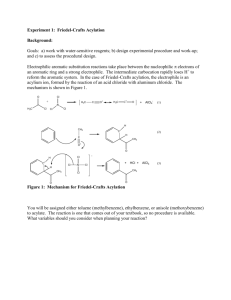
Acid, Base, Neutral Extraction
advertisement
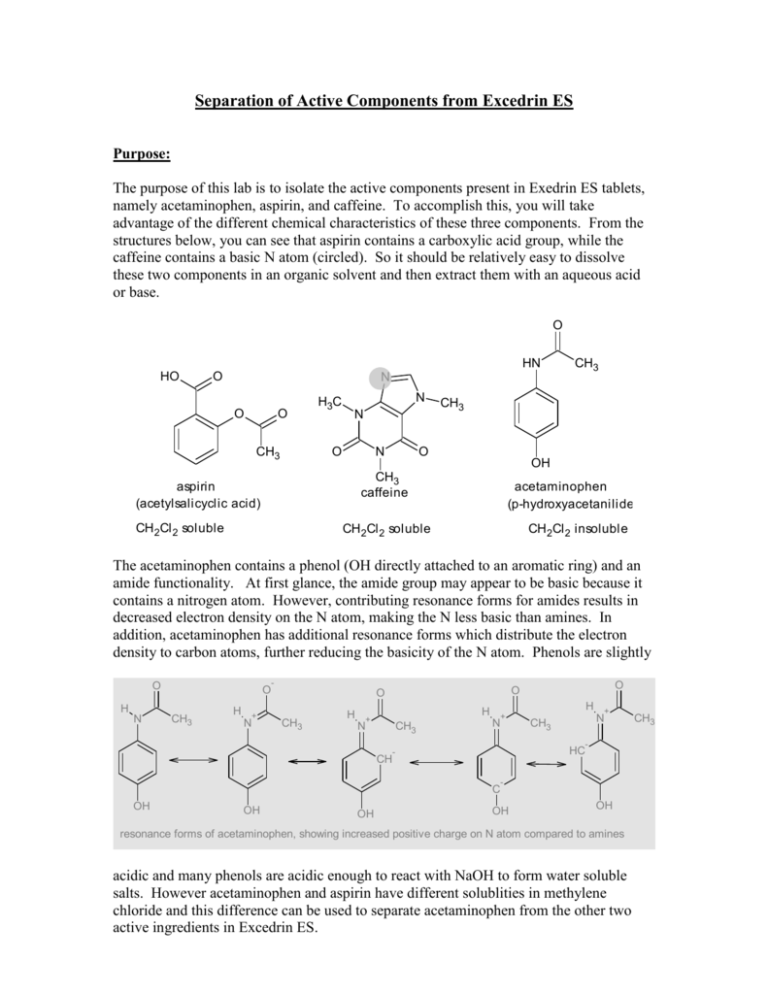
Separation of Active Components from Excedrin ES Purpose: The purpose of this lab is to isolate the active components present in Exedrin ES tablets, namely acetaminophen, aspirin, and caffeine. To accomplish this, you will take advantage of the different chemical characteristics of these three components. From the structures below, you can see that aspirin contains a carboxylic acid group, while the caffeine contains a basic N atom (circled). So it should be relatively easy to dissolve these two components in an organic solvent and then extract them with an aqueous acid or base. O HN HO O N O O CH3 H3C N N O N CH3 O OH CH3 caffeine aspirin (acetylsalicyclic acid) CH 2Cl 2 soluble CH3 acetaminophen (p-hydroxyacetanilide) CH 2Cl 2 soluble CH 2Cl 2 insoluble The acetaminophen contains a phenol (OH directly attached to an aromatic ring) and an amide functionality. At first glance, the amide group may appear to be basic because it contains a nitrogen atom. However, contributing resonance forms for amides results in decreased electron density on the N atom, making the N less basic than amines. In addition, acetaminophen has additional resonance forms which distribute the electron density to carbon atoms, further reducing the basicity of the N atom. Phenols are slightly O H N O CH3 H + N - CH3 H O O O H + N CH3 H + N + N CH3 - HC - CH - C OH OH OH OH OH resonance forms of acetaminophen, showing increased positive charge on N atom compared to amines acidic and many phenols are acidic enough to react with NaOH to form water soluble salts. However acetaminophen and aspirin have different solublities in methylene chloride and this difference can be used to separate acetaminophen from the other two active ingredients in Excedrin ES. CH3 Methylene chloride is not soluble in water and will form a separate liquid layer when shaken with water and allowed to settle in a separatory funnel. The two phases can be separated, thereby creating two fractions: one containing substances soluble in methylene chloride and one containing substances soluble in water. By adjusting the pH of the aqueous phase by adding NaOH or HCl, the nature of organic substance, and hence its solubility in water, can be manipulated. HO + O Na O O CH3 O - O NaOH O CH3 HCl water soluble CH 2Cl 2 soluble Cl O N N N + NH N H3C O O CH3 CH 2Cl 2 soluble CH3 HCl NaOH H3C O N N N CH3 O CH3 water soluble . You will use the properties described above to separate the components of Excedrin ES. As you follow the procedure, you will use your knowledge of the properties of each substance to determine the effect of each step on the separation scheme and the identity of the component(s) present at each step of the scheme. Based on these two properties, we can develop a scheme to separate these three compounds. First, use an organic solvent to dissolve the aspirin, and the caffeine. Since the acetaminophen is less soluble in organic solvents, it has effectively been isolated from the other two. Now we will extract the organic layer with an aqueous acid. The acid will react with the caffeine to produce a salt that is water soluble. Remove the aqueous layer and make it basic to regenerate the caffeine. The caffeine is once again soluble in organic solvent and so can be separated from the aqueous phase. Finally, extract the original organic layer with an aqueous base. It will react with the aspirin to produce a salt that is water soluble. Remove the aqueous layer and make it acidic to regenerate the aspirin. The aspirin is once again soluble in organic solvent and so can be separated from the aqueous phase. Unfortunately, there is no such thing as a 100% efficient extraction. Because of this you will not extract all of the original components. In addition, each crude product you collect will contain small amounts of one or more of the other compounds. To further purify you crude compounds, you will need to recrystallize them using the procedures you learned in previous labs. Equipment/supplies ExcedrinES: 2 tablets/student 3M NaOH (~10mL/student) 25mL E. flasks 5/student TLC plates Methylene chloride (~27ml/student) aspirin standard Ethanol (~5 mL/student) caffeine standard small beaker 5/student iodine chamber 1 hot plate/stirrer stir bar acetic acid/butyl acetate developing solvent 5% HCl (~10mL/student) acetaminophen standard boiling stick (3/student) Procedure I Sample Preparation 1. Carefully weigh 2 Excedrin ES tablets and grind the tablets into powder using a mortar and pestle. 2. Transfer the resulting powder into a clean, 25 mL Erlenmeyer flask and add ~7 mL methylene chloride. (Note: methylene chloride is a suspected carcinogen, handle with care!-keep the flask under a snorkel hood) 3. Add a magnetic stir bar and stir the contents on a magnetic stirrer/hot plate for 5 minutes with gentle warming. The caffeine and aspirin are soluble in the methylene chloride whereas the acetaminophen and binders are not. II Isolation of crude acetaminophen 4. Filter the material through a small Buchner funnel (pre-weigh the filter paper) and wash the solids with ~5mL additional methylene chloride. (this helps remove any methylene chloride soluble material still entrained (trapped) in the solids). Allow the solids to dry for 5 minutes and then weigh the filter paper/solids. Determine the mass of the solids. Label the solids ‘A’ 5. While the solids in step 4 are drying, transfer the filtrate from step 4 to a separatory funnel (make sure the stopcock is in the closed position). Label the filtrate ‘B’ 6. Transfer the solids ‘A’ in the funnel to a clean 25 mL Erlenmeyer flask and add ~5-7 mL ethanol. Add a boiling stick (provided) and gently boil the contents on a hot plate. Filter the contents while still warm by gravity filtration (pre-weigh the filer paper) into a small pre-weighed beaker and wash the contents of the filter paper with ~ 5mL warm ethanol. Place the filter paper on a watch glass and allow to air dry. Label the solids ‘C’. Obtain the weight of solids ’C’ when dry. 7. Concentrate the ethanol solution in the beaker to dryness by gently heating to a boil. Be careful not to over heat the material. After cooling, weigh the contents of the beaker. Calculate the amount of crude acetaminophen recovered. Label this ‘D’ III Isolation of aspirin 9. Add ~5mL 5% HCl to the separatory funnel described in step 5. Cap the separatory funnel and invert the funnel. While in the inverted position, open the stopcock to release any pressure buildup. Repeat this process once more, and then agitate the funnel to thoroughly mix the contents. Open the stopcock once more in the inverted position. Then close the stopcock and place the separatory funnel in a ring support and allow the layers to separate. 10. Drain the methylene chloride layer into a clean 25mL Erlenmeyer flask; drain the aqueous layer into another 25mL Erlenmeyer flask. Label the methylene chloride layer ‘E’ and the aqueous layer ‘F’ 11. Return the methylene chloride layer ‘E’ to the separatory funnel and repeat step 9. 12. Drain the aqueous layer from step 11 into flask ‘F’. Return the methylene chloride layer to flask ‘E’ 13. Remove trace amounts of water from the methylene chloride layer (E) by adding small amounts of anhydrous magnesium sulfate (MgSO4) until the MgSO4 easily disperses and remains suspended in the liquid. Allow the mixture to stand for 5 minutes. 14. Filter the MgSO4 suspension through a Kim-wipe placed in a short gravity funnel suspended over a pre-weighed beaker. The Kim-wipe should remove the magnesium sulfate. The filtrate in the beaker should be clear. If not, re-filter. 15. Evaporate the methylene chloride on a hot plate at low setting (2-3). Weigh the solids that remain in the beaker (‘E’) IV Isolation of caffeine 16. Transfer the contents of flask ‘F’ to the separatory funnel. Slowly add 10% NaOH(aq) with gentle swirling until the solution is basic to litmus paper. (use a glass rod to sample the mixture and touch the end of the rod to a piece of litmus paper) 17. Add 5mL methylene chloride to the separatory funnel and shake the contents to mix the contents. Allow the contents to settle into two phases. 18. Drain the methylene chloride layer into flask ‘G’ 19. Add 5mL fresh methylene chloride to the contents of the separatory funnel and repeat step 17. 20. Drain the methylene chloride layer into flask ‘G’; drain the aqueous layer into flask ‘H’ 21. Dry the methylene chloride layer and filter as described in steps 13-14 (make sure to pre-weigh the beaker.) 22. Evaporate the methylene chloride layer as described in step 15 . Weigh the residue (‘G’) VI Purification of crude substances. 23. Re-crystallize the crude solid ‘D as follows: Add ~2-3mL water and heat to near boiling. Add additional water slowly until the solids dissolve. Allow the solution to cool and then place in an ice bath. Recover the solids by vacuum filtration (Buchner funnel). Allow the solids to dry and obtain the mass. 24. Recrystallize the crude aspirin as follows: Add 2-3 mL of 95% ethanol to the beaker containing the crude aspirin and warm (do not boil) the mixture to dissolve the crystals. If the crystals do not all dissolve, add 2-3 mL more of the ethanol and continue to warm the mixture. When the crystals are all dissolved, add 8-10 mL of warm water, cover the beaker with a watch glass, and let the solution cool slowly. Crystals of aspirin will form. Complete the recrystallization by cooling in an ice bath. 25. Recrystallize the crude caffeine by adding just enough hot water to dissolve the crude material. Allow the resulting solution to cool to room temperature. Place the mixture in an ice bath to complete the recrystallization. VII Charactaerization of isolated substances 26. Obtain melting points for each of the separated components of Excedrin ES. Since caffeine sublimes readily, the capillary holding the caffeine sample should be sealed by melting the end of the capillary tube in a flame. Process Scheme Asp=aspirin asp, acet, caf, bind Acet=acetaminophen Caf=caffeine CH 2Cl 2 bind=binders insoluble B A soluble (filtrate) HCl/ H2O ethanol aq sol CH 2Cl 2 insoluble C D soluble (filtrate) sol E F NaOH/H2O/ CH2Cl 2 CH 2Cl 2 sol G aq sol H Report the amount and weight percent of aspirin in Excedrin ES. Also comment on its purity by comparing its m.p. and IR to literature values. Report the amount and weight percent of acetaminophen in Excedrin ES. Also comment on its purity by comparing its m.p. and IR to literature values. Report the amount and weight percent of caffeine in Excedrin ES. Also comment on its purity by comparing its m.p. and IR to literature values. Did the weight of all three components add up to the weight of the tablet? Why or why not? (Updated 10/18/2010)