, unpublished observation). The ventral component of the pancreas derives from endoderm that
is immediately adjacent and lateral to presumptive hepatic endoderm. Although
communication between liver and ventral pancreas has not been characterized,
it is possible that interaction between these adjacent structures plays a role in
subsequent morphogenesis.
?
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
401
The Hindgut Early in the development of the gut tube, the posterior endoderm
folds ventrally generating the hindgut (Figure 4). The hindgut forms posterior gut
structures including the large intestines and several studies have determined a role
for posterior Hox genes in proper A-P patterning of the hindgut. For example,
transgenic misexpression of the Hox3.1 gene more anteriorly results in profound
gastrointestinal tissue malformations indicative of a loss of endodermal positional
identity (Pollock et al 1992). What regulates A-P boundaries of Hox genes in the
gut? The chick hindgut represents one of the best-characterized examples where
regulated hox gene expression patterns endoderm (Figure 5, lower right panel).
In the hindgut, a reciprocal interaction between the endoderm and the mesoderm
results in spatially restricted hox gene expression and establishment of posterior
identity in endoderm. Specifically, hindgut endoderm expresses shh, and shh expression is sufficient to induce bmp4 and Hoxd-13 expression in adjacent posterior
mesoderm, but not in more anterior mesoderm. If Hoxd13 is misexpressed in more
anterior mesoderm, the adjacent stomach endoderm is transformed into an intestinal type of endoderm, as assayed by morphology and marker expression (Roberts
et al 1995, 1998). The molecules that transmit the signal from Hoxd-13 expressing
mesenchyme to endoderm are unidentified. What also remains undetermined in
this paradigm is how posterior identity is first established in the hindgut, since
A-P differences in mesoderm already exist at this time of development (Shh induces posterior mesoderm but not anterior mesoderm to express bmp4). These data
suggest that posterior pattern is established in mesoderm and endoderm prior to
hindgut formation and that Shh/Bmp4 refine that pattern through regulating Hox
gene expression. One promising candidate for a posterior determinant is Cdx2.
Cdx2 is expressed in the posterior endoderm and mesoderm of the primitive streak
stage mouse embryo, which is prior to expression of most hox genes. Furthermore,
a cdx homologue in frog regulates posterior Hox gene expression, which in turn
regulates development of the posterior gut (Isaacs et al 1998).
?
Transcription Factors that Mark Gut Domains
Formation and patterning of the gut is an ancient process and has likely employed the use of similar genes for the past 500 million years. In flies, the gene
caudal is an integral part of gut formation. In vertebrates, the caudal homologues (Cdx1, 2, 4) are similarly implicated in patterning and cell differentiation
in the gut (Chawengsaksophak et al 1997, Wicking et al 1998). The cephalochordate amphioxus contains an evolutionary relative of the Hox cluster called
a Parahox cluster. This Parahox cluster contains three genes that have co-linear
developmental expression from anterior to posterior coincident with their chromosomal location in the cluster (Brooke et al 1998). Remarkably, the vertebrate
homologues Cdx2 and Pdx1 are also expressed in an anterior to posterior pattern.
Cdx2, as mentioned above, is expressed in posterior structures that include endoderm and gut. Mutations in Cdx2 in humans and mice result in loss of gut growth
control and formation of colon tumors (Chawengsaksophak et al 1997, Wicking
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
402
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
et al 1998). Pdx1 is a homeobox gene expressed early in the posterior foregut and
midgut and later in the pancreatic islets of Langerhans (Ohlsson et al 1993). Mice
and humans that lack Pdx1 function fail to develop a pancreas (Jonsson et al 1994,
Stoffers et al 1997). Furthermore, humans that carry one null allele of PDX1 often
develop diabetes (Habener & Stoffers 1998). These findings suggest that the basic
mechanisms of gut patterning are shared among most chordates.
The vertebrate gastrointestinal tract is considerably more complex. It is not
surprising that the developing vertebrate gut tube expresses a wide variety of
transcriptional regulators (only some are mentioned below) that may contribute
to vertebrate cellular complexity (Figure 6; see color insert). The A-P and D-V
expression of many genes including the Hox cluster, Pax, Nkx, bHLH, HNF, and
nuclear receptor genes suggests a role in establishment of gut tube pattern. For
example, the anterior gut tube, which gives rise to several organs including thyroid,
parathyroid, thymus, esophagus, and lungs, expresses several Hoxb genes (Huang
et al 1998), Nkx2.6 (Biben et al 1998a, Nikolova et al 1997), Nkx 2.1 (Kimura
et al 1996, Minoo et al 1995, Rossi et al 1995), Pax 8 (Mansouri et al 1998), and
Pax 9 (Peters et al 1998). Numerous transcription factors are also expressed in
the domain of the gut that gives rise to the stomach, pancreas, and duodenum,
including Pdx1 (Ahlgren et al 1996, Jonsson et al 1994), Pax 4 and 6 (Sosa-Pineda
et al 1997, St-Onge et al 1997), Nkx 2.2 (Sussel et al 1998), Isl-1 (Ahlgren et al
1997), and NeuroD (Naya et al 1997). The posterior gut tube, which gives rise
to small intestine, large intestine, and colon, expresses Cdx 1, Cdx 2 (Beck et al
1995), and various genes in the Hoxd cluster (Roberts et al 1998).
A significant effort has been made to elucidate a role for these transcription factors in the morphogenesis of the gastrointestinal tract. To this end, many of these
genes have been genetically ablated in mice (Figure 6). Although most of these
transcription factors are expressed over broad domains of the gut tube, mutant
mouse phenotypes range from global patterning defects to loss of specific cell
types within an organ. For example, deletion of Nkx 2.1, which is expressed in
the foregut, resulted in mice lacking a thyroid gland and showing impaired lung
morphogenesis (Kimura et al 1996). Similarly, Pax 9-deficient mice have global
defects such as absence of thymus, parathyroid glands, and ultimobranchial bodies,
all of which derive from the pharyngeal pouches (Peters et al 1998). In contrast,
loss of Pax 8 results in a specific deletion of follicular cells of the thyroid gland
(Mansouri et al 1998). Transcription factor genes expressed in fore and midgut
include Pdx1, Pax 4, Pax 6, Neuro D, and Nkx2.2, and mice that lack these genes
show a wide range of phenotypes in the stomach, pancreas, and duodenum. Loss
of Pdx1 results in mice that lack a pancreas, and certain endocrine cell types in
the stomach and duodenum (Ahlgren et al 1996, Jonsson et al 1994, Larsson et al
1996). In contrast, absence of either Pax 4, Pax 6, or Nkx2.2 results in loss of specific
populations of hormone-producing (endocrine) cell types in the pancreas and duodenum. Specifically, deletion of Pax 4 or Nkx2.2 results in loss of insulin-producing
cells (Sosa-Pineda et al 1997, Sussel et al 1998), whereas deletion of Pax 6
results in loss of glucagon-producing cells in the pancreas (St-Onge et al 1997).
?
P1: FDS
October 22, 1999
10:2
Annual Reviews
AR094-11
?
Figure 6 Transcription factors in the early gut tube. A schematic representation of
the E9.5 gut tube shows expression of several transcription factors along the gut tube
and regions of the gut that contribute to specific organs. The anterior of the gut tube
is open at this time, and the four pharyngeal pouches are seen as out-pockets along the
anterior tube. Pharyngeal endoderm contributes to the oral cavity, thyroid, parathyroid,
and thymus. Many organs of the respiratory and gastrointestinal tracts are becoming
discernable. Transcription factors that are expressed in overlapping domains along the
foregut, midgut, or hindgut are listed below. For simplicity, transcription factors have
been grouped according to their relative A-P expression at this time of development.
Some genes have a D-V expression difference as well (not shown). The transcription
factors that have been genetically disrupted in mice show a corresponding phenotype,
indicated by the arrows. For example, mice that lack the gene encoding the transcription
factor Pdx1 have no pancreas.
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
403
Although the transcription factor Neuro D is not directly involved in endocrine
cell differentiation, it is necessary for later endocrine cell proliferation (Lee et al
1995, Naya et al 1997). Transcription factors have also been implicated in formation of the exocrine pancreas, which produces and secretes digestive enzymes
into the gut. Formation of the exocrine pancreas depends on PTF1-p48, a bHLHcontaining transcription factor involved in regulation of exocrine gene products
(Krapp et al 1998). Mice without this transcription factor lack the exocrine
pancreas.
It is suspected that these transcription factors dictate cell fate through activation
of specific target genes (Bossard & Zaret 1998, Drummond et al 1996, Huang et al
1998, Jin & Drucker 1996, Lazzaro et al 1991, Moller et al 1992, Ohlsson et al
1993, Thomas et al 1998, Zaret 1998). These studies argue against a simplistic
“one transcription factor one gut cell-type” model and instead suggest variable
transcriptional modulation through complex protein-protein interactions. One such
study suggests that PDX1 alone can bind and activate a specific promoter element
in endocrine cells, but activation of the same promoter element in exocrine cells
happens via formation of a trimeric complex of PDX1 with the transcription factors
PBX1b and MRG1 (MEIS2) (Swift et al 1998).
?
BUDDING OF ORGANS AND CELL DIFFERENTIATION
As discussed above, the E9 mouse embryo has a primitive gut tube with numerous
transcription factors expressed in overlapping domains along the A-P and D-V
axis. It seems plausible that these overlapping expression domains refine pattern in
the gut tube, and establish organ-specific domains, perhaps though transcriptional
activation of target genes. In fact, many of these downstream targets are expressed
in burgeoning organ domains: Albumin is expressed in the liver domain (Gualdi
et al 1996); insulin begins to be expressed in the pancreatic domain (Ohlsson et al
1993); and secretin, seratonin, and somatostatin (Gittes & Rutter 1992) expression
is evident in the duodenal and intestinal domains. Coincident with expression of
these genes is the morphogenesis of organ buds (Figure 1). Although it is not
known how overlapping gene expression may dictate where organ buds will arise,
it is known that organ budding and morphogenesis involves reciprocal interactions
between gut epithelium and the adjacent mesoderm (mesenchyme).
Mesenchymal/Epithelial Interactions
The Lung As the gut tube closes, lateral mesoderm migrates to the gut and condenses around it (E9+ in mouse). Condensed mesoderm (mesenchyme) communicates with gut endoderm to further pattern the gut and regulate differentiation of
organ-specific cell types. For example, gene targeting experiments in mouse have
determined that lung budding involves a Shh-mediated signal from endoderm to
the adjacent mesenchyme, which results in Fgf10 expression and signaling back to
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
404
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
the lung epithelium. These signals initiate branching morphogenesis, which establishes the primary airways and the lobes of each lung. Specifically, shh is expressed
by early foregut endoderm that gives rise to the lung, and mice that lack shh begin
to form a trachea but are missing defined lung buds (Pepicelli et al 1998). Furthermore, deletion of the shh-responsive transcription factors, Gli2 and Gli3, results in
embryos lacking esophagus, trachea, or lungs. shh overexpression in lung results
in induction of Fgf10 in the adjacent mesenchyme, thus implicating this cytokine
as a downstream target involved in outgrowth of the lung epithelium (Bellusci et al
1997a). In fact, FGF10 secreted by anterior mesenchyme does influence budding
and differentiation of lung epithelium (Bellusci et al 1997b), and inhibition of
FGF signaling by either expression of a dominant-negative FGF receptor, or by
genetic ablation of Fgf10, results in disrupted lung development (Min et al 1998,
Peters et al 1994). Although tracheal development was normal in Fgf10−/− mice,
main-stem bronchial formation and subsequent pulmonary branching morphogenesis were completely disrupted. The subsequent branching and growth that occurs
during lung morphogenesis seems to be negatively regulated by TGFβ ligands.
For example, overexpression of bmp4 in lungs inhibits secondary lung branching
of the primary lung buds (Bellusci et al 1996). In addition, abrogation of smad2
and smad3 in lung cultures effectively inhibits TGFβ signaling and results in an
increase of branching morphogenesis (Zhao et al 1998).
?
The Stomach, Pancreas, and Duodenum Several epithelial/mesenchymal interactions have been implicated in the organogenesis of the stomach, pancreas,
and duodenum. For example, epithelial cell proliferation in the stomach and
duodenum is increased in mice that lack the mesenchymal-specific, forkhead transcription factor Fkh6 (Kaestner et al 1997), and these mice have structural abnormalities of the stomach, duodenum, and jejunum. Other epithelial/mesenchymal
interactions regulate the spatial character of the target tissue. For example, glandular stomach mesenchyme of chick embryos can cause tracheal endoderm to
express the stomach marker pepsinogen (Hayashi et al 1988). Conversely, E11-day
mouse embryonic stomach epithelium is uniquely able to regulate the character of
the stomach mesenchyme as measured by induction of stomach-type smooth muscle (Takahashi et al 1998).
Several epithelium/mesenchymal interactions are implicated in the correct spatial development of the pancreatic region of the gut. Pancreatic mesenchyme
evolves from a region of mesoderm that also gives rise to spleen, and some observations suggest that formation of spleen and pancreas are linked. For example,
p48 knockout mice (see above), which lack the exocrine pancreas, have endocrine
cells, but interestingly these cells inappropriately colonize the spleen (Krapp et al
1998). Furthermore, the character of this pancreatic/spleen mesenchyme can be
altered by changing the underlying epithelium. This is illustrated by misexpression
of shh in pancreatic epithelium, which resulted in transformation of the surrounding mesenchyme into duodenal/stomach-type mesenchyme and loss of the spleen
(Apelqvist et al 1997). Although shh misexpression perturbs the architecture of
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
405
the pancreas, all exocrine and endocrine cell types develop, and these mice are
viable. It is not yet clear if spleen and pancreatic mesenchyme share a functional
association or are connected by proximity alone.
Pancreatic mesenchyme is also able to influence the differentiation of pancreatic
epithelium into exocrine or endocrine tissue. An example of this is seen in mice
that lack dorsal pancreatic mesenchyme as a result of disrupting the Islet-1 gene.
Endocrine and exocrine differentiation are also disrupted; however, only exocrine
development can be rescued in vitro by culturing Isl1−/− pancreatic epithelium
with wild-type mesenchyme (Ahlgren et al 1997). Consistent with this, the manual removal of mesenchyme results in pancreatic epithelia forming predominantly
endocrine cells in culture (Miralles et al 1998) or when grown under a kidney capsule (Gittes et al 1996). This mesenchymal regulation of exocrine and endocrine
pancreas development is mediated in part by growth factors. For example, pancreatic rudiments cultured in vitro with TGFβ will form predominantly endocrine
cells (Sanvito et al 1994), whereas the TGFβ antagonist follistatin, which is expressed by mesenchyme, will promote exocrine cell development (Miralles et al
1998). Similarly, the hepatocyte growth factor (HGF) secreted by the mesenchyme
can sustain in vitro growth of pancreatic epithelium (Birchmeier et al 1997) and
promote endocrine cell differentiation (K O’Donnell, personal communication).
These data suggest that early epithelial/mesenchymal interactions are fundamentally important for differentiation of organ-specific cell types and for subsequent
compartmentalization of endocrine cells into islets of Langerhans and exocrine
cells into acini.
?
PERSPECTIVES AND OBJECTIVES
Although endoderm differentiation is far from understood, this review highlights
the remarkable progress made in elucidating how a few early endoderm cells develop and give rise to such a multitude of functionally diverse cell types. In fact,
the endoderm and its organs are now receiving increased attention on two fronts.
First, the recognition that understanding lung, liver, pancreatic, and intestinal development will inform thinking about treating diseases of those organs has led to
increased research activity. Second, the possibility of replacing lost or dysfunctional tissues by stem cell differentiation and/or regeneration represents a broader
challenge to biologists, and this avenue is likely to be explored in the context of
endodermal development. With advances in functional genomics and experimental
ingenuity, there is every reason to believe that significant advances in these areas
will be forthcoming.
ACKNOWLEDGMENTS
We thank many people for invaluable discussion and exchange of ideas, but particular thanks go to Anne Grapin-Botton, Cheng-Jung Lai, Matthias Hebrok, Susanne
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
406
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
Wells, and Lee Henry for critical discussions about this manuscript. JMW is supported by a postdoctoral fellowship from the American Cancer Society. The work
in the authors’ laboratory is supported by the Howard Hughes Medical Institute
and the National Institutes of Health.
Visit the Annual Reviews home page at www.AnnualReviews.org
?
LITERATURE CITED
Ahlgren U, Jonsson J, Edlund H. 1996.
The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient
mice. Development 122:1409–16
Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. 1997. Independent requirement for
ISL1 in formation of pancreatic mesenchyme
and islet cells. Nature 385:257–60
Ang SL, Rossant J. 1993. Anterior mesendoderm induces mouse Engrailed genes in explant cultures. Development 118:139–49
Apelqvist A, Ahlgren U, Edlund H. 1997. Sonic
hedgehog directs specialised mesoderm differentiation in the intestine and pancreas.
Curr. Biol. 7:801–4
Beck F, Erler T, Russell A, James R. 1995.
Expression of Cdx-2 in the mouse embryo
and placenta: possible role in patterning of
the extra-embryonic membranes. Dev. Dyn.
204:219–27
Beddington RS, Smith JC. 1993. Control of
vertebrate gastrulation: inducing signals and
responding genes. Curr. Opin. Genet. Dev.
3:655–61
Bellusci S, Furuta Y, Rush MG, Henderson R,
Winnier G, Hogan BL. 1997a. Involvement
of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 124:53–63
Bellusci S, Grindley J, Emoto H, Itoh N,
Hogan BL. 1997b. Fibroblast growth factor
10 (FGF10) and branching morphogenesis
in the embryonic mouse lung. Development
124:4867–78
Bellusci S, Henderson R, Winnier G, Oikawa
T, Hogan BL. 1996. Evidence from normal
expression and targeted misexpression that
bone morphogenetic protein (Bmp-4) plays
a role in mouse embryonic lung morphogenesis. Development 122:1693–702
Biben C, Hatzistavrou T, Harvey RP. 1998a.
Expression of NK-2 class homeobox gene
Nkx2–6 in foregut endoderm and heart. Mech.
Dev. 73:125–27
Biben C, Stanley E, Fabri L, Kotecha S, Rhinn
M, et al. 1998b. Murine cerberus homologue mCer-1: a candidate anterior patterning molecule. Dev. Biol. 194:135–51
Birchmeier W, Brinkmann V, Niemann C,
Meiners S, DiCesare S, et al. 1997. Role of
HGF/SF and c-Met in morphogenesis and
metastasis of epithelial cells. Ciba Found.
Symp. 212:230–40
Bossard P, Zaret KS. 1998. GATA transcription
factors as potentiators of gut endoderm differentiation. Development 125:4909–17
Bouwmeester T, Kim S, Sasai Y, Lu B, De
Robertis EM. 1996. Cerberus is a headinducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature 382:595–601
Brooke NM, Garcia-Fernandez J, Holland PW.
1998. The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature
392:920–22
Burdsal CA, Flannery ML, Pedersen RA. 1998.
FGF-2 alters the fate of mouse epiblast from
ectoderm to mesoderm in vitro. Dev. Biol.
198:231–44
Chawengsaksophak K, James R, Hammond
VE, Kontgen F, Beck F. 1997. Homeosis and
intestinal tumours in Cdx2 mutant mice. Nature 386:84–87
Ciruna BG, Schwartz L, Harpal K, Yamaguchi
TP, Rossant J. 1997. Chimeric analysis of
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
fibroblast growth factor receptor-1 (Fgfr1)
function: a role for FGFR1 in morphogenetic
movement through the primitive streak. Development 124:2829–41
Conlon FL, Lyons KM, Takaesu N, Barth KS,
Kispert A, et al. 1994. A primary requirement
for nodal in the formation and maintenance
of the primitive streak in the mouse. Development 120:1919–28
Drummond F, Sowden J, Morrison K, Edwards
YH. 1996. The caudal-type homeobox protein Cdx-2 binds to the colon promoter of the
carbonic anhydrase 1 gene. Eur. J. Biochem.
236:670–81
Epstein M, Pillemer G, Yelin R, Yisraeli JK,
Fainsod A. 1997. Patterning of the embryo
along the anterior-posterior axis: the role of
the caudal genes. Development 124:3805–14
Faust C, Magnuson T. 1993. Genetic control of
gastrulation in the mouse. Curr. Opin. Genet.
Dev. 3:491–98
Feldman B, Poueymirou W, Papaioannou VE,
DeChiara TM, Goldfarb M. 1995. Requirement of FGF-4 for postimplantation mouse
development. Science 267:246–49
Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. 1996. Lineage-specific
morphogenesis in the developing pancreas:
role of mesenchymal factors. Development
122:439–47
Gittes GK, Rutter WJ. 1992. Onset of cellspecific gene expression in the developing
mouse pancreas. Proc. Natl. Acad. Sci. USA
89:1128–32
Green RP, Cohn SM, Sacchettini JC, Jackson
KE, Gordon JI. 1992. The mouse intestinal
fatty acid binding protein gene: nucleotide
sequence, pattern of developmental and regional expression, and proposed structure of
its protein product. DNA Cell Biol. 11:31–41
Gualdi R, Bossard P, Zheng M, Hamada Y,
Coleman JR, Zaret KS. 1996. Hepatic specification of the gut endoderm in vitro: cell
signaling and transcriptional control. Genes
Dev. 10:1670–82
Habener JF, Stoffers DA. 1998. A newly discovered role of transcription factors involved in
407
pancreas development and the pathogenesis
of diabetes mellitus. Proc. Assoc. Am. Phys.
110:12–21
Hayashi K, Yasugi S, Mizuno T. 1988.
Pepsinogen gene transcription induced in
heterologous epithelial-mesenchymal recombinations of chicken endoderms and
glandular stomach mesenchyme. Development 103:725–31
Hebrok M, Kim SK, Melton DA. 1998. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes
Dev. 12:1705–13
Hemmati-Brivanlou A, Melton D. 1997. Vertebrate neural induction. Annu. Rev. Neurosci.
20:43–60
Henry GL, Melton DA. 1998. Mixer, a homeobox gene required for endoderm development. Science 281:91–96
Hogan BL, Thaller C, Eichele G. 1992. Evidence that Hensen’s node is a site of retinoic
acid synthesis. Nature 359:237–41
Hogan BL, Beddington R, Costantini F, Lacy
E. 1994. Manipulating the Mouse Embryo. A
Laboratory Manual. New York: Cold Spring
Harbor Lab. Press. 2nd ed.
Huang D, Chen SW, Langston AW, Gudas LJ.
1998. A conserved retinoic acid responsive
element in the murine Hoxb-1 gene is required for expression in the developing gut.
Development 125:3235–46
Hudson C, Clements D, Friday RV, Stott D,
Woodland HR. 1997. Xsox17alpha and -beta
mediate endoderm formation in Xenopus.
Cell 91:397–405
Isaacs HV, Pownall ME, Slack JM. 1998. Regulation of Hox gene expression and posterior
development by the Xenopus caudal homologue Xcad3. EMBO J. 17:3413–27
Jin T, Drucker DJ. 1996. Activation of
proglucagon gene transcription through a
novel promoter element by the caudal-related
homeodomain protein cdx-2/3. Mol. Cell.
Biol. 16:19–28
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. 1999. Identification of a neural stem cell in the adult
?
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
408
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
mammalian central nervous system. Cell
96:25–34
Jonsson J, Carlsson L, Edlund T, Edlund H.
1994. Insulin-promoter-factor 1 is required
for pancreas development in mice. Nature
371:606–9
Kaestner KH, Silberg DG, Traber PG, Schutz G.
1997. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 11:1583–95
Keller RE. 1976. Vital dye mapping of the
gastrula and neurula of Xenopus laevis. II.
Prospective areas and morphogenetic movements of the deep layer. Dev. Biol. 51:118–
37
Kim SK, Hebrok M, Melton DA. 1997.
Notochord to endoderm signaling is required for pancreas development. Development 124:4243–52
Kim SK, Melton DA. 1998. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc. Natl. Acad. Sci.
USA 95:13036–41
Kimura S, Hara Y, Pineau T, FernandezSalguero P, Fox CH, et al. 1996. The T/ebp
null mouse: Thyroid-specific enhancerbinding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 10:60–69
Krapp A, Knofler M, Ledermann B, Berney C,
et al. 1999. The bHLH protein PTF1-p48 is
essential for the formation of the exocrine
and the correct spatial organization of the endocrine pancreas Genes Dev. 12:3752–63
Lai E, Darnell JE Jr. 1991. Transcriptional control in hepatocytes: a window on development. Trends Biochem. Sci. 16:427–30
Larsson LI, Madsen OD, Serup P, Jonsson J,
Edlund H. 1996. Pancreatic-duodenal homeobox 1-role in gastric endocrine patterning.
Mech. Dev. 60:175–84
Lawson KA, Meneses JJ, Pedersen RA. 1986.
Cell fate and cell lineage in the endoderm of
the presomite mouse embryo, studied with an
intracellular tracer. Dev. Biol. 115:325–39
Lawson KA, Meneses JJ, Pedersen RA. 1991.
Clonal analysis of epiblast fate during germ
layer formation in the mouse embryo. Development 113:891–911
Lawson KA, Pedersen RA. 1987. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of
germ layer formation in the mouse. Development 101:627–52
Lazzaro D, Price M, de Felice M, Di Lauro R.
1991. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the
foetal brain. Development 113:1093–104
Le Douarin N. 1968. Synthesis of glycogen
in hepatocytes undergoing differentiation:
role of homologous and heterologous mesenchyma. Dev. Biol. 17:101–14
Lee JE, Hollenberg SM, Snider L, Turner
DL, Lipnick N, Weintraub H. 1995. Conversion of Xenopus ectoderm into neurons by
NeuroD, a basic helix-loop-helix protein.
Science 268:836–44
Lin TP, Labosky PA, Grabel LB, Kozak CA,
Pitman JL, et al. 1994. The Pem homeobox
gene is X-linked and exclusively expressed in
extraembryonic tissues during early murine
development. Dev. Biol. 166:170–79
Mansouri A, Chowdhury K, Gruss P. 1998. Follicular cells of the thyroid gland require Pax8
gene function. Nat. Genet. 19:87–90
Min H, Danilenko DM, Scully SA, Bolon B,
Ring BD, et al. 1998. Fgf-10 is required for
both limb and lung development and exhibits
striking functional similarity to Drosophila
branchless. Genes Dev. 12:3156–61
Minoo P, Hamdan H, Bu D, Warburton D,
Stepanik P, deLemos R. 1995. TTF-1 regulates lung epithelial morphogenesis. Dev.
Biol. 172:694–98
Miralles F, Czernichow P, Scharfmann R. 1998.
Follistatin regulates the relative proportions
of endocrine versus exocrine tissue during pancreatic development. Development
125:1017–24
Moller CJ, Christgau S, Williamson MR, Madsen OD, Niu ZP, et al. 1992. Differential
expression of neural cell adhesion molecule
?
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
QC: FDS/anil
16:12
T1: FDX
Annual Reviews
AR092-13
ENDODERM DEVELOPMENT
and cadherins in pancreatic islets, glucagonomas, and insulinomas. Mol. Endocrinol.
6:1332–42
Narita N, Bielinska M, Wilson DB. 1997. Wildtype endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev. Biol. 189:270–74
Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo
FJ, et al. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroDdeficient mice. Genes Dev. 11:2323–34
Nikolova M, Chen X, Lufkin T. 1997. Nkx2.6
expression is transiently and specifically restricted to the branchial region of pharyngealstage mouse embryos. Mech. Dev. 69:215–18
Niswander L, Martin GR. 1992. Fgf-4 expression during gastrulation, myogenesis, limb
and tooth development in the mouse. Development 114:755–68
Ogawa M. 1993. Differentiation and proliferation of hematopoietic stem cells. Blood
81:2844–53
Ohlsson H, Karlsson K, Edlund T. 1993. IPF1,
a homeodomain-containing transactivator of
the insulin gene. EMBO J. 12:4251–59
Packer AI, Crotty DA, Elwell VA, Wolgemuth
DJ. 1998. Expression of the murine Hoxa4
gene requires both autoregulation and a conserved retinoic acid response element. Development 125:1991–98
Pepicelli CV, Lewis PM, McMahon AP. 1998.
Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr.
Biol. 8:1083–86
Peters H, Neubuser A, Kratochwil K, Balling
R. 1998. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit
craniofacial and limb abnormalities. Genes
Dev. 12:2735–47
Peters K, Werner S, Liao X, Wert S, Whitsett J, Williams L. 1994. Targeted expression of a dominant negative FGF receptor
blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO
J. 13:3296–301
Pollock RA, Jay G, Bieberich CJ. 1992. Al-
409
tering the boundaries of Hox3.1 expression:
evidence for antipodal gene regulation. Cell
71:911–23
Rhinn M, Dierich A, Shawlot W, Behringer RR,
Le Meur M, Ang SL. 1998. Sequential roles
for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction
and specification. Development 125:845–56
Roberts DJ, Johnson RL, Burke AC, Nelson CE,
Morgan BA, Tabin C. 1995. Sonic hedgehog is an endodermal signal inducing Bmp-4
and Hox genes during induction and regionalization of the chick hindgut. Development
121:3163–74
Roberts DJ, Smith DM, Goff DJ, Tabin
CJ. 1998. Epithelial-mesenchymal signaling
during the regionalization of the chick gut.
Development 125:2791–801
Rosenquist GC. 1971. The location of the pregut
endoderm in the chick embryo at the primitive streak stage as determined by radioautographic mapping. Dev. Biol. 26:323–35
Rossi DL, Acebron A, Santisteban P. 1995.
Function of the homeo and paired domain
proteins TTF-1 and Pax-8 in thyroid cell proliferation. J. Biol. Chem. 270:23139–42
Sanvito F, Herrera PL, Huarte J, Nichols A,
Montesano R, et al. 1994. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development 120:3451–62
Schultheiss TM, Burch JB, Lassar AB. 1997. A
role for bone morphogenetic proteins in the
induction of cardiac myogenesis. Genes Dev.
11:451–62
Schultheiss TM, Xydas S, Lassar AB. 1995. Induction of avian cardiac myogenesis by anterior endoderm. Development 121:4203–14
Slack JM. 1995. Developmental biology of the
pancreas. Development 121:1569–80
Sosa-Pineda B, Chowdhury K, Torres M, Oliver
G, Gruss P. 1997. The Pax4 gene is essential for differentiation of insulin-producing
beta cells in the mammalian pancreas. Nature 386:399–402
St-Onge L, Sosa-Pineda B, Chowdhury K,
Mansouri A, Gruss P. 1997. Pax6 is
?
P1: FKZ/FGP
P2: FNE/FGP
September 8, 1999
410
QC: FDS/anil
16:12
WELLS
■
T1: FDX
Annual Reviews
AR092-13
MELTON
required for differentiation of glucagonproducing alpha-cells in mouse pancreas.
Nature 387:406–9
Stepp MA, Urry LA, Hynes RO. 1994. Expression of alpha 4 integrin mRNA and protein
and fibronectin in the early chicken embryo.
Cell. Adhes. Commun. 2:359–75
Stoffers DA, Zinkin NT, Stanojevic V, Clarke
WL, Habener JF. 1997. Pancreatic agenesis
attributable to a single nucleotide deletion in
the human IPF1 gene coding sequence. Nat.
Genet. 15:106–10
Sussel L, Kalamaras J, Hartigan-O’Connor DJ,
Meneses JJ, Pedersen RA, et al. 1998. Mice
lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 125:2213–21
Swift GH, Liu Y, Rose SD, Bischof LJ, Steelman S, et al. 1998. An endocrine-exocrine
switch in the activity of the pancreatic homeodomain protein PDX1 through formation of
a trimeric complex with PBX1b and MRG1
(MEIS2). Mol. Cell. Biol. 18:5109–20
Takahashi Y, Imanaka T, Takano T. 1998. Spatial pattern of smooth muscle differentiation
is specified by the epithelium in the stomach
of mouse embryo. Dev. Dyn. 212:448–60
Tam PP, Behringer RR. 1997. Mouse gastrulation: the formation of a mammalian body
plan. Mech. Dev. 68:3–25
Thomas P, Beddington R. 1996. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse
embryo. Curr. Biol. 6:1487–96
Thomas PQ, Brown A, Beddington RS. 1998.
Hex: a homeobox gene revealing periimplantation asymmetry in the mouse em-
bryo and an early transient marker of
endothelial cell precursors. Development
125:85–94
Varlet I, Collignon J, Robertson EJ. 1997.
Nodal expression in the primitive endoderm
is required for specification of the anterior
axis during mouse gastrulation. Development
124:1033–44
Waldrip WR, Bikoff EK, Hoodless PA, Wrana
JL, Robertson EJ. 1998. Smad2 signaling in
extraembryonic tissues determines anteriorposterior polarity of the early mouse embryo.
Cell 92:797–808
Wicking C, Simms LA, Evans T, Walsh M,
Chawengsaksophak K, et al. 1998. CDX2,
a human homologue of Drosophila caudal,
is mutated in both alleles in a replication
error positive colorectal cancer. Oncogene
17:657–59
Winnier G, Blessing M, Labosky PA, Hogan
BL. 1995. Bone morphogenetic protein-4 is
required for mesoderm formation and patterning in the mouse. Genes Dev. 9:2105–
16
Yamaguchi TP, Rossant J. 1995. Fibroblast
growth factors in mammalian development.
Curr. Opin. Genet. Dev. 5:485–91
Zaret K. 1998. Early liver differentiation: genetic potentiation and multilevel growth control. Curr. Opin. Genet. Dev. 8:526–31
Zeynali B, Dixon KE. 1998. Effects of retinoic
acid on the endoderm in Xenopus embryos.
Dev. Genes Evol. 208:318–26
Zhao J, Lee M, Smith S, Warburton D. 1998.
Abrogation of Smad3 and Smad2 or of Smad4
gene expression positively regulates murine
embryonic lung branching morphogenesis in
culture. Dev. Biol. 194:182–95
?