Internal thoracic vein: friend or foe?
advertisement

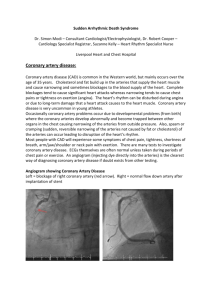
IMAGES IN MEDICINE Heart, Lung and Vessels. 2014; 6(3): 210-212 210 Internal thoracic vein: friend or foe? A. Roubelakis, D. Karangelis, S.K. Ohri Department of Cardiothoracic Surgery, Southampton University Hospitals NHS FoundationTrust, Southampton, UK Keywords: graft elongation, coronary artery bypass, revascularization. The internal thoracic vein is a conduit that has not been thoroughly investigated in literature as long term patency and outcomes are unknown. We present a case where the right internal thoracic vein (RITV) was used to extend a short right internal thoracic artery (RITA). The elongated composite conduit was then anastomosed to the right coronary artery (RCA). A 61-year-old male patient was referred electively for a coronary artery bypass grafting (CABG) operation. The patient had undergone extensive stenting to the left and right coronary artery systems. Unfortunately the stents to the right coronary artery had restenosed causing significant symptoms necessitating revascularization (Figure 1). From the conduit point of view, the patient had his long saphenous veins fully stripped bilaterally. His radial arteries were assessed with Allen’s test and use of saturation monitor: following the occlusion of the radial the saturations failed to rise, therefore they were deemed unusable. RITA was therefore elected to be the conduit of choice. Corresponding author: Dimos Karangelis MD, PhD Wessex Cardiac Centre Southampton University Hospitals FoundationT NHS Trust Tremona Road, Hampshire, UK e-mail: dimoskaragel@yahoo.gr Figure 1 - Preoperative angiogram showing an instent occlusion of the mid-distal RCA. RCA = right coronary artery. The operation was performed in a standard on-pump fashion. RITA was harvested initially as a pedicled graft. The target vessel was measuring approximately 1.5 mm in diameter and was opened distally due to the presence of the previous stents and the anatomy of the lesions. Unfortunately RITA intima was found to be of suboptimal quality and calibre distally and had to be shortened. This resulted in RITA length being insufficient to reach the target vessel, even with skeletonisation. RITA was then extended with a 2-3 cm segment of RITV which appeared to be of good quality and calibre. The reversed RITV and RITA were anastomosed in an end-to-end fashion using continuous 8-0 polypropylene suture (Figure 2). The composite graft was then anastomosed Heart, Lung and Vessels. 2014, Vol. 6 Internal thoracic vein: friend or foe? 211 Figure 2 - The end to end anastomosis (circled) between the RITA and RITV. There is no mismatch in the calibre of the two vessels. RITA=right internal thoracic artery; RITV = right internal thoracic vein. Figure 3 - The composite conduit as viewed from the patient’s head after the completion of the distal anastomosis. distally to the RCA (Figure 3) and proximally to the ascending aorta. There was excellent flow down this graft. The operation was completed uneventfully and the patient was discharged home on the 5th postoperative day. He remains symptomfree at a 6 month follow up. The ITV conduit studies in literature are very limited as they have not being used routinely, therefore long term results are unknown. The use of any other venous conduit (like short saphenous or cephalic veins) was not preferred due to patient’s age and would also lead to significant size mismatch between the RITA and the vein, if used as extentions. Left internal thoracic artery (LITA) needed to be preserved for possible future revascularization on the left coronary system. The use of gastroepiploic artery is not performed routinely in our unit and therefore experience is very limited. There is only one study in literature where ITVs were used as CABG grafts to the left anterior descending artery in a minipig model. The authors measured significant intimal hyperplasia in these grafts after 4 weeks (1). Similar studies to humans however have not been performed. A report from 1990 presented a 57 year old Heart, Lung and Vessels. 2014, Vol. 6 A. Roubelakis, et al. 212 patient who underwent CABG operation with the use of left and right ITAs and internal mammary vein (IMV). to an obtuse marginal. Angiography after 10 days revealed excellent patency of the IMV graft (2). It is difficult to justify the use of internal thoracic veins as conduits for CABG, as in most cases there is ample selection of other well established conduits. The calibre of these veins is similar to internal mammary arteries (IMAs) and could potentially be used as extensions. ITA elongation has been described before with the utilization of various grafts (3,4). To summarise, our method could potentially be useful when the internal mammary artery is not long enough to reach the target vessel when no other option is possible. The major limitation of this report is that a postoperative angiogram to assess the patency of the graft could not be obtained. There is no need of ethical committee approval for this case report. Written informed consent was obtained from the patient. REFERENCES 1. Popov AF, Dorge H, Hinz J, Schmitto JD, Stojanovic T, Seipelt R, et al. Accelerated intimal hyperplasia in aortocoronary internal mammary vein grafts in minipigs. J Cardiothorac Surg. 2008; 3: 20. 2. Stephan Y, Jebara VA, Fabiani JN, Carpentier A. The internal mammary vein: a new conduit for coronary artery bypass. J. Thorac. Cardiovasc. Surg. 1990; 99: 178. 3.Calafiore AM, Teodori G, Di Giammarco G, Vitolla G, Contini M, Maddestra N, et al. Left internal mammary elongation with inferior epigastric artery in minimally invasive coronary surgery. Eur J Cardiothorac Surg. 1997; 12: 393-6; discussion 397-8. 4.Bernet FH, Hirschmann MT, Reineke D, Grapow M, Zerkowski HR. Clinical outcome after composite grafting of calcified left anterior descending arteries. J Cardiovasc Surg (Torino) 2006; 47: 569-74. Cite this article as: Roubelakis A, Karangelis D, Ohri SK. Internal thoracic vein: friend or foe? Heart, Lung and Vessels. 2014; 6(3): 210-212. Source of Support: Nil. Disclosures: None declared. Acknowledgment: We thank Anne Gale for editorial assistance. Heart, Lung and Vessels. 2014, Vol. 6