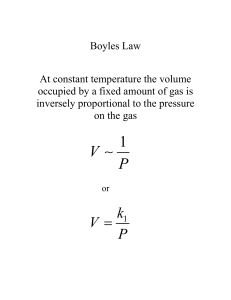
Chapter 17 Temperature and the Kinetic Theory of Gases
advertisement

Chapter 17 Temperature and the Kinetic Theory of Gases Conceptual Problems 1 (a) (b) (c) • True or false: The zeroth law of thermodynamics states that two objects in thermal equilibrium with each other must be in thermal equilibrium with a third object. The Fahrenheit and Celsius temperature scales differ only in the choice of the ice-point temperature. The Celsius degree and the kelvin are the same size. (a) False. If two objects are in thermal equilibrium with a third, then they are in thermal equilibrium with each other. (b) False. The Fahrenheit and Celsius temperature scales also differ in the number of intervals between the ice-point temperature and the steam-point temperature. (c) True. 2 • How can you determine if two objects are in thermal equilibrium with each other when putting them into physical contact with each other would have undesirable effects? (For example, if you put a piece of sodium in contact with water there would be a violent chemical reaction.) Determine the Concept Put each in thermal equilibrium with a third body; that is, a thermometer. If each body is in thermal equilibrium with the third, then they are in thermal equilibrium with each other. 3 • [SSM] ″Yesterday I woke up and it was 20 ºF in my bedroom,″ said Mert to his old friend Mort. ″That’s nothing,″ replied Mort. ″My room was –5.0 ºC.″ Who had the colder room, Mert or Mort? Picture the Problem We can decide which room was colder by converting 20°F to the equivalent Celsius temperature. Using the Fahrenheit-Celsius conversion, convert 20°F to the equivalent Celsius temperature: tC = 5 9 (t F − 32°) = 95 (20° − 32°) = −6.7°C so Mert' s room was colder. 1713 1714 Chapter 17 4 • Two identical vessels contain different ideal gases at the same pressure and temperature. It follows that (a) the number of gas molecules is the same in both vessels, (b) the total mass of gas is the same in both vessels, (c) the average speed of the gas molecules is the same in both vessels, (d) None of the above. Determine the Concept Because the vessels are identical (have the same volume) and the two ideal gases are at the same pressure and temperature, the ideal-gas law ( PV = NkT ) tells us that the number of gas molecules must be the same in both vessels. (a ) is correct. 5 • [SSM] Figure 17-18 shows a plot of volume versus absolute temperature for a process that takes a fixed amount of an ideal gas from point A to point B. What happens to the pressure of the gas during this process? Determine the Concept From the ideal-gas law, we have P = nR T V . In the process depicted, both the temperature and the volume increase, but the temperature increases faster than does the volume. Hence, the pressure increases. 6 • Figure 17-19 shows a plot of pressure versus absolute temperature for a process that takes a sample of an ideal gas from point A to point B. What happens to the volume of the gas during this process? Determine the Concept From the ideal-gas law, we have V = nR T P. In the process depicted, both the temperature and the pressure increase, but the pressure increases faster than does the temperature. Hence, the volume decreases. 7 • If a vessel contains equal amounts, by mass, of helium and argon, which of the following are true? (a) (b) (c) (d) The partial pressure exerted by each of the two gases on the walls of the container is the same. The average speed of a helium atom is the same as that of an argon atom. The number of helium atoms and argon atoms in the vessel are equal. None of the above. Determine the Concept (a) False. The partial pressure exerted by each gas in the mixture is the pressure it would exert if it alone occupied the container. Because the densities of helium and argon are not the same, these gases occupy different volumes and, hence, their partial pressures are not the same. (b) False. Assuming the gasses have been in the vessel for some time, their average kinetic energies would be the same. Because the densities of helium and Temperature and the Kinetic Theory of Gases 1715 argon are not the same, their average speeds must be different. (c) False. We know that the volumes of the two gasses are equal (they both occupy the full volume of the container) and that their temperatures are equal. Because their pressures are different, the number of atoms of each gas must be different, too. (d) Because none of the above is true, (d ) is true. 8 • By what factor must the absolute temperature of a gas be increased to double the rms speed of its molecules? Determine the Concept We can use vrms = 3RT M to relate the temperature of a gas to the rms speed of its molecules. Express the dependence of the rms speed of the molecules of a gas on their absolute temperature: 3RT M where R is the gas constant, M is the molar mass, and T is the absolute temperature. vrms = Because v rms ∝ T , the temperature must be quadrupled in order to double the rms speed of the molecules. 9 • Two different gases are at the same temperature. What can be said about the average translational kinetic energies of the molecules? What can be said about the rms speeds of the gas molecules? Determine the Concept The average kinetic energies are equal. The ratio of their rms speeds is equal to the square root of the reciprocal of the ratio of their molecular masses. 10 • A vessel holds a mixture of helium (He) and methane (CH4). The ratio of the rms speed of the He atoms to that of the CH4 molecules is (a) 1, (b) 2, (c) 4, (d) 16 Picture the Problem We can express the rms speeds of the helium atoms and the methane molecules using vrms = 3RT M . Express the rms speed of the helium atoms: vrms, He = 3RT M He 1716 Chapter 17 Express the rms speed of the methane molecules: Divide the first of these equations by the second to obtain: Use Appendix C to find the molar masses of helium and methane: vrms. CH 4 = vrms, He vrms. CH 4 vrms, He vrms. CH 4 3RT M CH 4 = M CH 4 = 16 g/mol =2 4 g/mol M He and (b) is correct. 11 • True or false: If the pressure of a fixed amount of gas increases, the temperature of the gas must increase. False. Whether the pressure changes also depends on whether and how the volume changes. In an isothermal process, the pressure can increase while the volume decreases and the temperature is constant. 12 • Why might the Celsius and Fahrenheit scales be more convenient than the absolute scale for ordinary, nonscientific purposes? Determine the Concept For the Celsius scale, the ice point (0°C) and the boiling point of water at 1 atm (100°C) are more convenient than 273 K and 373 K; temperatures in roughly this range are normally encountered. On the Fahrenheit scale, the temperature of warm-blooded animals is roughly 100°F; this may be a more convenient reference than approximately 300 K. Throughout most of the world, the Celsius scale is the standard for nonscientific purposes. 13 • An astronomer claims that the temperature at the center of the Sun is 7 about 10 degrees. Do you think that this temperature is in kelvins, degrees Celsius, or doesn’t it matter? Determine the Concept Because T = tC + 273.15 K and 107 >> 273, it doesn’t matter. 14 • Imagine that you have a fixed amount of ideal gas in a container that expands to maintain constant pressure. If you double the absolute temperature of the gas, the average speed of the molecules (a) remains constant, (b) doubles, (c) quadruples, (d) increases by a factor of 2 . Determine the Concept The average speed of the molecules in an ideal gas depends on the square root of the kelvin temperature. Because vav ∝ T , doubling Temperature and the Kinetic Theory of Gases 1717 the temperature while maintaining constant pressure increases the average speed by a factor of 2 . (d ) is correct. 15 • [SSM] Suppose that you compress an ideal gas to half its original volume, while also halving its absolute temperature. During this process, the pressure of the gas (a) halves, (b) remains constant, (c) doubles, (d) quadruples. Determine the Concept From the ideal-gas law, PV = nRT , halving both the temperature and volume of the gas leaves the pressure unchanged. (b) is correct. 16 • The average translational kinetic energy of the molecules of a gas depends on (a) the number of moles and the temperature, (b) the pressure and the temperature, (c) the pressure only, (d) the temperature only. Determine the Concept The average translational kinetic energy of the molecules of an ideal gas Kav depends on its temperature T according to K av = 32 kT . (d ) is correct. 17 •• [SSM] Which speed is greater, the speed of sound in a gas or the rms speed of the molecules of the gas? Justify your answer, using the appropriate formulas, and explain why your answer is intuitively plausible. Determine the Concept The rms speed of molecules of an ideal gas is given by 3RT γRT and the speed of sound in a gas is given by vsound = . vrms = M M The rms speed of the molecules of an ideal-gas is given by: vrms = The speed of sound in a gas is given by: vsound = Divide the first of these equations by the second and simplify to obtain: For a monatomic gas, γ = 1.67 and: vrms = vsound 3RT M γRT M 3RT M = 3 γ γRT M vrms, monatomic vsound = 3 = 1.34 1.67 1718 Chapter 17 For a diatomic gas, γ = 1.40 and: vrms, diatomic vsound = 3 = 1.46 1.40 In general, the rms speed is always somewhat greater than the speed of sound. However, it is only the component of the molecular velocities in the direction of propagation that is relevant to this issue. In addition, In a gas the mean free path is greater than the average intermolecular distance. 18 •• Imagine that you increase the temperature of a gas while holding its volume fixed. Explain in terms of molecular motion why the pressure of the gas on the walls of its container increases. Determine the Concept The pressure is a measure of the change in momentum per second of a gas molecule on collision with the wall of the container. When the gas is heated, the average velocity and the average momentum of the molecules increase and, as a consequence, the pressure exerted by the molecules increases. 19 •• Imagine that you compress a gas while holding it at fixed temperature (perhaps by immersing the container in cool water). Explain in terms of molecular motion why the pressure of the gas on the walls of its container increases. Determine the Concept If the volume decreases the pressure increases because more molecules hit a unit of area of the walls in a given time. The fact that the temperature does not change tells us the molecular speed does not change with volume. 20 •• Oxygen has a molar mass of 32 g/mol and nitrogen has a molar mass of 28 g/mol. The oxygen and nitrogen molecules in a room have (a) (b) (c) (d) (e) (f) equal average translational kinetic energies, but the oxygen molecules have a larger average speed than the nitrogen molecules have. equal average translational kinetic energies, but the oxygen molecules have a smaller average speed than the nitrogen molecules have. equal average translational kinetic energies and equal average speeds. equal average speeds, but the oxygen molecules have a larger average translational kinetic energy than the nitrogen molecules have. equal average speeds, but the oxygen molecules have a smaller average translational kinetic energy than the nitrogen molecules have. None of the above. Picture the Problem The average kinetic energies of the molecules are given by K av = (12 mv 2 )av = 32 kT . Assuming that the room’s temperature distribution is Temperature and the Kinetic Theory of Gases 1719 uniform, we can conclude that the oxygen and nitrogen molecules have equal average kinetic energies. Because the oxygen molecules are more massive, they must be moving slower than the nitrogen molecules. (b) is correct. 21 •• [SSM] Liquid nitrogen is relatively cheap, while liquid helium is relatively expensive. One reason for the difference in price is that while nitrogen is the most common constituent of the atmosphere, only small traces of helium can be found in the atmosphere. Use ideas from this chapter to explain why it is that only small traces of helium can be found in the atmosphere. Determine the Concept The average molecular speed of He gas at 300 K is about 1.4 km/s, so a significant fraction of He molecules have speeds in excess of earth’s escape velocity (11.2 km/s). Thus, they "leak" away into space. Over time, the He content of the atmosphere decreases to almost nothing. Estimation and Approximation 22 • Estimate the total number of air molecules in your classroom. Picture the Problem The number of air molecules in your classroom is given by the ideal-gas law. From the ideal-gas law, the number of molecules N in a given volume V at pressure P and temperature T is given by: PV kT where k is Boltzmann’s constant. Assuming that a typical classroom is a rectangular parallelepiped, its volume is given by: V = Awh N= Substituting for V yields: N= PAwh kT Assume atmospheric pressure, room temperature and that the room is 15 m square and 5 m high to obtain: 101.325 kPa ⎞ ⎛ ⎜1 atm × ⎟ (15 m )(15 m )(5 m ) atm ⎝ ⎠ N= ≈ 3 × 10 28 1.381× 10 −23 J/K (293 K ) ( ) Chapter 17 1720 23 •• Estimate the density of dry air at sea level on a warm summer day. Picture the Problem We can use the definition of mass density, the definition of the molar mass of a gas, and the ideal-gas law to estimate the density of air at sea level on a warm day. The density of air at sea level is given by: ρ= m V The mass of the air molecules occupying a given volume is the product of the number of moles n and the molar mass M of the molecules. Substitute for m to obtain: ρ= nM V n= PV RT ρ= M ⎛ PV ⎞ PM ⎜ ⎟= V ⎝ RT ⎠ RT From the ideal-gas law, the number of moles in a given volume depends on the pressure and temperature according to: Substitute in the expression for ρ and simplify to obtain: Assuming atmospheric pressure, a temperature of 27°C (93°F), and 29 g/mol for the molar mass of air, substitute numerical values and evaluate ρ: 101.325 kPa ⎞ ⎛ ⎜1 atm × ⎟(29 g/mol) atm ⎝ ⎠ ρ= (8.314 J/mol ⋅ K )(300 K ) = 1.2 kg/m 3 24 •• A stoppered test tube that has a volume of 10.0 mL has 1.00 mL of water at its bottom. The water has a temperature of 100ºC and is initially at a pressure of 1.00 atm. The test tube is held over a flame until the water has completely boiled away. Estimate the final pressure inside the test tube. Picture the Problem Assuming the steam to be an ideal gas at a temperature of 373 K, we can use the ideal-gas law to estimate the pressure inside the test tube when the water is completely boiled away. Using the ideal-gas law, relate the pressure inside the test tube to its volume and the temperature: P= NkT V Temperature and the Kinetic Theory of Gases 1721 Relate the number of particles N to the mass of water, its molar mass M, and Avogadro’s number NA: N m M = ⇒N = m A N NA M Relate the mass of 1.00 mL of water to its density: m = ρV = 1.00 × 103 kg/m3 ( ( ) × 1.00 × 10− 6 m3 ) = 1.00 g Substitute for m, NA, and M (18 g/mol) and evaluate N: N = (1.00 g ) 6.022 × 1023 particles/mol 18 g/mol = 3.346 × 1022 particles Substitute numerical values and evaluate P: (3.346 ×10 )( ) particles 1.381× 10 −23 J/K (373 K ) 10 − 6 m 3 10.0 mL × mL 1 atm = 1.723 ×10 7 N/m 2 × 101.325 ×10 3 N/m 2 P= 22 = 170 atm 25 •• [SSM] In Chapter 11, we found that the escape speed at the surface of a planet of radius R is ve = 2gR , where g is the acceleration due to gravity at the surface of the planet. If the rms speed of a gas is greater than about 15 to 20 percent of the escape speed of a planet, virtually all of the molecules of that gas will escape the atmosphere of the planet. (a) (b) (c) (d) At what temperature is vrms for O2 equal to 15 percent of the escape speed for Earth? At what temperature is vrms for H2 equal to 15 percent of the escape speed for Earth? Temperatures in the upper atmosphere reach 1000 K. How does this help account for the low abundance of hydrogen in Earth’s atmosphere? Compute the temperatures for which the rms speeds of O2 and H2 are equal to 15 percent of the escape speed at the surface of the moon, where g is about one-sixth of its value on Earth and R = 1738 km. How does this account for the absence of an atmosphere on the moon? Picture the Problem We can find the escape temperatures for the earth and the moon by equating, in turn, 0.15ve and vrms of O2 and H2. We can compare these 1722 Chapter 17 temperatures to explain the absence from Earth’s upper atmosphere and from the surface of the moon. See Appendix C for the molar masses of O2 and H2. (a) Express vrms for O2: vrms, O 2 = 3RT M O2 where R is the gas constant, T is the absolute temperature, and M O 2 is the molar mass of oxygen. Equate 0.15ve and v rms, O 2 : 3RT M 0.15 2 gRearth = Solve for T to obtain: T= 0.045 gRearth M 3R (1) Substitute numerical values and evaluate T for O2: T= ( )( )( ) 0.045 9.81m/s 2 6.37 × 106 m 32.0 × 10−3 kg/mol = 3.60 × 103 K 3(8.314 J/mol ⋅ K ) (b) Substitute numerical values and evaluate T for H2: T= ( )( )( ) 0.045 9.81 m/s 2 6.37 ×106 m 2.02 ×10 −3 kg/mol = 230 K 3(8.314 J/mol ⋅ K ) (c) Because hydrogen is lighter than air it rises to the top of the atmosphere. Because the temperature is high there, a greater fraction of the molecules reach escape speed. (d) Express equation (1) at the surface of the moon: 0.045 g moon Rmoon M 3R 1 0.045( 6 g earth )Rmoon M = 3R 0.0025 g earth Rmoon M = R T= Substitute numerical values and evaluate T for O2: ( )( )( ) 0.0025 9.81 m/s 2 1.738 × 106 m 32.0 × 10−3 kg/mol T= = 160 K 8.314 J/mol ⋅ K Temperature and the Kinetic Theory of Gases 1723 Substitute numerical values and evaluate T for H2: T= ( )( )( ) 0.0025 9.81 m/s 2 1.738 × 106 m 2.02 × 10−3 kg/mol = 10 K 8.314 J/mol ⋅ K Because g is less on the moon, the escape speed is lower. Thus, a larger percentage of the molecules are moving at escape speed. 26 •• The escape speed for gas molecules in the atmosphere of Mars is 5.0 km/s and the surface temperature of Mars is typically 0ºC. Calculate the rms speeds for (a) H2, (b) O2, and (c) CO2 at this temperature. (d) Are H2, O2, and CO2 likely to be found in the atmosphere of Mars? Picture the Problem We can use vrms = 3RT M to calculate the rms speeds of H2, O2, and CO2 at 273 K and then compare these speeds to 20% of the escape velocity on Mars to decide the likelihood of finding these gases in the atmosphere of Mars. See Appendix C for molar masses. Express the rms speed of an atom as a function of the temperature: vrms = (a) Substitute numerical values and evaluate vrms for H2: v rms,H2 = 3RT M 3(8.314 J/mol ⋅ K )(273 K ) 2.02 × 10 −3 kg/mol = 1.84 km/s (b) Evaluate vrms for O2: v rms,O2 = 3(8.314 J/mol ⋅ K )(273 K ) 32.0 × 10 −3 kg/mol = 461 m/s (c) Evaluate vrms for CO2: v rms,CO2 = 3(8.314 J/mol ⋅ K )(273 K ) 44.0 × 10 −3 kg/mol = 393 m/s (d) Calculate 20% of vesc for Mars: v = 15 vesc = 1 5 (5.0 km/s) = 1.0 km/s Because v is greater than v rms CO2 and O2 but less than v rms for H2, O2 and CO2, but not H2 should be present. 1724 Chapter 17 27 ••[SSM] The escape speed for gas molecules in the atmosphere of Jupiter is 60 km/s and the surface temperature of Jupiter is typically –150ºC. Calculate the rms speeds for (a) H2, (b) O2, and (c) CO2 at this temperature. (d) Are H2, O2, and CO2 likely to be found in the atmosphere of Jupiter? Picture the Problem We can use vrms = 3RT M to calculate the rms speeds of H2, O2, and CO2 at 123 K and then compare these speeds to 20% of the escape velocity on Jupiter to decide the likelihood of finding these gases in the atmosphere of Jupiter. See Appendix C for the molar masses of H2, O2, and CO2. Express the rms speed of an atom as a function of the temperature: vrms = (a) Substitute numerical values and evaluate vrms for H2: v rms,H2 = 3RT M 3(8.314 J/mol ⋅ K )(123 K ) 2.02 × 10 −3 kg/mol = 1.23 km/s (b) Evaluate vrms for O2: v rms,O2 = 3(8.314 J/mol ⋅ K )(123 K ) 32.0 × 10 −3 kg/mol = 310 m/s (c) Evaluate vrms for CO2: v rms,CO2 = 3(8.314 J/mol ⋅ K )(123 K ) 44.0 ×10 −3 kg/mol = 264 m/s (d) Calculate 20% of vesc for Jupiter: v = 15 vesc = 1 5 (60 km/s) = 12 km/s Because ve is greater than vrms for O2, CO2 and H2, O2, all three gasses should be found on Jupiter. 28 •• Estimate the average pressure on the front wall of a racquetball court, due to the collisions of the ball with the wall during a game. Use any reasonable numbers for the mass of the ball, its typical speed, and the dimensions of the court. Is the average pressure from the ball significant compared to that from the air? Picture the Problem The average pressure exerted by the ball on the wall is the ratio of the average force it exerts on the wall to the area of the wall. The average force, in turn, is the rate at which the momentum of the ball changes during each collision with the wall. Assume that the mass of a racquetball is 100 g, that the court measures 5 m by 5 m, and that the speed of the racquetball is 10 m/s. We’ll Temperature and the Kinetic Theory of Gases 1725 also assume that the interval Δt between collisions with the wall is 1 s. The average pressure exerted on the wall by the racquetball is given by: Fav Fav = A wh where w and h are the width and height of the wall, respectively. Assuming head-on collisions with the wall, the change in momentum of the ball during each collision is 2mv and the average force exerted by the ball on the wall is: Δp 2mv = Δt Δt where Δt is the elapsed time between collisions of the ball with the wall. Substituting for Fav yields: Substitute numerical values and evaluate Pav: Pav = Fav = Pav = 2mv whΔt Pav = 2(.1 kg )(10 m/s ) = 0.08 N/m 2 (5 m )(5 m )(1 s ) ≈ 0.1 Pa Express the ratio of Pav and Patm to obtain: Pav 0.1 Pa ≈ 5 = 10 − 6 Patm 10 Pa or Pav ≈ 10 −6 Patm and the average pressure from the ball is not significant compared to atmospheric pressure. To a first approximation, the Sun consists of a gas of equal numbers of 29 •• protons and electrons. (The masses of these particles can be found in Appendix B.) The temperature at the center of the Sun is about 1 × 107 K, and the density of the Sun is about 1 × 105 kg/m3. Because the temperature is so high, the protons and electrons are separate particles (rather than being joined together to form hydrogen atoms). (a) Estimate the pressure at the center of the Sun. (b) Estimate the rms speeds of the protons and the electrons at the center of the Sun. Picture the Problem We’ll assume that we can model the plasma as an ideal gas, at least to a first approximation. Then we can apply the ideal gas law to an arbitrary volume of the material, say one cubic meter. (a) To the extent that the plasma acts like an ideal gas, the pressure at the center of the Sun is given by: P= nRT V 1726 Chapter 17 The mass of our 1 m3 volume of matter is 105 kg and this mass consists mostly of protons. One mole of protons has a mass of 1 g, so in 105 kg, the number of moles of protons would be: 105 kg nprotons = = −3 = 108 mol M protons 10 kg or, counting electrons, n = 2nprotons = 2 × 108 mol Substitute numerical values and evaluate P: (2 ×10 P= mprotons ) ( mol (8.314 J/mol ⋅ K ) 10 7 K 1 m3 1 atm ≈ 2 ×1016 Pa × 101.325 kPa 8 ≈ 2 ×1011 atm (b) To one significant figure, the average speed of the protons is the same as the rms speed: vrms, protons = 3RT M protons Substitute numerical values and evaluate v rms, protons : v rms, protons = 3(8.314 J/mol ⋅ K ) 107 K 0.001 kg ( ) = 5 × 105 m/s The rms speed of an electron at the center of the Sun is given by: vrms, electrons = 3RT = M electrons Substitute numerical values and evaluate v rms, electrons : vrms, electrons = 3(8.314 J/mol ⋅ K ) 10 7 K 1 2000 (0.001 kg ) 1 2000 3RT M protons ( ) = 2 × 10 7 m/s Remarks: The huge pressure we calculated in (a) is required to support the tremendous weight of the rest of the sun. The very high speed we calculated for the plasma electrons is still an order-of-magnitude smaller than the speed of light. You are designing a vacuum chamber for fabricating reflective 30 •• coatings. Inside this chamber, a small sample of metal will be vaporized so that its atoms travel in straight lines (the effects of gravity are negligible during the brief time of flight) to a surface where they land to form a very thin film. The sample of metal is 30 cm from the surface to which the metal atoms will adhere. How low must the pressure in the chamber be so that the metal atoms only rarely collide with air molecules before they land on the surface? ) Temperature and the Kinetic Theory of Gases 1727 Picture the Problem We can use the expression for the mean free path of a molecule to eliminate the number of molecules per unit volume nV from the idealgas law. Assume that the air in the chamber is at room temperature (300 K) and that the average diameter of an air molecule is 4 × 10−10 m. Apply the ideal gas law to express the pressure in terms of the number of molecules per unit volume and the temperature: The mean free path of an air molecule is given by: Substitute for nV to obtain: Substitute numerical values and evaluate P: P= λ= P= P= NkT = nV kT V 1 2nV π d 2 1 ⇒ nV = 2λπ d 2 kT 2λπ d 2 (1.381×10 −23 ( ) J/K (300 K ) 2 (30 cm )π 4 × 10 −10 m ) 2 ≈ 20 mPa 31 ••• [SSM] In normal breathing conditions, approximately 5 percent of each exhaled breath is carbon dioxide. Given this information and neglecting any difference in water-vapor content, estimate the typical difference in mass between an inhaled breath and an exhaled breath. Picture the Problem One breath (one’s lung capacity) is about half a liter. The only thing that occurs in breathing is that oxygen is exchanged for carbon dioxide. Let’s estimate that of the 20% of the air that is breathed in as oxygen, ¼ is exchanged for carbon dioxide. Then the mass difference between breaths will be 5% of a breath multiplied by the molar mass difference between oxygen and carbon dioxide and by the number of moles in a breath. Because this is an estimation problem, we’ll use 32 g/mol as an approximation for the molar mass of oxygen and 44 g/mol as an approximation for the molar mass of carbon dioxide. Express the difference in mass between an inhaled breath and an exhaled breath: Δm = mO 2 − mCO 2 ( ) = f CO 2 M O 2 − M CO 2 nbreath where f CO 2 is the fraction of the air breathed in that is exchanged for carbon dioxide. The number of moles per breath is given by: nbreath = Vbreath 22.4 L/mol 1728 Chapter 17 Substituting for nbreath yields: ( ) ⎛ Vbreath ⎞ Δm = f CO 2 M O 2 − M CO 2 ⎜ ⎟ ⎝ 22.4 L/mol ⎠ Substitute numerical values and evaluate Δm: Δm = (0.05)(44 g/mol − 32 g/mol) (0.5 L ) 22.4 L/mol ≈ 1× 10 −4 g Temperature Scales 32 • A certain ski wax is rated for use between –12 and –7.0 ºC. What is this temperature range on the Fahrenheit scale? Picture the Problem We can use the fact that 5 C° = 9 F° to set up a proportion that allows us to make easy interval conversions from either the Celsius or Fahrenheit scale to the other. The proportion relating a temperature range on the Fahrenheit scale of a temperature range on the Celsius scale is: Δt F 9 F° ⎛ 9 F° ⎞ = ⇒ Δt F = ⎜ ⎟Δt C Δt C 5 C° ⎝ 5 C° ⎠ Substitute numerical values and evaluate ΔtF: ⎛ 9 F° ⎞ Δt F = ⎜ ⎟(− 7°C − (− 12°C )) = 9 F° ⎝ 5 C° ⎠ Remarks: An equivalent but slightly longer solution involves converting the two temperatures to their Fahrenheit equivalents and then subtracting these temperatures. [SSM] The melting point of gold is 1945.4 ºF. Express this 33 • temperature in degrees Celsius. Picture the Problem We can use the Fahrenheit-Celsius conversion equation to find this temperature on the Celsius scale. Convert 1945.4°F to the equivalent Celsius temperature: tC = 5 9 (tF − 32°) = 95 (1945.4° − 32°) = 1063°C 34 • A weather report indicates that the temperature is expected to drop by 15.0°C over the next four hours. By how many degrees on the Fahrenheit scale will the temperature drop? Temperature and the Kinetic Theory of Gases 1729 Picture the Problem We can use the fact that 5 C° = 9 F° to set up a proportion that allows us to make easy interval conversions from either the Celsius or Fahrenheit scale to the other. The proportion relating a temperature range on the Fahrenheit scale of a temperature range on the Celsius scale is: Δt F 9 F° ⎛ 9 F° ⎞ = ⇒ Δt F = ⎜ ⎟Δt C Δt C 5 C° ⎝ 5 C° ⎠ Substitute numerical values and evaluate ΔtF: ⎛ 9 F° ⎞ Δt F = ⎜ ⎟(15.0 C°) = 27.0 F° ⎝ 5 C° ⎠ The length of the column of mercury in a thermometer is 4.00 cm 35 • when the thermometer is immersed in ice water at 1 atm of pressure, and 24.0 cm when the thermometer is immersed in boiling water at 1 atm of pressure. Assume that the length of the mercury column varies linearly with temperature. (a) Sketch a graph of the length of the mercury column versus temperature (in degrees Celsius). (b) What is the length of the column at room temperature (22.0ºC)? (c) If the mercury column is 25.4 cm long when the thermometer is immersed in a chemical solution, what is the temperature of the solution? Picture the Problem We can use the equation of the graph plotted in (a) to (b) find the length of the mercury column at room temperature and (c) the temperature of the solution when the height of the mercury column is 25.4 cm. (a) A graph of the length of the mercury column versus temperature (in degrees Celsius) is shown to the right. The equation of the line is: cm ⎞ ⎛ L = ⎜ 0.200 ⎟ t C + 4.00 cm (1) °C ⎠ ⎝ L, cm 24.0 4.00 0 (b) Evaluate L(22.0°C ) t C , °C cm ⎞ ⎛ L = ⎜ 0.200 ⎟ (22.0°C ) + 4.00 cm °C ⎠ ⎝ = 8.40 cm (c) Solve equation (1) for tC to obtain: 100 tC = L − 4.00 cm cm 0.200 °C 1730 Chapter 17 Substitute numerical values and evaluate t C (25.4 cm ) : t C (25.4 cm ) = 25.4 cm − 4.00 cm cm 0.200 °C = 107°C 36 • The temperature of the interior of the Sun is about 1.0 × 107 K. What is this temperature in (a) Celsius degrees, (b) kelvins, and (c) Fahrenheit degrees? Picture the Problem We can use the temperature conversion equations tF = 95 tC + 32° and tC = T − 273.15 K to convert 107 K to the Fahrenheit and Celsius temperatures. Express the kelvin temperature in terms of the Celsius temperature: T = tC + 273.15 K (a) Solve for and evaluate tC: t C = T − 273.15 K = 1.0 × 10 7 K − 273.15 K ≈ 1.0 × 10 7 °C (b) Use the Celsius to Fahrenheit conversion equation to evaluate tF: ( ) t F = 95 1.0 × 107°C + 32° ≈ 1.8 × 107°F 37 • The boiling point of nitrogen, N2, is 77.35 K. Express this temperature in degrees Fahrenheit. Picture the Problem While we could convert 77.35 K to a Celsius temperature and then convert the Celsius temperature to a Fahrenheit temperature, an alternative solution is to use the diagram to the right to set up a proportion for the direct conversion of the kelvin temperature to its Fahrenheit equivalent. Use the diagram to set up the proportion: F K 212 373.15 32 273.15 tF 77.35 32°F − t F 273.15 K − 77.35 K = 212°F − 32°F 373.15 K − 273.15 K or 32°F − t F 195.8 = 180 F° 100 Temperature and the Kinetic Theory of Gases 1731 Solving for tF yields: t F = 32°F − 195.8 × 180 F° = − 320 °F 100 38 • The pressure of a constant-volume gas thermometer is 0.400 atm at the ice point and 0.546 atm at the steam point. (a) Sketch a graph of pressure versus Celsius temperature for this thermometer. (b) When the pressure is 0.100 atm, what is the temperature? (c) What is the pressure at 444.6 ºC (the boiling point of sulfur)? Picture the Problem We can use the equation of the graph plotted in (a) to (b) find the temperature when the pressure is 0.100 atm and (c) the pressure when the temperature is 444.6ºC. (a) A graph of pressure (in atm) versus temperature (in degrees Celsius) for this thermometer is shown to the right. The equation of this graph is: atm ⎞ ⎛ P = ⎜1.46 × 10−3 ⎟t C + 0.400 atm °C ⎠ ⎝ P , atm 0.546 0.400 0 100 t C , °C P − 0.400 atm atm 1.46 × 10 −3 °C (b) Solving for tC yields: tC = Substitute numerical values and evaluate t C (0.100 atm ) : t C (0.100 atm ) = 0.100 atm − 0.400 atm atm 1.46 × 10−3 °C = − 205°C Substitute numerical values and evaluate P (444.6°C ) : atm ⎞ ⎛ P (444.6°C ) = ⎜1.46 × 10−3 ⎟ (444.6°C ) + 0.400 atm = 1.05 atm °C ⎠ ⎝ 39 • [SSM] A constant-volume gas thermometer reads 50.0 torr at the triple point of water. (a) Sketch a graph of pressure vs. absolute temperature for this thermometer. (b) What will be the pressure when the thermometer measures a temperature of 300 K? (c) What ideal-gas temperature corresponds to a pressure of 678 torr? 1732 Chapter 17 Picture the Problem We can use the equation of the graph plotted in (a) to (b) find the pressure when the temperature is 300 K and (c) the ideal-gas temperature when the pressure is 678 torr. (a) A graph of pressure (in torr) versus temperature (in kelvins) for this thermometer is shown to the right. The equation of this graph is: ⎛ 50.0 torr ⎞ P =⎜ ⎟T ⎝ 273 K ⎠ (1) (b) Evaluate P when T = 300 K: P , torr 50.0 0 273 0 T, K ⎛ 50.0 torr ⎞ P (300 K ) = ⎜ ⎟ (300 K ) ⎝ 273 K ⎠ = 54.9 torr (c) Solve equation (1) for T to obtain: ⎛ 273 K ⎞ T =⎜ ⎟P ⎝ 50.0 torr ⎠ Evaluate T (678 torr ) : ⎛ 273 K ⎞ T (678 torr ) = ⎜ ⎟ (678 torr ) ⎝ 50.0 torr ⎠ = 3.70 × 103 K 40 • A constant-volume gas thermometer has a pressure of 30.0 torr when it reads a temperature of 373 K. (a) Sketch a graph of pressure vs. absolute temperature for this thermometer. (b) What is its triple-point pressure P3? (c) What temperature corresponds to a pressure of 0.175 torr? Picture the Problem We can use the equation of the graph plotted in (a) to (b) find the triple-point pressure P3 and (c) the temperature when the pressure is 0.175 torr. Temperature and the Kinetic Theory of Gases 1733 (a) A graph of pressure versus absolute temperature for this thermometer is shown to the right. The equation of this graph is: ⎛ 30.0 torr ⎞ P =⎜ ⎟T ⎝ 373 K ⎠ (1) P , torr 30.0 0 T, K 0 373 (b) Solve for and evaluate the thermometer’s triple-point (273.16 K) pressure: ⎛ 30.0 torr ⎞ P (273.16 K ) = ⎜ ⎟ (273.16 K ) ⎝ 373 K ⎠ (c) Solve equation (1) for T to obtain: ⎛ 373 K ⎞ T =⎜ ⎟P ⎝ 30.0 torr ⎠ Evaluate T (0.175 torr ) : ⎛ 373 K ⎞ T (0.175 torr ) = ⎜ ⎟ (0.175 torr ) ⎝ 30.0 torr ⎠ = 22.0 torr = 2.18 K 41 • At what temperature do the Fahrenheit and Celsius temperature scales give the same reading? Picture the Problem We can find the temperature at which the Fahrenheit and Celsius scales give the same reading by setting tF = tC in the temperatureconversion equation. Set tF = tC in tC = 5 9 (tF − 32°) : Solve for and evaluate tF: tF = 5 9 (tF − 32°) t C = t F = − 40.0°C = − 40.0°F Remarks: If you’ve not already thought of doing so, you might use your graphing calculator to plot tC versus tF and tF = tC (a straight line at 45°) on the same graph. Their intersection is at (−40, −40). 42 • Sodium melts at 371 K. What is the melting point of sodium on the Celsius and Fahrenheit temperature scales? 1734 Chapter 17 Picture the Problem We can use the Celsius-to-absolute conversion equation to find 371 K on the Celsius scale and the Celsius-to-Fahrenheit conversion equation to find the Fahrenheit temperature corresponding to 371 K. Express the absolute temperature as a function of the Celsius temperature: T = tC + 273.15 K Solve for and evaluate tC: t C = T − 273.15 K = 371 K − 273.15 K = 98°C Use the Celsius-to-Fahrenheit conversion equation to find tF: tF = 95 tC + 32° = 9 5 (97.9°) + 32° = 208°F 43 • The boiling point of oxygen at 1.00 atm is 90.2 K. What is the boiling point of oxygen at 1.00 atm on the Celsius and Fahrenheit scales? Picture the Problem We can use the Celsius-to-absolute conversion equation to find 90.2 K on the Celsius scale and the Celsius-to-Fahrenheit conversion equation to find the Fahrenheit temperature corresponding to 90.2 K. Express the absolute temperature as a function of the Celsius temperature: T = tC + 273.15 K Solve for and evaluate tC: t C = T − 273.15 K = 90.2 K − 273.15 K = − 183°C Use the Celsius-to-Fahrenheit conversion equation to find tF: tF = 95 tC + 32° = 9 5 (− 183°) + 32° = − 297°F 44 •• On the Réaumur temperature scale, the melting point of ice is 0ºR and the boiling point of water is 80ºR. Derive expressions for converting temperatures on the Réaumur scale to the Celsius and Fahrenheit scales. Picture the Problem We can use the following diagram to set up proportions that will allow us to convert temperatures on the Réaumur scale to Celsius and Fahrenheit temperatures. Temperature and the Kinetic Theory of Gases 1735 100 tC 0 R F C 212 80 tF tR 32 0 Referring to the diagram, set up a proportion to convert temperatures on the Réaumur scale to Celsius temperatures: tC − 0°C t − 0°R = R 100°C − 0°C 80°R − 0°R Simplify to obtain: tC t = R ⇒ tC = 100 80 Referring to the diagram, set up a proportion to convert temperatures on the Réaumur scale to Fahrenheit temperatures: t F − 32°F t − 0°R = R 212°F − 32°F 80°R − 0°R Simplify to obtain: t F − 32 t R = ⇒ tF = 180 80 5 4 R t t + 32 9 4 R 45 ••• [SSM] A thermistor is a solid-state device widely used in a variety of engineering applications. Its primary characteristic is that its electrical resistance varies greatly with temperature. Its temperature dependence is given approximately by R = R0eB/T, where R is in ohms (Ω), T is in kelvins, and R0 and B are constants that can be determined by measuring R at calibration points such as the ice point and the steam point. (a) If R = 7360 Ω at the ice point and 153 Ω at the steam point, find R0 and B. (b) What is the resistance of the thermistor at t = 98.6 ºF? (c) What is the rate of change of the resistance with temperature (dR/dT) at the ice point and the steam point? (d) At which temperature is the thermistor most sensitive? Picture the Problem We can use the temperature dependence of the resistance of the thermistor and the given data to determine R0 and B. Once we know these quantities, we can use the temperature-dependence equation to find the resistance at any temperature in the calibration range. Differentiation of R with respect to T will allow us to express the rate of change of resistance with temperature at both the ice point and the steam point temperatures. 1736 Chapter 17 (a) Express the resistance at the ice point as a function of temperature of the ice point: 7360 Ω = R0e B 273 K (1) Express the resistance at the steam point as a function of temperature of the steam point: 153 Ω = R0e B 373 K (2) Divide equation (1) by equation (2) to obtain: 7360 Ω = 48.10 = e B 273 K − B 373 K 153 Ω Solve for B by taking the logarithm of both sides of the equation: 1 ⎞ −1 ⎛ 1 − ln 48.1 = B⎜ ⎟K ⎝ 273 373 ⎠ and ln 48.1 = 3.944 × 10 3 K B= 1 ⎞ −1 ⎛ 1 − ⎜ ⎟K ⎝ 273 373 ⎠ = 3.94 × 10 3 K Solve equation (1) for R0 and substitute for B: R0 = 7360 Ω = (7360 Ω )e − B 273 K B 273 K e = (7360 Ω )e −3.944×10 3 K 273 K = 3.913 ×10 −3 Ω = 3.91×10 −3 Ω ( ) 3 (b) From (a) we have: R = 3.913 × 10 −3 Ω e 3.944×10 Convert 98.6 °F to kelvins to obtain: T = 310 K Substitute for T to obtain: R(310 K ) = 3.913 × 10 −3 Ω e 3.944×10 ( K T ) 3 K 310 K = 1.31 kΩ (c) Differentiate R with respect to T to obtain: dR d d ⎛B⎞ R0e B T ) = R0e B T = ( ⎜ ⎟ dT dT dT ⎝ T ⎠ RB −B = 2 R0e B T = − 2 T T Temperature and the Kinetic Theory of Gases 1737 Evaluate dR/dT at the ice point: ( (7360 Ω ) 3.943 ×103 K ⎛ dR ⎞ = − ⎜ ⎟ (273.16 K )2 ⎝ dT ⎠ice point ) = − 389 Ω / K Evaluate dR/dT at the steam point: ( (153 Ω ) 3.943 ×103 K ⎛ dR ⎞ = − ⎜ ⎟ (373.16 K )2 ⎝ dT ⎠steam point ) = − 4.33 Ω / K (d) The thermistor is more sensitive (has greater sensitivity) at lower temperatures. The Ideal-Gas Law 46 • An ideal gas in a cylinder fitted with a piston (Figure 17-20) is held at fixed pressure. If the temperature of the gas increases from 50° to 100°C, by what factor does the volume change? Picture the Problem Let the subscript 50 refer to the gas at 50°C and the subscript 100 to the gas at 100°C. We can apply the ideal-gas law for a fixed amount of gas to find the ratio of the final and initial volumes. Apply the ideal-gas law for a fixed amount of gas: P100V100 P50V50 = T100 T50 or, because P100 = P50, V100 T100 = V50 T50 Substitute numerical values and evaluate V100/V50: V100 (273.15 + 100 )K = = 1.15 (273.15 + 50)K V50 or a 15% increase in volume. 47 • [SSM] A 10.0-L vessel contains gas at a temperature of 0.00ºC and a pressure of 4.00 atm. How many moles of gas are in the vessel? How many molecules? Picture the Problem We can use the ideal-gas law to find the number of moles of gas in the vessel and the definition of Avogadro’s number to find the number of molecules. 1738 Chapter 17 Apply the ideal-gas law to the gas: PV = nRT ⇒ n = Substitute numerical values and evaluate n: n= PV RT (4.00 atm )(10.0 L ) (8.206 ×10 −2 ) L ⋅ atm/mol ⋅ K (273 K ) = 1.786 mol = 1.79 mol N = nN A Relate the number of molecules N in the gas in terms of the number of moles n: Substitute numerical values and evaluate N: ( ) N = (1.786 mol) 6.022 × 10 23 molecules/mol = 1.08 × 10 24 molecules 48 •• A pressure as low as 1.00 × 10–8 torr can be achieved using an oil diffusion pump. How many molecules are there in 1.00 cm3 of a gas at this pressure if its temperature is 300 K? Picture the Problem We can use the ideal-gas law to relate the number of molecules in the gas to its pressure, volume, and temperature. Solve the ideal-gas law for the number of molecules in a gas as a function of its pressure, volume, and temperature: N= PV kT Substitute numerical values and evaluate N: N= (1.00 ×10 −8 ) ( ) torr (133.32 Pa/torr ) 1.00 × 10 −6 m 3 = 3.22 × 108 1.381× 10−23 J/K (300 K ) ( ) You copy the following paragraph from a Martian physics textbook: 49 •• ″1 snorf of an ideal gas occupies a volume of 1.35 zaks. At a temperature of 22 glips, the gas has a pressure of 12.5 klads. At a temperature of –10 glips, the same gas now has a pressure of 8.7 klads.″ Determine the temperature of absolute zero in glips. Picture the Problem Because the gas is ideal, its pressure is directly proportional to its temperature. Hence, a graph of P versus T will be linear and the linear equation relating P and T can be solved for the temperature corresponding to zero pressure. We’ll assume that the data was taken at constant volume. Temperature and the Kinetic Theory of Gases 1739 A graph of P, in klads, as function of T, in glips, is shown to the right. The equation of this graph is: P, klads 12.5 ⎛ 3.8 klads ⎞ ⎟⎟T + 9.9 klads P = ⎜⎜ ⎝ 32 glips ⎠ 8.7 T0 −10 0 22 When P = 0: ⎛ 3.8 klads ⎞ ⎟⎟T0 + 9.9 klads 0 = ⎜⎜ ⎝ 32 glips ⎠ Solve for T0 to obtain: T0 = − 83 glips T , glips 50 •• A motorist inflates the tires of her car to a gauge pressure of 180 kPa on a day when the temperature is –8.0ºC. When she arrives at her destination, the tire pressure has increased to 245 kPa. What is the temperature of the tires if we assume that (a) the tires do not expand or (b) that the tires expand so the volume of the enclosed air increases by 7 percent? Picture the Problem Let the subscript 1 refer to the tires when their gauge pressure is 180 kPa and the subscript 2 to conditions when their gauge pressure is 245 kPa. Assume that the air in the tires behaves as an ideal gas. Then, we can apply the ideal-gas law for a fixed amount of gas to relate the temperatures to the pressures and volumes of the tires. (a) Apply the ideal-gas law for a fixed amount of gas to the air in the tires: P2V2 P1V1 = T2 T1 (1) where the temperatures and pressures are absolute. Solve for T2: T2 = T1 Substitute numerical values to obtain: T2 = (265 K ) P2 because V1 = V2. P1 245 kPa + 101 kPa 180 kPa + 101 kPa = 326.3 K = 53°C 1740 Chapter 17 (b) Use equation (1) with V2 = 1.07 V1. Solve for T2: Substitute numerical values and evaluate T2: T2 = ⎛P ⎞ P2V2 T1 = (1.07 ) ⎜⎜ 2 T1 ⎟⎟ P1V1 ⎝ P1 ⎠ T2 = (1.07 )(326.3 K ) = 349.1 K = 76°C 51 •• A room is 6.0 m by 5.0 m by 3.0 m. (a) If the air pressure in the room is 1.0 atm and the temperature is 300 K, find the number of moles of air in the room. (b) If the temperature increases by 5.0 K and the pressure remains constant, how many moles of air leave the room? Picture the Problem We can apply the ideal-gas law to find the number of moles of air in the room as a function of the temperature. (a) Use the ideal-gas law to relate the number of moles of air in the room to the pressure, volume, and temperature of the air: n= PV RT Substitute numerical values and evaluate n: n= (101.325 kPa )(6.0 m )(5.0 m )(3.0 m ) (8.314 J/mol ⋅ K )(300 K ) (1) = 3.66 ×10 3 mol = 3.7 ×10 3 mol (b) Letting n′ represent the number of moles in the room when the temperature rises by 5 K, express the number of moles of air that leave the room: Δn = n − n' Apply the ideal-gas law to obtain: n' = Divide equation (2) by equation (1) to obtain: n' T T and n' = n = n T' T' Substitute for n′ to obtain: PV RT ' Δn = n − n (2) T ⎛ T⎞ = n⎜1 − ⎟ T' ⎝ T' ⎠ Temperature and the Kinetic Theory of Gases 1741 Substitute numerical values and evaluate Δn: ⎛ 300 K ⎞ ⎟⎟ Δn = 3.66 × 103 mol ⎜⎜1 − ⎝ 305 K ⎠ ( ) = 60 mol 52 •• Image that 10.0 g of liquid helium, initially at 4.20 K, evaporate into an empty balloon that is kept at 1.00 atm pressure. What is the volume of the balloon at (a) 25.0 K and (b) 293 K? Picture the Problem Let the subscript 1 refer to helium gas at 4.2 K and the subscript 2 to the gas at 293 K. We can apply the ideal-gas law to find the volume of the gas at 4.2 K and a fixed amount of gas to find its volume at 293 K. See Appendix C for the molar mass of helium. (a) Apply the ideal-gas law to the helium gas to express its volume: m RT nRT1 M 1 mRT1 V1 = = = P1 P1 MP1 Substitute numerical values and evaluate V1: V1 = (10.0 g )(0.08206 L ⋅ atm/mol ⋅ K )(25.0 K ) = 5.125 L = (4.003 g/mol)(1.00 atm) (b) Apply the ideal-gas law for a fixed amount of gas and solve for the volume of the helium gas at 293 K: Substitute numerical values and evaluate V2: 5.13 L P2V2 P1V1 = T2 T1 and, because P1 = P2, T V2 = 2 V1 T1 V2 = 293 K (5.125 L ) = 60.1L 25.0 K 53 •• A closed container with a volume of 6.00 L holds 10.0 g of liquid helium at 25.0 K and enough air to fill the rest of its volume at a pressure of 1.00 atm. The helium then evaporates and the container warms to room temperature (293 K). What is the final pressure inside the container? Picture the Problem We can apply the law of partial pressures to find the final pressure inside the container. See Appendix C for the molar mass of helium and of air. 1742 Chapter 17 The final pressure inside the container is the sum of the partial pressures of helium gas and air: Pfinal = PHe gas + Pair The pressure exerted by the air molecules at room temperature is given by the ideal-gas law: Pair = = (1) nair RT mair RT ρ airVRT = = V M airV M airV ρ air RT M air The pressure exerted by the helium molecules at room temperature is also given by the ideal-gas law: PHe gas = Substituting in equation (1) yields: Pfinal = nHe RT mHe RT = V M HeV mHe RT ρ air RT + M HeV M air ⎛ m ρ = ⎜⎜ He + air ⎝ M HeV M air ⎞ ⎟⎟ RT ⎠ Substitute numerical values and evaluate P2: Pfinal ⎛ kg ⎞⎟ ⎜ 1.293 3 10.0 g ⎜ m ⎟ ⎛⎜ 8.314 J ⎞⎟ (293 K ) =⎜ + 3 −3 g ⎟⎝ mol ⋅ K ⎠ ⎛ ⎞ ⎜ ⎛⎜ 4.003 g ⎞⎟ ⎜ 6.00 L × 10 m ⎟ 28.81 ⎟ ⎜⎝ mol ⎟ mol ⎠ ⎜⎝ L ⎟⎠ ⎝ ⎠ 1 atm = 1.124 × 106 Pa × = 11.1 atm 101.325 kPa 54 •• An automobile tire is filled to a gauge pressure of 200 kPa when its temperature is 20ºC. (Gauge pressure is the difference between the actual pressure and atmospheric pressure.) After the car has been driven at high speeds, the tire temperature increases to 50ºC. (a) Assuming that the volume of the tire does not change and that air behaves as an ideal gas, find the gauge pressure of the air in the tire. (b) Calculate the gauge pressure if the tire expands so the volume of the enclosed air increases by 10 percent. Picture the Problem Let the subscript 1 refer to the tire when its temperature is 20°C and the subscript 2 to conditions when its temperature is 50°C. We can apply the ideal-gas law for a fixed amount of gas to relate the temperatures to the pressures of the air in the tire. Temperature and the Kinetic Theory of Gases 1743 (a) Apply the ideal-gas law for a fixed amount of gas and solve for pressure at the higher temperature: P2V2 P1V1 = T2 T1 (1) and P2 = T2 P1 T1 because V1 = V2 Substitute numerical values to obtain: 323 K (200 kPa + 101 kPa ) 293 K = 332 kPa P2 = and P2,gauge = 332 kPa − 101 kPa = 231 kPa (b) Solve equation (1) for P2: P2 = V1T2 P1 V2T1 Because V2 = 1.10 V1: P2 = T2 V1T2 P1 = P1 1.10V1T1 1.10 T1 Substitute numerical values and evaluate P2 and P2,gauge: P2 = 323 K (200 kPa + 101kPa ) (1.10)(293 K ) = 302 kPa and P2,gauge = 302 kPa − 101 kPa = 201 kPa 55 •• [SSM] After nitrogen (N2) and oxygen (O2), the most abundant molecule in Earth's atmosphere is water, H2O. However, the fraction of H2O molecules in a given volume of air varies dramatically, from practically zero percent under the driest conditions to as high as 4 percent where it is very humid. (a) At a given temperature and pressure, would air be denser when its water vapor content is large or small? (b) What is the difference in mass, at room temperature and atmospheric pressure, between a cubic meter of air with no water vapor molecules, and a cubic meter of air in which 4 percent of the molecules are water vapor molecules? Picture the Problem (a) At a given temperature and pressure, the ideal-gas law tells us that the total number of molecules per unit volume, N/V, is constant. The denser gas will, therefore, be the one in which the average mass per molecule is greater. (b) We can apply the ideal-gas law and use the relationship between the masses of the dry and humid air and their molar masses to find the difference in 1744 Chapter 17 mass, at room temperature and atmospheric pressure, between a cubic meter of air containing no water vapor, and a cubic meter of air containing 4% water vapor. See Appendix C for the molar masses of N2, O2, and H2O. (a) The molecular mass of N2 is 28.014 amu (see Appendix C), that of O2 is 31.999 amu, and that of H2O is 18.0152 amu. Because H2O molecules are lighter than the predominant molecules in air, a given volume of air will be less when its water vapor content is lower. (b) Express the difference in mass between a cubic meter of air containing no water vapor, and a cubic meter of air containing 4% water vapor: Δm = mdry − mhumid The masses of the dry air and humid air are related to their molar masses according to: mdry = 0.04nM dry and m humid = 0.04nM humid Substitute for mdry and mhumid and simplify to obtain: Δm = 0.04nM dry − 0.04nM humid From the ideal-gas law we have: PatmV RT and so Δm can be written as 0.04 PatmV (M dry − M humid ) Δm = RT = 0.04n(M dry − M humid ) n= Substitute numerical values (28.811 g/mol is an 80% nitrogen and 20% oxygen weighted average) and evaluate Δm: ( 0.04)(101.325 kPa ) (1.00 m 3 ) (28.811 g/mol − 18.0152 g/mol) ≈ Δm = (8.314 J/mol ⋅ K )(300 K ) 18 g 56 •• A scuba diver is 40 m below the surface of a lake, where the temperature is 5.0ºC. He releases an air bubble that has a volume of 15 cm3. The bubble rises to the surface, where the temperature is 25ºC. Assume that the air in the bubble is always in thermal equilibrium with the surrounding water, and assume that there is no exchange of molecules between the bubble and the surrounding water. What is the volume of the bubble right before it breaks the surface? Hint: Remember that the pressure also changes. Temperature and the Kinetic Theory of Gases 1745 Picture the Problem Let the subscript 1 refer to the conditions at the bottom of the lake and the subscript 2 to the surface of the lake and apply the ideal-gas law for a fixed amount of gas. Apply the ideal-gas law for a fixed amount of gas: P2V2 P1V1 VT P = ⇒ V2 = 1 2 1 T1 P2 T2 T1 The pressure at the bottom of the lake is the sum of the pressure at its surface (atmospheric) and the pressure due to the depth of the lake: P1 = Patm + ρgh Substituting for P1 yields: V2 = V1T2 (Patm + ρgh ) T1 P2 Substitute numerical values and evaluate V2: V2 = (15 cm )(298 K )[101.325 kPa + (1.00 ×10 3 )( ] ) kg/m 3 9.81 m/s 2 (40 m ) (278 K )(101.325 kPa ) 3 = 78 cm3 57 •• [SSM] A hot-air balloon is open at the bottom. The balloon has a volume of 446 m3 is filled with air with an average temperature of 100°C. The air outside the balloon has a temperature of 20.0°C and a pressure of 1.00 atm. How large a payload (including the envelope of the balloon itself) can the balloon lift? Use 29.0 g/mol for the molar mass of air. (Neglect the volume of both the payload and the envelope of the balloon.) Picture the Problem Assume that the volume of the balloon is not changing. Then the air inside and outside the balloon must be at the same pressure of about 1.00 atm. The contents of the balloon are the air molecules inside it. We can use Archimedes principle to express the buoyant force on the balloon and we can find the weight of the air molecules inside the balloon. You’ll need to determine the molar mass of air. See Appendix C for the molar masses of oxygen and nitrogen. Express the net force on the balloon and its contents: Fnet = B − wair inside the balloon Using Archimedes principle, express the buoyant force on the balloon: B = wdisplaced fluid = mdisplaced fluid g (1) or B = ρoVballoon g where ρo is the density of the air outside the balloon. 1746 Chapter 17 Express the weight of the air inside the balloon: wair inside the balloon = ρiVballoon g where ρi is the density of the air inside the balloon. Substitute in equation (1) for B and wair inside the balloon to obtain: Fnet = ρ oVballoon g − ρiVballoon g Express the densities of the air molecules in terms of their number densities, molecular mass, and Avogadro’s number: ρ= Using the ideal-gas law, relate the number density of air N/V to its temperature and pressure: Substitute for (2) = (ρ o − ρ i )Vballoon g M ⎛N⎞ ⎜ ⎟ NA ⎝ V ⎠ PV = NkT and N to obtain: V ρ= Substitute in equation (2) and simplify to obtain: N P = V kT M ⎛ P ⎞ ⎜ ⎟ N A ⎝ kT ⎠ MP ⎛ 1 1 ⎞ ⎜ − ⎟Vballoon g N A k ⎜⎝ To Ti ⎟⎠ MP ⎛ 1 1 ⎞ 1 ⎜⎜ − ⎟⎟ 6 π d 3 g = N A k ⎝ To Ti ⎠ Fnet = ( ) Assuming that the average molar mass of air is 28.81 g/mol, substitute numerical values and evaluate Fnet: [ ⎛ Fnet = ]( 1 1 ⎞ 1 ⎟⎟ 6 π (15.0 m )3 9.81 m/s 2 − ⎝ 297 K 348 K ⎠ 23 6.022 × 10 particles/mol 1.381 × 10 −23 J/K (28.81g/mol)(101.325 kPa )⎜⎜ ( )( ) ) = 3.0 kN 58 ••• A helium balloon is used to lift a load of 110 N. The weight of the envelope of the balloon is 50.0 N and the volume of the helium when the balloon is fully inflated is 32.0 m3. The temperature of the air is 0ºC and the atmospheric pressure is 1.00 atm. The balloon is inflated with a sufficient amount of helium gas that the net upward force on the balloon and its load is 30.0 N. Neglect any effects due to the changes of temperature as the altitude changes. (a) How many moles of helium gas are contained in the balloon? (b) At what altitude will the balloon be fully inflated? (c) Does the balloon ever reach the altitude at which it is fully inflated? (d) If the answer to (c) is ″Yes,″ what is the maximum altitude attained by the balloon? Temperature and the Kinetic Theory of Gases 1747 Picture the Problem (a) We can find the number of moles of helium gas in the balloon by applying the ideal-gas law to relate n to the pressure, volume, and temperature of the helium and Archimedes principle to find the volume of the helium. In Part (b), we can apply the result of Problem 13-91 to relate atmospheric pressure to altitude and use the ideal-gas law to determine the pressure of the gas when the balloon is fully inflated. In Part (c), we’ll find the net force acting on the balloon at the altitude at which it is fully inflated in order to decide whether it can rise to that altitude. (a) Apply the ideal-gas law to the helium in the balloon and solve for n: n= Relate the net force on the balloon to its weight: FB − wskin − wload − wHe = 30 N Use Archimedes principle to express the buoyant force on the balloon in terms of the volume of the balloon: FB = wdisplaced air Substitute to obtain: ρ airVg − wskin − wload − ρ HeVg = 30.0 N Solve for the volume of the helium: V = 30.0 N + wskin + wload (ρ air − ρ He )g Substituting for V in equation (1) yields: n= P(30.0 N + wskin + wload ) RT (ρ air − ρ He )g PV RT (1) = ρ airVg Substitute numerical values and evaluate n: (1.00 atm )(30.0 N + 50.0 N + 110 N )⎛⎜⎜ 1L ⎞ ⎟ 10 −3 m 3 ⎟⎠ ⎝ n= 8.206 × 10−2 L ⋅ atm/mol ⋅ K (273 K ) 1.293 kg/m 3 − 0.179 kg/m 3 9.81 m/s 2 ( ) ( = 776 mol (b) Using the result of Problem 13-91, express the variation in atmospheric pressure with altitude: P(h ) = P0 e −Ch where C = 0.13 km −1 . )( ) 1748 Chapter 17 h= 1 ⎡ P0 ⎤ ln C ⎢⎣ P(h ) ⎥⎦ Using the ideal-gas law, express P: P= nRT V Substitute for P in equation (2) and simplify to obtain: ⎡ 1 ⎢ P h = ln ⎢ 0 C ⎢ nRT ⎣ V Solving for h yields: (2) ⎤ ⎥ 1 ⎡ P0V ⎤ ⎥ = ln ⎢ ⎥ ⎥ C ⎣ nRT ⎦ ⎦ Substitute numerical values and evaluate h: ⎡ ⎤ ⎛ ⎞ (1.00 atm )⎜⎜ 32.0 m 3 × 1−3L 3 ⎟⎟ ⎢ ⎥ 10 m ⎠ 1 ⎛ ⎞ ⎢ ⎝ ⎥ h=⎜ ln −1 ⎟ −2 ⎢ ⎥ ( ) ( ) × ⋅ ⋅ 0 . 13 km 776 mol 8.206 10 L atm/mol K 273 K ⎝ ⎠ ⎢ ⎥ ⎢⎣ ⎥⎦ ( ) = 4.84 km = 4.8 km (c) Express the condition that must be satisfied if the balloon is to reach its fully inflated altitude: Fnet = FB − wtot ≥ 0 The total weight is the sum of the weights of the load, balloon skin, and helium: w tot = wload + wskin + wHe or, because wHe = ρ HeVg , w tot = w load + w skin + ρ HeVg Express the buoyant force on the balloon at h = 4.84 km: FB = ρ air, hVg Express the dependence of the density of the air on atmospheric pressure: P P ρ air, h = ⇒ ρair, h = ρ air (5) P0 ρ air P0 Substitute for ρair,h in equation (4) to obtain: FB = P ρ airVg P0 (3) (4) Temperature and the Kinetic Theory of Gases 1749 Substitute for wtot and FB in equation (3) and simplify to obtain: Fnet = P Vg (ρ air − ρ He ) − wload − wskin P0 or, because P(h ) = P0 e −Ch , Fnet = e −ChVg (ρ air − ρ He ) − wload − wskin Substitute numerical values and evaluate Fnet: −1 Fnet = e − (0.13 km )(4.84 km ) (32.0 m 3 )(9.81 m/s 2 )(1.293 kg/m 3 − 0.179 kg/m 3 ) − 110 N − 50 N = 30 N Because Fnet = 30 N > 0, the balloon will rise higher than the altitude at which it is fully inflated. (d) The balloon will rise until the net force acting on it is zero. Because the buoyant force depends on the density of the air, the balloon will rise until the density of the air has decreased sufficiently for the buoyant force to just equal the total weight of the balloon. ρ 1 ln air C ρ air, h Substitute equation (5) in equation (2) to obtain: h= Using equation (4), express the density of the air in terms of FB: ρ air, h = Substituting for ρ air, h and ⎡ ⎤ ⎢ ρ ⎥ 1 ⎡Vgρ air ⎤ 1 h = ln ⎢ air ⎥ = ln ⎢ ⎥ C ⎢ FB ⎥ C ⎣ FB ⎦ ⎢⎣ Vg ⎥⎦ simplifying yields: FB Vg Substitute numerical values and evaluate h: ( )( )( ) 3 2 3 1 ⎛ ⎞ ⎡ 32.0 m 9.81 m/s 1.293 kg/m ⎤ h=⎜ ln ⎢ ⎥ = 6.0 km −1 ⎟ 190.5 N ⎝ 0.13 km ⎠ ⎣ ⎦ Kinetic Theory of Gases 59 • [SSM] (a) One mole of argon gas is confined to a 1.0-liter container at a pressure of 10 atm. What is the rms speed of the argon atoms? (b) Compare your answer to the rms speed for helium atoms under the same conditions. 1750 Chapter 17 Picture the Problem We can express the rms speeds of argon and helium atoms by combining PV = nRT and vrms = 3RT M to obtain an expression for vrms in terms of P, V, and M. See Appendix C for the molar masses of argon and helium. Express the rms speed of an atom as a function of the temperature and its molar mass: From the ideal-gas law we have: RT = Substitute for RT to obtain: 3RT M vrms = PV n 3PV nM vrms = (a) Substitute numerical values and evaluate vrms for argon atoms: v rms, Ar = ( ) 3(10 atm )(101.325 kPa/atm ) 1.0 × 10 −3 m 3 = 0.28 km/s (1mol) 39.948 ×10−3 kg/mol ( ) (b) Substitute numerical values and evaluate vrms for helium atoms: v rms, He = ( ) 3(10 atm )(101.325 kPa/atm ) 1.0 × 10−3 m 3 = 0.87 km/s (1mol) 4.003 ×10−3 kg/mol ( ) The rms speed of argon atoms is slightly less than one third the rms speed of helium atoms. 60 • Find the total translational kinetic energy of the molecules of 1.0 L of oxygen gas at a temperature of 0.0ºC and a pressure of 1.0 atm. Picture the Problem We can express the total translational kinetic energy of the oxygen gas by combining K = 32 nRT and the ideal-gas law to obtain an expression for K in terms of the pressure and volume of the gas. Relate the total translational kinetic energy of translation to the temperature of the gas: K = 32 nRT Using the ideal-gas law, substitute for nRT to obtain: K = 32 PV Temperature and the Kinetic Theory of Gases 1751 Substitute numerical values and evaluate K: K= 3 2 ⎛ (101.325 kPa )⎜⎜1.0 L × 10 ⎝ m3 ⎞ ⎟ L ⎟⎠ −3 = 0.15 kJ 61 • Estimate the rms speed and the average kinetic energy of a hydrogen atom in a gas at a temperature of 1.0 × 107 K. (At this temperature, which is approximately the temperature in the interior of a star, hydrogen atoms are ionized and become protons.) Picture the Problem Because we’re given the temperature of the hydrogen atom and know its molar mass, we can find its rms speed using vrms = 3RT M and its average kinetic energy from K av = 32 kT . See Appendix C for the molar mass of hydrogen. Relate the rms speed of a hydrogen atom to its temperature and molar mass: Substitute numerical values and evaluate vrms: vrms = 3RT MH v rms = 3(8.314 J/mol ⋅ K ) 1.0 × 107 K 1.0079 × 10−3 kg/mol ( ) )( ) = 5.0 × 105 m/s Express the average kinetic energy of the hydrogen atom as a function of its temperature: K av = 32 kT Substitute numerical values and evaluate Kav: K av = 32 1.381×10 −23 J/K 1.0 × 10 7 K ( = 2.1×10 −16 J 62 • Liquid helium has a temperature of only 4.20 K and is in equilibrium with its vapor at atmospheric pressure. Calculate the rms speed of a helium atom in the vapor at this temperature, and comment on the result. Picture the Problem The rms speed of helium atoms is given by vrms = See Appendix C for the molar mass of helium. 3RT . M He 1752 Chapter 17 The rms speed of helium atoms is given by: v rms = 3 RT M He Substitute numerical values and evaluate vrms: v rms = 3(8.314 J/mol ⋅ K )(4.20 K ) 4.003 g/mol = 162 m/s Thermal speeds, even at temperatures as low as 4.20 K, are very large compared to most of the speeds we experience directly. 63 • Show that the mean free path for a molecule in an ideal gas at temperature T and pressure P is given by λ = kT 2 Pπ d 2 . Picture the Problem We can combine λ = 1 and PV = nRT to express 2nvπ d 2 the mean free path for a molecule in an ideal gas in terms of the pressure and temperature. Express the mean free path of a molecule in an ideal gas: λ= 1 2nvπ d 2 (1) where N nN A nv = = V V Solve the ideal-gas law for the volume of the gas: V = nRT P Substitute for V in the expression for nv to obtain: nv = nN A P P= nRT kT Substitute for nv in equation (1) to obtain: λ= kT 2 Pπ d 2 64 •• State-of-the-art vacuum equipment can attain pressures as low as –11 7.0 × 10 Pa. Suppose that a chamber contains helium at this pressure and at room temperature (300 K). Estimate the mean free path and the collision time for helium in the chamber. Assume the diameter of a helium atom is 1.0 × 10–10 m. Picture the Problem We can find the collision time from the mean free path and the average (rms) speed of the helium molecules. We can use the result of Problem Temperature and the Kinetic Theory of Gases 1753 63 to find the mean free path of the molecules and vrms = 3RT M to find the average speed of the molecules. See Appendix C for the molar mass of helium. Express the collision time in terms of the mean free path for and the average speed of a helium molecule: τ= From Problem 63, the mean free path of the gas is given by: λ= Substitute numerical values and evaluate the mean free path λ: λ vav λ= ≈ λ (1) vrms kT 2 Pπ d 2 ( (1.381×10 2 7.0 ×10 −11 −23 ) J/K (300 K ) ) ( Pa π 1.0 ×10 −10 m ) 2 = 1.332 ×10 9 m = 1.3 ×10 9 m Express the rms speed of the helium molecules: vrms = Substitute for vrms in equation (1) and simplify to obtain: τ= 3RT M He λ 3RT M He =λ M He 3RT Substitute numerical values and evaluate τ : τ = (1.332 × 109 m ) 4.007 g/mol = 9.7 × 10 5 s 3(8.314 J/mol ⋅ K )(300 K ) 65 •• [SSM] Oxygen (O2) is confined to a cube-shaped container 15 cm on an edge at a temperature of 300 K. Compare the average kinetic energy of a molecule of the gas to the change in its gravitational potential energy if it falls 15 cm (the height of the container). Picture the Problem We can use K = 32 kT and ΔU = mgh = Mgh N A to express the ratio of the average kinetic energy of a molecule of the gas to the change in its gravitational potential energy if it falls from the top of the container to the bottom. See Appendix C for the molar mass of oxygen. Express the average kinetic energy of a molecule of the gas as a function of its temperature: K av = 32 kT 1754 Chapter 17 Letting h represent the height of the container, express the change in the potential energy of a molecule as it falls from the top of the container to the bottom: ΔU = mgh = Express the ratio of Kav to ΔU and simplify to obtain: M O 2 gh NA 3 kT K av 3N A kT = 2 = ΔU M O 2 gh 2M O 2 gh NA Substitute numerical values and evaluate Kav/ΔU: ( )( )( ) K av 3 6.022 ×10 23 particles/mol 1.381×10 −23 J/K (300 K ) = = 7.9 ×10 4 2 −3 ΔU 2 32.0 × 10 kg/mol 9.81 m/s (0.15 m ) ( ) *The Distribution of Molecular Speeds 66 •• Use calculus to show that f(v), given by Equation 17-36, has its maximum value at a speed v = 2 kT / m . Picture the Problem Equation 17-36 gives the Maxwell-Boltzmann speed distribution. Setting its derivative with respect to v equal to zero will tell us where the function’s extreme values lie. Differentiate Equation 17-36with respect to v: 32 df d ⎡ 4 ⎛ m ⎞ 2 − mv 2 = ⎜ ⎟ ve ⎢ dv dv ⎢⎣ π ⎝ 2kT ⎠ 4 ⎛ m ⎞ = ⎜ ⎟ π ⎝ 2kT ⎠ Set df/dv = 0 for extrema and solve for v: 2v − 32 2 kT ⎤ ⎥ ⎦⎥ ⎛ mv3 ⎞ − mv 2 ⎜⎜ 2v − ⎟e kT ⎟⎠ ⎝ mv3 =0⇒ v= kT 2 kT 2kT m Examination of the graph of f(v) makes it clear that this extreme value is, in fact, a maximum. See Figure 17-17 and note that it is concave downward at v = 2kT m . Remarks: An alternative to the examination of f(v) in order to conclude that v = 2kT m maximizes the Maxwell-Boltzmann speed distribution function is to show that d2f/dv2 < 0 at v = 2kT m . Temperature and the Kinetic Theory of Gases 1755 67 •• [SSM] The fractional distribution function f(v) is defined in Equation 17-36. Because f(v) dv gives the fraction of molecules that have speeds in the range between v and v + dv, the integral of f(v) dv over all the possible ranges of speeds must equal 1. Given that the integral ∫ ∞ 0 2 v 2 e− av dv = π 4 a−3/ 2 , show that ∫ f (v )dv = 1 , where f(v) is given by Equation 17-36. ∞ 0 Picture the Problem We can show that f(v) is normalized by using the given integral to integrate it over all possible speeds. Express the integral of Equation 1736: ∞ Let a = m 2kT to obtain: ∞ ∫ 0 ∫ 4 ⎛ m ⎞ f (v )dv = ⎜ ⎟ π ⎝ 2kT ⎠ f (v )dv = 0 π ∞ ∫v e 2 − mv 2 2 kT dv 0 a 3 2 ∫ v 2e − av dv 2 0 ⎛ π −3 2 ⎞ a 3 2 ⎜⎜ a ⎟⎟ = 1 π 0 ⎝ 4 ⎠ That is, f(v) is normalized. Use the given integral to obtain: ∞ ∫ f (v )dv = ∞ 3 − av 2 ∫0 v 4 1 dv = 2 , calculate the average 2a speed vav of molecules in a gas using the Maxwell–Boltzmann distribution function. 68 •• Given that the integral 4 3 2∞ e Picture the Problem In Problem 67 we showed that f(v) is normalized. Hence we ∞ can evaluate vav using ∫ vf (v )dv . 0 The average speed of the molecules in the gas is given by: ∞ vav = ∫ vf (v )dv 0 = Substitute a = m 2kT : vav = 4 ⎛ m ⎞ ⎜ ⎟ π ⎝ 2kT ⎠ 4 π ∞ a 32 3 2∞ ∫v e 3 − mv 2 2 kT 0 3 − av ∫ v e dv 0 2 dv 1756 Chapter 17 Use the given integral to obtain: ⎛ a −2 ⎞ 2 1 ⎟⎟ = a 3 2 ⎜⎜ π π a ⎝ 2 ⎠ 4 vav = 2 = 2kT m π The translational kinetic energies of the molecules of a gas are 69 •• distributed according to the Maxwell-Boltzmann energy distribution, Equation 17-38. (a) Determine the most probable value of the translational kinetic energy (in terms of the temperature T) and compare this value to the average value. (b) Sketch a graph of the translational kinetic energy distribution [f(E) versus E] and label the most probable energy and the average energy. (Do not worry about calibrating the vertical scale of the graph.) (c) Your teacher says, ″Just looking at the graph f(E) versus E of allows you to see that the average translational kinetic energy is considerably greater than the most probable translational kinetic energy.″ What feature(s) of the graph support her claim? Picture the Problem We can set the derivative of f(E) with respect to E equal to zero in order to determine the most probable value of the kinetic energy of the gas molecules. (a) The Maxwell-Boltzmann energy distribution function is: ⎛ 2 ⎞⎛ 1 ⎞ f (E ) = ⎜⎜ ⎟⎟ ⎜ ⎟ ⎝ π ⎠ ⎝ kT ⎠ 32 E e − E kT Differentiate this expression with respect to E to obtain: d ⎛ 2 ⎞⎛ 1 ⎞ f (E ) = ⎜⎜ ⎟⎟ ⎜ ⎟ dE ⎝ π ⎠ ⎝ kT ⎠ 32 ⎛ 1 −1 2 − E kT ⎞ ⎛ −1 ⎞ ⎜⎜ 2 E e + E 1 2 ⎜ ⎟e − E kt ⎟⎟ ⎝ kT ⎠ ⎝ ⎠ Set this derivative equal to zero for extrema values and simplify to obtain: 1 2 Solving for Epeak yields: E peak = The average value of the energy of the gas molecules is given by: Eav = 32 kT Express the ratio of Epeak to Eav: E peak −1 2 Epeak e Eav = − E peak kT 1 2 1 2 3 2 1 2 ⎛ − 1 ⎞ − E peak + Epeak ⎜ ⎟e ⎝ kT ⎠ kt =0 kT kT 1 = 3 ⇒ E peak = kT 1 3 Eav (b) The following graph of the energy distribution was plotted using a spreadsheet program. Note that kT was set equal to 1 and that, as predicted by our result in (a), the peak value occurs for E = 0.5. Temperature and the Kinetic Theory of Gases 1757 0.50 0.45 0.40 0.35 f (E ) 0.30 0.25 0.20 0.15 0.10 0.05 0.00 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 E (c) The graph rises from zero to the peak much more rapidly than it falls off to the right of the peak. Because the distribution is so strongly skewed to the right of the peak, the outlying molecules with relatively high energies pull the average (3kT/2) far to the right of the most probable value (kT/2). General Problems 70 • Find the temperature at which the rms speed of a molecule of hydrogen gas equals 343 m/s. Picture the Problem We can use vrms = 3RT M to relate the temperature of the H2 molecules to their rms speed. See Appendix C for the molar mass of hydrogen. Relate the rms speed of the hydrogen molecules to its temperature: vrms = Substitute numerical values and evaluate T: T= 2 M H 2 vrms 3RT ⇒T = M H2 3R (2.016 ×10 ) kg/mol (343 m/s ) 3(8.314 J/mol ⋅ K ) −3 2 = 9.51K 71 •• (a) If 1.0 mol of a gas in a cylindrical container occupies a volume of 10 L at a pressure of 1.0 atm, what is the temperature of the gas in kelvins? (b) The cylinder is fitted with a piston so that the volume of the gas (Figure 1720) can vary. When the gas is heated at constant pressure, it expands to a volume of 20 L. What is the temperature of the gas in kelvins? (c) Next, the volume is fixed at 20 L, and the gas’s temperature is increased to 350 K. What is the pressure of the gas now? 1758 Chapter 17 Picture the Problem We can use the ideal-gas law to find the initial temperature of the gas and the ideal-gas law for a fixed amount of gas to relate the volumes, pressures, and temperatures resulting from the given processes. (a) Apply the ideal-gas law to express the temperature of the gas: T= PV nR Substitute numerical values and evaluate T: T= (101.325 kPa ) (10 ×10 −3 m 3 ) (1.0 mol)(8.314 J/mol ⋅ K ) = 121.9 K = 1.2 × 10 2 K (b) Use the ideal-gas law for a fixed amount of gas to relate the temperatures and volumes: Solve for and evaluate T2: P1V1 P2V2 = T1 T2 or, because P1 = P2, V1 V2 = T1 T2 T2 = V2 T1 = 2(121.9 K ) = 243.7 K V1 = 2.4 × 10 2 K (c) Use the ideal-gas law for a fixed amount of gas to relate the temperatures and pressures: P1V1 P2V2 = T1 T2 or, because V1 = V2, P1 P2 = T1 T2 Solve for T2: P2 = T2 P1 T1 Substitute numerical values and evaluate P2: P2 = 350 K (1.0 atm) = 1.4 atm 243.7 K 72 •• (a) The volume per molecule of a gas is the reciprocal of the number density (the number of molecules per unit volume). (a) Find the average volume per molecule for dry air at room temperature and atmospheric pressure. (b) Take the cube root of your answer to Part (a) to obtain a rough estimate of the average distance d between air molecules. (c) Find or estimate the average diameter D of an air molecule, and compare it to your answer to Part (b). (d) Sketch the molecules in a cube-shaped volume of air, with the edge length of the cube equal Temperature and the Kinetic Theory of Gases 1759 to 3d. Make your figure to scale and place the molecules in what you think is a typical configuration. (e) Use your picture to explain why the mean free path of an air molecule is much greater than the average distance between molecules. Picture the Problem (a) We can use the ideal-gas law to find the average volume per molecule. (b) If one were to divide a container of air into little cubes, with one cube per molecule, then this distance would be the width of each cube or, equivalently, the distance from the center of one cube to the centers of the neighboring cubes. On average, we can imagine that the molecules are at the centers of their respective cubes, so this distance is also the average distance between neighboring molecules. (a) The ideal-gas law relates the number of molecules N in a gas to the volume V they occupy: PV = NkT ⇒ Substitute numerical values and evaluate V/N: 1.381× 10 −23 J/K (293 K ) V = N 101.325 kPa V kT = N P ( ) = 3.99 × 10 −26 m 3 (b) Taking the cube root of V/N yields: d = 3 3.99 × 10−26 m 3 ≈ 3.42 nm (c) Example 17-10 gives the average diameter of an air molecule as: D = 3.75 × 10 −10 m = 0.375 nm or about 1/10 the average distance between molecules. (d) A sketch of the molecules in a cube-shaped volume of air, with the edge length of the cube equal to 3d, follows. The random distribution of the molecules is a typical configuration. 3d 3d 3d 1760 Chapter 17 (e) If a particular molecule in the diagram is moving in a random direction, its chance of colliding with a neighbor is very small because it can miss in either of the two directions perpendicular to its motion. So the mean free path, or average distance between collisions, should be many times larger than the distance to the nearest neighbor. 73 •• [SSM] The Maxwell-Boltzmann distribution applies not just to gases, but also to the molecular motions within liquids. The fact that not all molecules have the same speed helps us understand the process of evaporation. (a) Explain in terms of molecular motion why a drop of water becomes cooler as molecules evaporate from the drop’s surface. (Evaporative cooling is an important mechanism for regulating our body temperatures, and is also used to cool buildings in hot, dry locations.) (b) Use the Maxwell-Boltzmann distribution to explain why even a slight increase in temperature can greatly increase the rate at which a drop of water evaporates. Determine the Concept (a) To escape from the surface of a droplet of water, molecules must have enough translational kinetic energy to overcome the attractive forces from their neighbors. Therefore the molecules that escape will be those that are moving faster, leaving the slower molecules behind. The slower molecules have less kinetic energy, so the temperature of the droplet, which is proportional to the average translational kinetic energy per molecule, decreases. (b) As long as the temperature isn’t too high, the molecules that evaporate from a surface will be only those with the most extreme speeds, at the high-energy ″tail″ of the Maxwell-Boltzmann distribution. Within this part of the distribution, increasing the temperature only slightly can greatly increase the percentage of molecules with speeds above a certain threshold. For example, suppose that we set an initial threshold at E = 5kT1, then imagine increasing the temperature by 10% so T2 = 1.1T1. At the threshold, the ratio of the new energy distribution to the old one is −3 − 5 F (T2 ) ⎛ T1 ⎞ kT2 kT1 = ⎜⎜ ⎟⎟e e = (1.1) 2 e 1.1e 5 = 1.366 F (T1 ) ⎝ T2 ⎠ −E +E an increase of almost 37%. 74 •• A cubic metal box that has 20-cm–long edges contains air at a pressure of 1.0 atm and a temperature of 300 K. The box is sealed so that the enclosed volume remains constant, and it is heated to a temperature of 400 K. Find the force due to the internal air pressure on each wall of the box. Temperature and the Kinetic Theory of Gases 1761 Picture the Problem We can use the definition of pressure to express the net force on each wall of the box in terms of its area and the pressure differential between the inside and the outside of the box. We can apply the ideal-gas law for a fixed amount of gas to find the pressure inside the box after it has been heated. Using the definition of pressure, express the net force on each wall of the box: F = AΔP = A(Pinside − Patm ) Use the ideal-gas law for a fixed amount of gas to relate the initial and final pressures of the gas: PatmVinitial PinsideVfinal = Tinitial Tfinal (1) or, because Vinitial = Vfinal, Patm P = inside Tinitial Tfinal Solve for Pinside to obtain: Pinside = Substituting in equation (1) and simplifying yields: ⎛T ⎞ F = A⎜⎜ final Patm − Patm ⎟⎟ ⎝ Tinitial ⎠ ⎛T ⎞ = A⎜⎜ final − 1⎟⎟ Patm ⎝ Tinitial ⎠ Substitute numerical values and evaluate F: ⎞ 2 ⎛ 400 K F = (0.20 m ) ⎜ − 1⎟ (101.325 kPa ) ⎝ 300 K ⎠ Tfinal Patm Tinitial = 1.4 kN 75 •• [SSM] In attempting to create liquid hydrogen for fuel, one of the proposals is to convert plain old water (H2O) into H2 and O2 gases by electrolysis. How many moles of each of these gases result from the electrolysis of 2.0 L of water? Picture the Problem We can use the molar mass of water to find the number of moles in 2.0 L of water. Because there are two hydrogen atoms in each molecule of water, there must be as many hydrogen molecules in the gas formed by electrolysis as there were molecules of water and, because there is one oxygen atom in each molecule of water, there must be half as many oxygen molecules in the gas formed by electrolysis as there were molecules of water. See Appendix C for the molar masses of hydrogen and oxygen. 1762 Chapter 17 Express the electrolysis of water into H2 and O2: Express the number of moles in 2.0 L of water: nH 2 O → nH 2 + 12 nO 2 g L = 110 mol = 18.02 g/mol 2.0 L × 1000 nH 2 O Because there are two hydrogen atoms for each water molecule: nH 2 = 110 mol Because there is one oxygen atom for each water molecule: nO 2 = 12 nH 2 O = 1 2 (110 mol) = 55 mol 76 •• A hollow 40-cm-long cylinder of negligible mass rests on its side on a horizontal frictionless table. The cylinder is divided into two equal sections by a vertical non-porous membrane. One section contains nitrogen and the other contains oxygen. The pressure of the nitrogen is twice that of the oxygen. How far will the cylinder move if the membrane breaks? Picture the Problem The diagram shows the cylinder before removal of the membrane. We’ll assume that the gases are at the same temperature. The approximate location of the center of mass (CM) is indicated. We can find the distance the cylinder moves by finding the location of the CM after the membrane is removed. See Appendix C for the molar masses of oxygen and nitrogen. N2 O2 x , cm 0 CM 40 Express the distance the cylinder will move in terms of the movement of the center of mass when the membrane is removed: Δx = xcm,after − xcm,before Apply the ideal-gas law to both collections of molecules to obtain: PN 2 VN 2 = nN 2 kT and PO 2 VO 2 = nO 2 kT Temperature and the Kinetic Theory of Gases 1763 Divide the first of these equations by the second to obtain: PN 2 PO 2 = nN 2 nO 2 or, because PN 2 = 2 PO 2 , 2 PO 2 PO 2 = nN 2 nO 2 ⇒ nN 2 = 2nO 2 Express the mass of O2 in terms of its molar mass and the number of moles of oxygen: mO 2 = nO 2 M O 2 Express the mass of N2 in terms of its molar mass and the number of moles of nitrogen: mN 2 = nO 2 M N 2 Using the definition of center of mass, express the center of mass before the membrane is removed: ∑x m = ∑m i xcm,before i i i = n(N 2 )M (N 2 )xcm, N 2 + n(O 2 )M (O 2 )xcm,O 2 n(N 2 )M (N 2 ) + n(O 2 )M (O 2 ) i = = 2n(O 2 )M (N 2 )xcm, N 2 + n(O 2 )M (O 2 )xcm,O 2 2n(O 2 )M (N 2 ) + n(O 2 )M (O 2 ) 2 M (N 2 )xcm, N 2 + M (O 2 )xcm,O 2 2M (N 2 ) + M (O 2 ) Substitute numerical values and evaluate xcm,before: xcm,before = 2(10 cm )(28.01g ) + (30 cm )(32.00 g ) = 17.27 cm 2(28.01g ) + 32.00 g Locate the center of mass after the membrane is removed: xcm,after = Substitute to obtain: 2(20 cm )(28.01g ) + (20 cm )(32.00 g ) = 20.00 cm 2(28.01g ) + 32.00 g Δx = 20.00 cm − 17.27 cm = 2.7 cm 1764 Chapter 17 Because momentum must be conserved during this process and the center of mass moved to the right, the cylinder moved 2.7 cm to the left. 77 •• A cylinder of fixed volume contains a mixture of helium gas (He) and hydrogen gas (H2) at a temperature T1 and pressure P1. If the temperature is doubled to T2 = 2T1, the pressure would also double, except for the fact that at temperature the H2 is essentially 100 percent dissociated into H1. In reality, at pressure P2 = 2P1 the temperature is T2 = 3T1. If the mass of the hydrogen in the cylinder is m, what is the mass of the nitrogen in the cylinder? Picture the Problem We can apply the ideal-gas law to the two processes to find the number of moles of hydrogen in terms of the number of moles of nitrogen in the gas. Using the definition of molar mass, we can relate the mass of each gas to the number of moles of each gas and their molar masses. See Appendix C for the molar masses of nitrogen gas and hydrogen gas. [ ] Apply the ideal-gas law to the first case: P1V = 2nN 2 + nH 2 RT1 Apply the ideal-gas law to the second case: 3P1V = 2nN 2 + 2nH 2 2 RT1 Divide the second of these equations by the first and simplify to express nH 2 in terms of nN2 : nH 2 = 2nN 2 Relate the mN to nN2 : mN = nN 2 M N 2 [ ] = n N 2 (28.01g/mol) and nN 2 = Relate the m to nH2 : mN 2 28.01g/mol m = nH 2 M H 2 = nH 2 (2.016 g/mol) and nH 2 = mH 2 2.016 g/mol (1) Temperature and the Kinetic Theory of Gases 1765 Substitute in equation (1) and solve for mN: 2m N 2 m = 2.016 g/mol 28.01g/mol and mN 2 ≈ 7m 78 •• The mean free path for O2 molecules at a temperature of 300 K and at 1.00 atm pressure is 7.10 × 10–8 m. Use this data to estimate the size of an O2 molecule. Picture the Problem Because the O2 molecule resembles 2 spheres stuck together, which in cross section look something like two circles, we can estimate the radius of the molecule from the formula for the area of a circle. We can express the area, and hence the radius, of the circle in terms of the mean free path and the number density of the molecules and use the ideal-gas law to express the number density. Express the area of two circles of diameter d that touch each other: ⎛ πd 2 ⎞ πd 2 ⎟⎟ = ⇒d = A = 2⎜⎜ 4 2 ⎝ ⎠ Relate the mean free path of the molecules to their number density and cross-sectional area: λ= Substitute for A in equation (1) to obtain: Use the ideal-gas law to relate the number density of the O2 molecules to their temperature and pressure: Substituting for nv yields: Substitute numerical values and evaluate d: d= 2A π (1) 1 1 ⇒A= nv A nv λ 2 πnv λ PV = NkT or nv = N P = V kT d= 2kT πPλ d= 2 1.381×10 − 23 J/K (300 K ) π (101.325 kPa ) 7.10 ×10 −8 m ( ( ) ) = 0.605 nm 79 •• [SSM] Current experiments in atomic trapping and cooling can create low-density gases of rubidium and other atoms with temperatures in the nanokelvin (10–9 K) range. These atoms are trapped and cooled using magnetic 1766 Chapter 17 fields and lasers in ultrahigh vacuum chambers. One method that is used to measure the temperature of a trapped gas is to turn the trap off and measure the time it takes for molecules of the gas to fall a given distance. Consider a gas of rubidium atoms at a temperature of 120 nK. Calculate how long it would take an atom traveling at the rms speed of the gas to fall a distance of 10.0 cm if (a) it were initially moving directly downward and (b) if it were initially moving directly upward. Assume that the atom doesn’t collide with any others along its trajectory. Picture the Problem Choose a coordinate system in which downward is the positive direction. We can use a constant-acceleration equation to relate the fall distance to the initial velocity of the molecule, the acceleration due to gravity, the fall time, and vrms = 3kT m to find the initial velocity of the rubidium molecules. (a) Using a constant-acceleration equation, relate the fall distance to the initial velocity of a molecule, the acceleration due to gravity, and the fall time: Relate the rms speed of rubidium atoms to their temperature and mass: Substitute numerical values and evaluate vrms: y = v0t + 12 gt 2 (1) vrms = 3kT mRb v rms = 3 1.381× 10−23 J/K (120 nK ) (85.47 u ) 1.6606 ×10−27 kg/u ( ) ( = 5.918 × 10−3 m/s Letting vrms = v0, substitute in equation (1) to obtain: ( ) ( ) 0.100 m = 5.918 × 10 −3 m/s t + 12 9.81m/s 2 t 2 Use the quadratic formula or your graphing calculator to solve this equation for its positive root: t = 0.14218 s = 142 ms (b) If the atom is initially moving upward: v rms = v0 = −5.918 ×10 −3 m/s ) Temperature and the Kinetic Theory of Gases 1767 Substitute in equation (1) to obtain: ( ) ( ) 0.100 m = − 5.918 × 10−3 m/s t + 12 9.81 m/s 2 t 2 Use the quadratic formula or your graphing calculator to solve this equation for its positive root: t = 0.1464 s = 146 ms 80 ••• A cylinder is filled with 0.10 mol of an ideal gas at standard temperature and pressure, and a 1.4-kg piston seals the gas in the cylinder (Figure 17-21) with a frictionless seal. The trapped column of gas is 2.4-m high. The piston and cylinder are surrounded by air, also at standard temperature and pressure. The piston is released from rest and starts to fall. The motion of the piston ceases after the oscillations stop with the piston and the trapped air in thermal equilibrium with the surrounding air. (a) Find the height of the gas column. (b) Suppose that the piston is pushed down below its equilibrium position by a small amount and then released. Assuming that the temperature of the gas remains constant, find the frequency of vibration of the piston. Picture the Problem (a) Let A be the cross-sectional area of the cylinder. We can use the ideal-gas law to find the height of the piston under equilibrium conditions. In (b), we can apply Newton’s 2nd law and the ideal-gas law for a fixed amount of gas to the show that, for small displacements from its equilibrium position, the piston executes simple harmonic motion. (a) Express the pressure inside the cylinder: Pin = Patm + Apply the ideal-gas law to obtain a second expression for the pressure of the gas in the cylinder: Pin = Equating these two expressions yields: Patm + Solve for h to obtain: h= = mg A nRT nRT = V hA mg nRT = A hA (2.4 m )APatm nRT = APatm + mg APatm + mg 2.4 m mg 1+ APatm (1) 1768 Chapter 17 At STP, 0.10 mol of gas occupies 2.24 L. Therefore: Substitute numerical values and evaluate h: (2.4 m )A = 2.24 × 10−3 m3 and A = 9.333 × 10 −4 m 2 h= 2.4 m ( 1.4 kg ) 9.81 m/s 2 1+ 9.333 ×10 − 4 m 2 (101.325 kPa ) ( ( ) ) = 2.096 m = 2.1 m (b) Relate the frequency of vibration of the piston to its mass and a ″stiffness″ constant: 1 k (2) 2π m where m is the mass of the piston and k is a constant of proportionality. Letting y be the displacement from equilibrium, apply ∑ Fy = ma y to Pin A − mg − Patm A = 0 f = the piston in its equilibrium position: For a small displacement y above equilibrium: P' in A − mg − Patm A = ma y or P' in A − Pin A = ma y Using the ideal-gas law for a fixed amount of gas and constant temperature, relate Pin' to Pin : P'inV' = PinV or Substituting for V and simplifying yields: P' in A = Pin A Substitute in equation (3) to obtain: y⎞ ⎛ Pin A⎜1 + ⎟ − Pin A = ma y ⎝ h⎠ or, for y << h, y⎞ ⎛ Pin A⎜1 − ⎟ − Pin A ≈ ma y ⎝ h⎠ Simplify equation (4) to obtain: (3) P' in (V + Ay ) = PinV ⇒ P'in = Pin V V + Ay Ah 1 = Pin A y Ah + Ay 1+ h −1 − Pin A y ≈ ma y h (4) Temperature and the Kinetic Theory of Gases 1769 Substitute in equation (1) to obtain: ⎛ nRT ⎞ y ⎛ nRT ⎞ −⎜ ⎟ A ≈ ma y ⇒ − ⎜ 2 ⎟ y ≈ ma y ⎝ Ah ⎠ h ⎝ h ⎠ Solving for ay yields: ay = − nRT y mh 2 or k y , the condition for SHM m k nRT where = m mh 2 ay = − Substitute in equation (2) to obtain: f = 1 2π nRT mh 2 Substitute numerical values and evaluate f: f = 1 2π (0.10 mol)(8.314 J/mol ⋅ K )(300 K ) = (1.4 kg )(2.096 m )2 1.0 Hz 81 ••• During this problem, you will use a spreadsheet to study the distribution of molecular speeds in a gas. Figure 17-22 should help you get started. (a) Enter the values for constants R, M, and T as shown. Then in column A, enter values of speed ranging from 0 to 1200 m/s, in increments of 1 m/s. (This spreadsheet will be long.) In cell B7, enter the formula for the MaxwellBoltzmann fraction fractional speed distribution. This formula contains parameters v, R, M and T. Substitute A7 for v, B$1 or R, B$2 for M and B$3 for T. Then use the FILL DOWN command to enter the formula in the cells below B7. Create a graph of f(v) versus v using the data in columns A and B. (b) Explore how the graph changes as you increase and decrease the temperature, and describe the results. (c) Add a third column in which each cell contains the cumulative sum of all f(v) values, multiplied by the interval size dv (which equals 1), in the rows above and including the row in question. What is the physical interpretation of the numbers in this column? (d) For nitrogen gas at 300 K, what percentage of the molecules has speeds less than 200 m/s? (e) For nitrogen gas at 300 K, what percentage of the molecules has speeds greater than 700 m/s? (a) The first few rows of the spreadsheet are shown below. Note that the column for Part (c) is included. A B C 1 R= 8.31 J/mol-K 2 M= 0.028 kg/mol 3 T= 300 K 4 5 f(v) sum f(v)dv v 1770 Chapter 17 6 7 8 9 10 11 12 (m/s) 0 1 2 3 4 5 (s/m) 0.00E+00 3.00E−08 1.20E−07 2.70E−07 4.80E−07 7.51E−07 (unitless) 0.00E+00 3.00E−08 1.50E−07 4.20E−07 9.01E−07 1.65E−06 A graph of f(v) for nitrogen at 300 K follows: 0.0025 f (v ), s/m 0.0020 0.0015 0.0010 0.0005 0.0000 0 200 400 600 800 1000 1200 v , m/s (b) As the temperature is increased the horizontal position of the peak moves to the right in proportion to the square root of the temperature, while the height of the peak drops by the same factor, preserving the total area under the graph (which must be 1.0). (c) Each number in column C of the spreadsheet (shown in (a)) is approximately equal to the integral of f(v) from zero up to the corresponding v value. This integral represents the probability of a molecule having a speed less than or equal to this value of v. Temperature and the Kinetic Theory of Gases 1771 1.2 1.0 f (v ) dv 0.8 0.6 0.4 0.2 0.0 0 200 400 600 800 1000 1200 v , m/s (d) Looking in cell C207, we find that the probability (at 300 K) of a nitrogen molecule having a speed less than 200 m/s is 0.0706 , or about 7%. Note that this value is consistent with the graph of f (v )dv shown immediately above. (e) Looking in cell C707, we find that the probability of a nitrogen molecule having a speed less than 700 m/s is approximately 0.862, so the probability of it having a speed greater than this would be 1 – 0.862 or 0.138 , or a little under 14%. 1772 Chapter 17