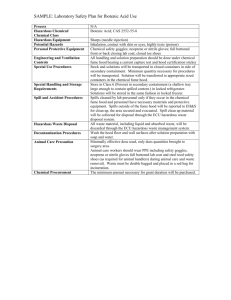
Chemical & Laboratory Safety Manual
advertisement

University of Georgia Chemical and Laboratory Safety Manual Previous Revision 1997 Last Revision 2002 Last Reviewed 2013 E MERGENCY P HONE N UMBERS UGA Police 2-2200 Environmental Safety 2-5801 Fire/Ambulance 9-911 Poison Control 9-800-282-5846 St. Mary’s Hospital 9-548-7581 Athens Regional Hospital 9-549-9977 Biosafety Office 2-7265 Fire Safety 9-369-5706 TABLE OF CONTENTS SECTION 1. Authority and Responsibility I. II. III. IV. Chemical and Laboratory Safety Committee Environmental Safety Division College dean, department or unit head The laboratory supervisor SECTION 2. Standard Operating Procedures I. II. III. IV. V. VI. VII. VIII. IX. X. XI. XII. XIII. XIV. XV. Chemical procurement, distribution, and storage Transportation and shipment of hazardous chemicals on- and off-campus Safe work practices Chemical fume hood use Housekeeping, maintenance, and inspections Personal protective equipment Records Signs and labels Spills and other laboratory accidents Electrical safety Mechanical hazards Synthesized chemicals Laboratory decommissioning Hazardous chemical and waste disposal Fire Safety SECTION 3. The Laboratory Facilities I. II. III. IV. V. Minimum design provision Construction and renovation review General laboratory ventilation Other ventilation devices Exhaust stacks SECTION 4. Particularly Hazardous Substances I. II. General requirements Standard operating procedures for particularly hazardous substances Appendix A. Chemical and Laboratory Safety Committee members Appendix B. Unsafe Laboratory Closure Policy I. Notice of unacceptable laboratory operations i II. III. Corrective actions suggested by the Committee Appeal of Committee action Appendix C. Flammable, Oxidizing and other Pressurized Gases I. II. II. Guidelines for using and storing pressurized gases Gas cylinder storage and labeling Proper handling of gas cy linders Appendix D. Signs, Forms, and Labels I. Forms A. B. C. D. E. II. Postings A. B. C. D. E. F. G. III. UGA Laboratory Safety Survey Form CAUTION Sign Request Form Fume Hood Certification Request Form Employee On-going Chemical Specific Right to Know Training Record Request to Open (commission) a Laboratory or Request to Close (decommission) a Laboratory Emergency Phone Numbers [posted by lab phone] Eyewash Location Safety Shower Location Chemical Spill Kit Location Chemical Storage Plan Gas Cylinder Tags Unattended Laboratory Operations Labeling Systems (RTK) A. B. C. Hazardous Chemical Container Labeling Acceptable Abbreviations for Primary Hazards Acceptable Chemical Abbreviations for Chemical Secondary Container Labeling Appendix E. Respirator Protection Program Appendix F. Board of Regents Fume Hood Standards Appendix G. Hazardous Waste Summary Procedures Appendix H. Waste Minimization, Bench-top Treatment and Surplus Redistribution ii Appendix I. I. II. III. Appendix J. I. Particularly Hazardous Substances Peroxide-forming Chemicals Specific Chemicals Suspected and Known Carcinogens Reproductive Toxins Recommended Laboratory Standard Operating Procedures and Other Resource Information Suggested Outline of Needed Standard Operating Procedures A. B. C. D. E. F. II. III. IV. V. VI. VII. VIII. IX. X. XI. XII. XIII. Safety Procedures and Protocols Personal injury procedures Chemical storage plan Chemical waste disposal Lab apparatus protocols and operating procedures Particularly hazardous substances How to Properly Complete a Caution Sign Chemical Spill Proced ure -Developing a General Lab Spill Kit SOP for Selection and Use of PPE Chemical Fume Hood SOP In Case Of Fire Portable Fire Extinguisher SOP Emergency Eyewash Station SOP Safety Shower SOP Respiratory Injury Protocol Hazardous Chemical Ingestion Protocol Chemical Storage Plan Perchloric Acid SOP The back section of this manual “Laboratory Standard Operating Procedures (SOP)” is provided for laboratory supervisors to place their laboratory’s SOPs. Within each section is a model SOP which may be modified to meet individual laboratory needs. iii SECTION 1. Authority and Responsibility I. Chemical and Laboratory Safety Committee (hereafter referred to as Committee) A. B. Appointment of the Committee 1. The Committee is appointed by the president of the University of Georgia (UGA). 2. The Committee will report to the vice presidents for academic affairs, research, and business and finance. The aforementioned vice presidents shall serve as the administrative Committee to receive and act upon the Committee’s findings, conclusions, and recommendations. Membership 1. The voting members of the Committee will include (see Appendix A for current membership) a. A minimum of 11 faculty members and laboratory directors from the Athens campus of UGA who are knowledgeable and active users of chemicals and laboratories and represent the diverse disciplines that use chemicals and laboratories b. Representatives from the following off-campus facilities: Coastal Plain Station, Marine Extension Service, Skidaway Institute of Oceanography, Georgia Agricultural Experiment Stations, and Savannah River Ecology Laboratory c. The associate vice president of Environmental Safety Division d. The University manager of chemical and lab safety e. The University director of Research Services f. The University hazardous waste coordinator 2. The non-voting members of the Committee will include the vice president for legal affairs with others designated as necessary. 3. The Committee will choose a chair and vice chair from among the appointed faculty members or laboratory directors every three years. Section 1 - 1 C. 4. The Committee will select a secretary from among its voting members. 5. A majority of the Committee eligible to vote will constitute a voting quorum. 6. A voting member of the Committee who cannot attend a meeting may designate a qualified alternate. This alternate will be a voting member of the Committee. 7. A Committee member may be replaced upon the recommendation of the chair to the president. The chair may also recommend the name(s) of qualified candidates for any vacancy. 8. Any Committee member wishing to resign his/her seat on the Committee is requested to submit the resignation in writing two meetings before the resignation is to take effect. 9. The chair may recommend the replacement of any Committee member who has missed two meetings during one calendar year. Meetings The Committee will meet, with at least a seven (7) calendar day notice, once each quarter during each academic year. Additional meetings may be called by the chair. The minutes of each meeting will be provided to the president, vice presidents for academic affairs, research, legal affairs, business and finance and to all members of the Committee. D. Duties and responsibilities 1. The Committee shall establish and review laboratory safety policies, procedures, and safety survey audit forms. No changes shall be made to this Chemical and Laboratory Safety Manual, appendices, or portions of the manual contained on the web site without Committee approval. The Committee shall approve all procedures used to evaluate laboratory safety and evaluate compliance of laboratory supervisors. 2. The policies will be designed to: a. b. Keep the University in compliance with local, state, and federal regulations regarding laboratory safety, the purchase, transportation, use, handling, storage, and disposal of all chemicals Protect and optimize safety for all faculty, staff, students, visitors, and members of the public from hazardous agents Section 1 - 2 c. 3. E. Recommend and approve training programs on laboratory safety practices that will result in faculty, staff, and students having a continuing conscientious awareness of and for safe laboratory practices, chemical storage, chemical use, and chemical disposal The Committee will review and advise on corrective actions recommended by the laboratory safety staff from ESD. To facilitate this review, the Committee will maintain an e-mail site which laboratory supervisors can utilize to contact the Committee: clsc@esd.uga.edu. a. Laboratory supervisors may appeal decisions to the Committee made by ESD in the implementation of the laboratory safety program. (See Appendix B, Unsafe Laboratory Closure Policy.) b. ESD, through the associate vice president of ESD, may bring to the Committee for resolution, problems with laboratory supervisors whom they feel are not in compliance with the University of Georgia Chemical and Laboratory Safety Manual. 4. The Committee will bring to the attention of UGA administration problems that need to be addressed by administrative procedures and advise them of options available and the desirability of various options. 5. The Committee will have the authority, after informing the vice presidents for academic affairs, research, legal affairs, and business and finance, to close any laboratory determined to be unsafe per the laboratory closure policy (see Appendix B) or which is not storing, using or disposing of chemicals safely or according to University policy. Any closed laboratory will be reopened for use only after a review by the Committee that results in affirming that the practices in the laboratory have been modified to result in a safe environment for faculty, staff, and students. Investigation of incidents 1. An incident that causes an excessive chemical or hazardous agent exposure will be investigated by ESD to determine the cause, and necessary remedial action will be recommended. 2. At the discretion of the Committee, a Committee member may be appointed to examine the circumstances in conjunction with ESD. Section 1 - 3 3. II. In the event of a serious disagreement between ESD and a laboratory supervisor over the causes or circumstances of an incident, the Committee may designate one or more Committee members to review the situation and make recommendations to the full Committee. Environmental Safety Division A. B. Mission 1. Provide advice and consultation to the Committee which is solely responsible for establishing University policies for chemical and laboratory safety as presented in this manual 2. Provide advice, consultation, and assistance to laboratory supervisors in complying with the policies and guidelines of this manual 3. Advise the Committee as to unsafe conditions in University laboratories using the guidelines and procedures provided for in the laboratory closure policy (see Appendix B) Authority 1. The associate vice president of the Environmental Safety Division has been designated environmental coordinator for the University of Georgia in response to a Regents’ directive to establish a central point of coordination for environmental matters. Therefore, any University unit receiving any communication from a regulatory agency regarding an environmental concern should immediately notify the associate vice president of Environmental Safety Division and the Office of Legal Affairs. 2. When the Environmental Safety Division determines that there is a serious violation of law, governmental regulation, or Committee policy in the control, use, or storage of hazardous materials, the cognizant vice president, the Office of Legal Affairs, and the Committee will be advised. In those instances where an investigation, either by the Environmental Safety Division or a governmental regulatory agency, is indicated, the cognizant vice president and the Committee will be advised of the investigation. 3. Once an investigation is complete and the circumstances surrounding the violation(s) have been determined, responsibility will be assigned for taking appropriate action to minimize the likelihood of recurrence. The Environmental Safety Division will initiate one of the following steps and inform the Committee of their action. Section 1 - 4 C. a. If a legal issue is involved the Environmental Safety Division will consult with the Office of the Vice President for Legal Affairs; both offices will then jointly determine the next action to be taken. b. If a legal issue is not involved, but internal action is needed, the Environmental Safety Division will refer the occurrence along with a copy of an audit or report of investigation to the cognizant vice president who will have responsibility for taking appropriate action to minimize the likelihood of recurrence. The Environmental Safety Division will be available to assist and advise. A copy of a referral will be sent to the president to whom a report of action will be filed by the cognizant vice president. 4. ESD has the responsibility and authority for conducting internal safety audits. These internal audits are governed by existing regulations and the policies set by the Committee which are contained in this manual. ESD shall provide a copy of the current Committee-approved survey form as part of this manual. These audits must be filed in a timely manner and as deemed necessary with the associate vice president of Environmental Safety Division/unit head. The response of the laboratory supervisor to these audits must be filed in a timely manner with the associate vice president of Environmental Safety Division and the unit head. (See Appendix D for a current copy of the laboratory safety survey form.) 5. ESD has the responsibility and authority to take immediate and necessary action to protect the health and safety of University employees, the public, and the environment in those situations that pose an immediate threat to life and health. These actions shall be governed using the provisions and guidelines of the laboratory closure procedure (see Appendix B). 6. ESD has the responsibility and authority for conducting internal audits of the centralized inventory system administered by the director of Central Research Stores (CRS) and filing results of such audits with the vice president for research, associate vice president of CRS, and the associate vice president of Environmental Safety Division. Duties 1. Conduct surveys of University laboratories for compliance with the policies and provisions of this manual Section 1 - 5 III. 2. Advise, as appropriate, laboratory supervisors, deans, department/unit heads, and the Committee of problems found in individual laboratories 3. Provide technical assistance to laboratory personnel in establishing safety programs in their individual laboratories 4. Provide testing for proper operation of safety equipment in chemical laboratories (i.e., safety showers, chemical fume hoods, and eye wash stations) 5. Provide consultation on the safe design of chemical laboratories and their associated safety equipment 6. Provide programs for chemical exposure monitoring, respiratory equipment issuance and fit testing, right to know, and other relevant safety education 7. Respond to chemical emergencies, providing guidance, consultation, and appropriate assistance 8. Dispose of known hazardous chemical waste in compliance with existing Hazardous Materials Treatment Facility (HMTF) regulations 9. Assist in the development and maintenance of a central inventory system 10. Assist departments and laboratories in developing plans for the use, storage, and disposal of hazardous chemicals and for the training of laboratory workers, ensuring that those plans are compatible with University policy 11. Ensure that there are appropriate safety manuals, approved by the Committee for all research and service laboratories and academic units. These manuals must address all relevant aspects of compliance including laboratory safety survey forms, receiving, shipping, disposal, and safety training College dean, department or unit head A. Responsibility 1. Each organizational head is responsible to the next higher administrator for the control, use, storage, and disposal of hazardous materials in laboratories under the program administration and control of all personnel, including laboratory supervisors, within the organizational unit for which each Section 1 - 6 respective head is responsible. IV. 2. Any hazardous material left in any laboratory without a proper supervisor in attendance for a period of 60 or more calendar days will be made the responsibility of the unit. The department head will initiate disposition by HMTF. 3. In the event that a supervisor abandons hazardous material upon leaving the University as in Section 1.IV.B.4 and fails to arrange for proper disposal or transfer of materials, the following course of action shall be taken: a. Within 30 calendar days of the termination date of the laboratory supervisor, the department head, in consultation with staff members of ESD, will submit a plan of remediation to the appropriate vice president and inform the Committee. b. After remediation, a final safety inspection shall be performed and a report sent to the Committee. The laboratory supervisor A. Definition A laboratory supervisor is defined as a faculty member (assistant professor, associate professor, professor, or instructor), a research professional, an academic professional, or laboratory director who is associated with or provides guidance to a laboratory or laboratories using hazardous agents. Graduate students and postdoctoral associates will not be considered a supervisor except under special circumstances at the discretion of the unit head. B. Supervisor responsibility 1. The supervisor shall train or provide for the training of all new personnel before allowing them to work in a laboratory using hazardous materials. Training shall include the following: a. Reading of this manual b. Job specific safety protocol for chemicals and equipment. See Appendix J for recommendations and guidelines for development of standard operating procedures. c. The proper use of job-specific personal protective equipment (PPE) Section 1 - 7 C. d. All training required by the Georgia Public Employee Hazardous Chemical Protection and Right to Know Act of 1988 and the University right to know compliance plan e. Directions for notifying the proper authorities in the event of an emergency or accident i. University Police 2-2200 ii. Athens Regional Medical Center 9-549-9977 iii. St. Mary’s Hospital 9-548-7581 iv. Environmental Safety Division 2-5801 v. Biosafety Office 2-7265 2. The supervisor shall see that records are kept as required by this manual. (See sample laboratory safety survey form, Appendix D, which lists necessary records.) 3. The supervisor shall remove chemical and biological substances under his/her control that may pose a hazard prior to maintenance personnel working on furnishings, equipment, or laboratory systems. 4. When leaving the University, or terminating his/her supervisory position, the supervisor shall relinquish all hazardous chemicals in his/her possession by disposal or transfer to another supervisor who has facilities capable of safely handling the material in question. (See Section 2.XIII, laboratory decommissioning plan.) Supervisor on leave or absent more than 60 calendar days 1. A supervisor on leave or absent for a period greater than 60 calendar days may assign responsibility for his/her program to a temporary supervisor who will be in charge of the laboratory in his/her absence. This person will be: a faculty member, a laboratory director, a research professional, or an academic professional who agrees, in writing, to accept responsibility for the laboratory. 2. If the laboratory supervisor does not choose the option listed above, his/her laboratory will be placed under the temporary supervision of another faculty member, research professional, academic professional, or laboratory director selected by the Section 1 - 8 department head. D. 3. The departing supervisor will ensure that all door signs reflect the change in supervisory status. 4. The temporary supervisor may not be utilized for a period exceeding 12 months without approval of the department head. Supervisor retirement When a supervisor retires, with or without emeritus status, his/her supervisory status will terminate. The laboratory supervisor may apply to the department head for continued supervisory status. If the department head can ascertain that the laboratory supervisor will have adequate facilities to store and handle the hazardous materials safely and that the funds will be available, if needed, for disposal, the retired laboratory supervisor may be given the role of supervisor for a specific time period to be determined by the department head. Section 1 - 9 Section 2. I. Standard Operating Procedures Chemical procurement, distribution, and storage A. Procurement The director of CRS is designated as the sole agent for submitting purchase requests for chemicals to the University procurement office, receiving, and distributing all chemicals to on-campus research and science laboratories and academic units of UGA. B. Inventory The director of CRS is charged with setting up and maintaining a centralized inventory system of chemicals for campus units. The associate vice president of Environmental Safety Division is directed to ensure that the individual units’ inventory reporting practices are coordinated with the CRS inventory system to ensure that all compliance requirements are met. C. Program audits ESD has the responsibility and authority for conducting internal audits of the centralized inventory system and filing the results of such audits with the vice president for research, the associate vice president of ESD, and the director of CRS. D. Distribution CRS will distribute purchased chemicals throughout campus. Only personnel who have received chemical specific right to know training shall receive chemical shipments. E. Storage 1. General All incoming containers of chemicals must have appropriate manufacturers labels that are not removed or defaced. Each container should be labeled with the date it was received and the date it was opened, as some chemicals form peroxides or other unstable products/explosives when stored for relatively short periods (see Appendix I for a listing of peroxide forming chemicals.) Chemicals in the laboratory shall be segregated by hazard class and compatibility. Acids, bases, flammables, reactives requiring separate and special storage, highly toxic compounds, and general non-hazardous chemical storage shall be separated from each other. The higher shelves shall be used for Section 2 - 1 containers containing chemicals which present the lowest hazard. Open shelves used for the storage of hazardous chemicals shall be well-anchored, painted, or made of, or covered with, chemicalresistant materials. (See Appendix J - Short List of Incompatible Materials) Work areas should not be used for long-term storage. Storage of glass chemical containers on the laboratory work area floor shall be strictly prohibited. 2. Flammable liquids The total allowable quantities of flammable liquids, including waste, in research laboratories which are separated from nonlaboratory areas according to existing fire codes, shall be the following: a. Twenty gallons are allowed per 100 square feet of a properly fire separated laboratory unit. Ten gallons are allowed per 100 square feet in non-fire separated lab units. This volume includes flammable liquids stored in safety cans and proper storage cabinets. The maximum allowable volume of flammable liquids is 120 gallons in a single laboratory unit. b. Up to 35 gallons of flammable solvents which are outside of flammable storage cabinets are allowed in a laboratory. Of this amount, 25 gallons must be contained in 2 gallon or smaller approved safety cans. The remaining ten gallons may be kept in other containers such as the original five-gallon shipping container, glassware and squeeze bottles. c. No more than two 60-gallon capacity cabinets are allowed per laboratory unit. d. Quantities allowed within an instructional laboratory unit shall be restricted to one-half that allowed in a research laboratory unit. e. Dispensing of flammable liquids from containers larger than five-gallon capacity, shall only be performed in a proper dispensing area per National Fire Protection Association (NFPA) 30. The dispensing area, if also used for bulk storage, shall be separated from the laboratory work area, per NFPA 45. (Please consult ESD for copies of applicable regulations.) Dispensing from larger containers can be arranged by contacting CRS. Section 2 - 2 3. f. No containers larger than five-gallon capacity are allowed for storage inside the laboratory area. Containers larger than five gallons used for dispensing shall be properly bonded and grounded to prevent a static discharge as an ignition source. g. Storage of flammable liquids in refrigerators not specifically designed and approved for that use by a recognized testing agency shall be strictly prohibited (please consult ESD for acceptable specifications). A flammable materials storage refrigerator/freezer has a spark proof interior that separates the contents from the compressor and motor. The explosion proof refrigerator/freezer is for the storage of volatile materials in areas away from possible spark hazards from electrical devices or other potential fire hazards. Flammable and other pressurized gases Storage of pressurized gas cylinders shall comply with NFPA 45 (see Appendix C for guidelines.) 4. Acids and bases Acids shall be separated from caustics and from active metals such as sodium, magnesium and potassium. Acids shall be separated from chemicals that can generate toxic gases on contact, such as sodium cyanide and iron sulfide. Large bottles of acids shall be stored on lower shelves or in acid cabinets. Oxidizing acids (nitric, perchloric) shall be separated from organic acids, flammable, and combustible materials. Separation of nitric and perchloric acid from other acids may be accomplished by placing in an unbreakable chemical resistant carrier. (See Appendix J for specific precautions for perchloric acid use.) Mild acids and bases such as citric acid and sodium carbonate may be stored with other low-hazard reagents. Opened containers of acids and bases should be stored in a chemical resistant secondary container (pan or tray), i.e., nalgene. H2 SO4 should be kept separate (separate secondary containers) from HCL. 5. Peroxide-forming chemicals Peroxide-forming chemicals shall be stored in a dark, cool, and dry place. Peroxide-forming chemicals shall be labeled with the date received and date opened (see Appendix I for lists of peroxide forming chemicals.) It is recommended that opened containers not be kept longer than six months except when inhibitors are present (see manufacturers recommendations). Section 2 - 3 6. Water-reactive chemicals Water-reactive chemicals shall be kept in a cool and dry place. Metal specific class D extinguishers shall be made available in laboratories where on pound or greater of water-reactive materials are used or stored. See Appendix J and reference this list of Common Water Reactives. 7. Oxidizers Oxidizers shall be stored away from flammable, combustible, and reducing agents (e.g., zinc, alkaline metals). 8. Toxic chemicals Toxic chemicals shall be stored according to the nature of the chemical, with appropriate warnings and security. Toxic chemicals shall not be stored with flammable chemicals, i.e., chloroform, etc. II. III. Transportation and shipment of hazardous chemicals on- and off-campus. If you plan on shipping hazardous chemicals while traveling abroad or from a location off of the UGA main campus, you MUST follow the procedures outlined below in subset A. A. Contact ESD Hazardous Material Treatment Facility for information concerning the transportation or shipment of any hazardous material to an off-site location which will require the services of any common carrier by ground or air. Please reference the HM Shipping procedures. B. Personal vehicles shall not be used to transport hazardous materials. When transporting hazardous materials in a UGA vehicle, please contact Hazardous Materials at 706-369-5706. C. Any hazardous chemical transported by hand between laboratories or oncampus buildings is to be contained in a chemical-resistant unbreakable carrier capable of containing the entire volume of the chemical being transported. D. When receiving gas cylinders or transporting them from a common storage area, ensure that they are secured to a hand truck. Never roll cylinders across the floor. Protective caps should be in place prior to transport. See “A Guide to Relocating Hazardous Materials”. Safe work practices A. Exposure minimization 1. General precautions for handling all laboratory chemicals outlined in this manual should be adopted, along with specific guidelines for particular chemicals as needed. Section 2 - 4 B. 2. Exposure to hazardous chemicals should be minimized. For work with substances that present special hazards, special precautions shall be taken. One should assume that any mixture will be more hazardous than its most hazardous component and that all substances of unknown hazard are hazardous. Refer to the material safety data sheet (MSDS) for specific information about a chemical or product containing hazardous chemicals. (MSDS are available at http://esd.uga.edu/chemical-lab-safety/right-know/msds-access. 3. The best way to prevent exposure to airborne substances is to prevent their escape into the working atmosphere by use of fume hoods and other local ventilation devices. All individuals handling hazardous chemicals in the laboratory shall be trained in the proper operation and use of fume hoods and other local ventilation devices (see Section 2.IV, chemical fume hood use.) 4. Develop and encourage safe habits and avoid unnecessary exposure to chemicals by any route. Do not smell or taste chemicals. Vent any apparatus that may discharge particularly hazardous chemicals into local exhaust devices. Chemicals shall be properly stored and used to prevent exposure. Appropriate personal protective equipment (PPE) shall be provided to employees working in areas where hazardous substances are in use (see Section 2.VI. for PPE requirements.) Employees shall be trained in the safe use and maintenance of PPE provided in the laboratory. Test positive pressure glove boxes for leaks before use. Do not allow release of toxic substances into any building area, only into an appropriate local exhaust device ducted to the outdoors. PELs, TLVs, or RELs The permissible exposure limits (PEL) of the Occupational Safety and Health Administration, the threshold limit values (TLV) of the American Conference of Governmental Industrial Hygienists, and the recommended exposure limits (REL) of the National Institute for Occupational Safety and Health shall not be exceeded. These levels may be found on the MSDS of any hazardous chemical or by contacting ESD. ESD will address any occupational exposure concerns. C. Chemical selection Use only those chemicals that can be used safely in the available ventilation system of the facility being used. D. Eating, etc. Eating, drinking, smoking, or application of cosmetics is not allowed in Section 2 - 5 laboratories that use hazardous agents. Hands shall be washed before conducting these activities outside of the laboratory. No food or product intended for consumption shall be stored in areas where chemicals are stored. Glassware or utensils that are also used for laboratory operations shall not be used with food or beverages. E. Glassware Handle and store laboratory glassware with care to avoid damage; do not use damaged glassware. Use extra care with Dewar flasks and other evacuated or pressurized glass apparatus; shield or wrap them to contain chemicals and fragments should implosion occur. F. G. Sharps and needles 1. All sharps (needles, knives, scalpels, broken glassware, Pasteur pipettes, etc.) shall be placed in approved, impervious sharps containers (available from CRS). Sharps and sharps containers should never be disposed of in the general trash. Contact the UGA Biosafety Office for instructions regarding disposal methods. 2. Sharps containers shall not be overfilled. 3. Needles shall not be recapped but placed directly into an appropriate sharps container after each use. 4. Needles shall not be cut as a form of disposal. Personal hygiene Thoroughly wash hands immediately after working with chemicals. Liquid rather than bar soap and paper towels, appropriately protected from contamination, are to be supplied at hand washing areas. H. Visitors No unsupervised children under 16 years of age shall be allowed into any laboratory or unseparated office space. No pets shall be allowed into the laboratory. Visitors should be warned of hazards present in the laboratory. I. Horseplay Practical jokes or other behavior that might confuse, startle, or distract another worker shall be prohibited in laboratories. J. Mouth pipetting Section 2 - 6 Mouth suction for pipetting or starting a siphon shall not be allowed. K. Personal apparel Low-heeled, closed-toe shoes shall be worn when handling hazardous chemicals. Appropriate apparel, as described in Section 2.VI shall be worn when working with hazardous chemicals. L. Personal housekeeping Work areas shall be kept clean and uncluttered. M. Planning Seek information and advice about hazards, plan appropriate protective procedures, and plan positioning of equipment before beginning any new operation. A dry run is highly recommended for new procedures or for personnel unfamiliar with the techniques. (See Appendix J for guidelines and recommendations for formulating protocols.) N. Vigilance Be alert for unsafe conditions and notify the appropriate supervisor if a problem is detected. O. Working alone Working alone with hazardous chemicals in a laboratory is discouraged. Potentially dangerous operations should be noted on the lab door and how to contact responsible lab personnel. (See Appendix D for recommended posting forms.) P. First aid Each laboratory facility should have a well supplied first aid kit readily available and prominently displayed or location posted. The kit should be checked regularly and supplies replenished. It is recommended that any injury occurring in a laboratory be checked out be a physician (see Section 2.IX.A). Q. Gas cylinders All gas cylinders shall be handled in accordance with NFPA procedures given in Appendix C. IV. Chemical fume hood use Section 2 - 7 A. Purchasing The purchase of all laboratory fume hoods shall follow Board of Regents’ standards. ESD is responsible for inspecting fume hoods after installation or modification to determine if they conform to these standards. It is recommended that ESD be consulted during the purchasing process. B. Airflow Airflow into and within the fume hood shall not be excessively turbulent; fume hood face velocity shall be adequate as specified by the Board of Regents, namely averaging 100 linear feet per minute at full open sash with no greater than a ± 20% variation across the open face of the hood. Air disturbances at the face of the fume hood should be avoided. C. Testing and certification Quality and quantity of hood performance shall be evaluated by ESD on installation, regularly certified (at least annually) and whenever a change in local ventilation devices is made. D. Hood sash The fume hood sash should be closed when the hood is not in use. As much as possible, work in the hood should be performed with the sash open 10—12 inches. E. Use Fume hoods should be kept clean and uncluttered. Work within the hood should be carried out at least six inches back from the front opening. Electrical receptacles or other spark sources shall be protected from flammable vapors. No permanent electrical receptacles shall be permitted in the hood. No chemical fume hood shall be used for the storage of chemicals or equipment unless they are a component of the operation for which the hood is being used or the hood is for the sole purpose of storage. Hood sashes should be closed as much as possible. The slots in the hood baffle shall be kept free of obstruction by apparatus or containers. Measures should be taken to prevent Kimwipes, tissues, or other articles from being drawn up into the exhaust duct. Bench coat surface protectors or other materials shall not obstruct hood air foils. Laboratory doors opening into main corridors shall be kept closed unless specifically designed and permitted by codes to be left open. The heating of perchloric acid in any hood other than a special purpose perchloric acid hood shall be prohibited. No cutting of holes or other unauthorized alteration of a chemical fume hood or its duct work shall be performed. Hoods that are malfunctioning or posted with a Danger - Inadequate Air Flow sign shall not be used for any operation using hazardous chemicals. Section 2 - 8 Any signs of reduced flow or other problems shall be promptly reported to a supervisor. F. Laminar flow, biosafety cabinets Please refer to UGA Biosafety Manual V. Housekeeping, maintenance, and inspections A. Work spaces Laboratory aisle spaces must be maintained unobstructed and work stations uncluttered. B. Inspections Inspections (audits) will be conducted by ESD laboratory safety personnel. It is recommended that laboratory personnel conduct regular self inspections. (See Appendix D for the current ESD laboratory safety survey form.) C. Maintenance Emergency showers and eyewash stations shall be tested annually for functionality by ESD with a record of testing attached. Make sure each emergency shower and eyewash has a test tag attached to it. If not, please contact Environmental Safety for testing. Eyewash stations should be flushed on a weekly basis by laboratory personnel. It is recommended that an eyewash testing log be kept. Other safety equipment (e.g., gloves, guards, goggles, glasses, carriers, etc.) should be inspected by lab personnel prior to use. D. Passageways Stairways and hallways shall not be used as storage areas. Access to exits, emergency equipment, and utility controls shall never be blocked or obstructed. E. Exit doors Doors that open into exit corridors or enclosures must be kept closed unless permitted by fire codes to be kept open. VI. Personal protective equipment (PPE) A. Skin absorption protection (gloves, aprons, and lab coats) Section 2 - 9 B. 1. Personnel performing procedures that use chemicals that can irritate or be absorbed by the skin shall wear appropriate PPE. 2. PPE such as gloves and aprons resistant to the chemical to be used shall be provided to workers when the potential for skin absorption exists. Check manufacturer’s specifications to determine breakthrough time for the specific glove and chemical. Laboratory coats are intended to prevent contact with the minor chemical splashes and spills encountered in a laboratory. Laboratory coats which do not significantly resist penetration by organic liquids shall be removed immediately when they become contaminated. Laboratory coats shall be worn in the immediate areas where hazardous chemicals are handled or used. Laboratory coats used during the handling of hazardous chemicals, biologicals, or radioactive substances should not be worn in other areas outside the laboratory such as offices, cafeterias, or libraries. Eye protection All employees and students who participate in or observe any of the following functions shall be provided with and shall wear protection devices: chemical, physical, or combined chemical-physical operations involving caustic, toxic, irritant, or explosive materials, hot liquids or solids, injurious radiation, or any dispensing of hazardous chemicals. Ensure when ordering that the manufacturer of the eye protection specifies that it conforms to ANSI Z 87.1. C. 1. Chemical splash goggles which have splash proof sides to fully protect the eyes or a face shield shall be worn when participating in or observing procedures using liquid hazardous chemicals which are corrosive or have a health hazard rating of 3 or 4. 2. Impact-resistant safety glasses with side shields must be worn when performing or observing procedures where powders, chips, or other flying particles are the primary hazard. 3. Eyewear should be cleaned before being issued to a different employee. It is recommended that contact lenses not be worn (even with additional eye protection) in environments involving chemical splash or vapor exposure. Respiratory protection Advice regarding the purchase of respirators is available from ESD. Any laboratory operation in which respirators are provided must conform to the University’s respirator program (see Appendix E). All operations within a laboratory facility that involve the transfer or alteration of a Section 2 - 10 hazardous chemical which may generate air contaminants at or above the appropriate TLV shall be carried out in a chemical fume hood appropriate for the work being performed. Contact ESD with any questions concerning respirator use. VII. Records A. Inventory A hazardous chemical list for each laboratory will be maintained by the lab supervisor, updated periodically and made accessible to laboratory personnel. The list should include CAS number, hazard class, NFPA hazard rating, if available, and storage codes. The chemical inventory database maintained by CRS will provide information regarding chemicals purchased and delivered to the labs and should serve as the basis for the hazardous chemical list. Contact CRS for details on accessing and modifying this database. VIII. B. All laboratories using hazardous chemicals should develop specific chemical standard operating procedures as provided for in the chemicalspecific right to know training implementation plan as part of the mandated University right to know plan. (See Appendix J for sample operating procedures which must be kept as part of this manual and which may be expanded based on individual laboratory operating procedures.) C. Signed documentation of annual chemical-specific right to know training, as required by the University’s right to know plan, shall be maintained by the laboratory supervisor. This does not include the human resources new employee orientation training. Check with your departmental or unit right to know coordinator for your departments record keeping system. (See Appendix D for right to know training forms.) Online training is offered at http://esd.uga.edu/chemical-lab-safety/training. Signs and labels A. Laboratory corridor doors shall display approved CAUTION door signs (see Appendix D for request form and Appendix J for instructions on “How to Properly Complete a Caution Sign). All required laboratory emergency information shall be provided on the CAUTION sign. Laboratory CAUTION door signs and labels shall be updated as necessary and at least annually. Principal hazardous materials and their amounts shall be listed. Requests for Caution door signs. B. Laboratory refrigerators and microwave ovens All laboratory refrigerators, freezers, and microwaves shall be affixed with an approved Notice - Laboratory Use Only sign available from Section 2 - 11 ESD. Only refrigerators specifically designed and approved by a recognized testing agency as explosion-proof or explosion-resistant shall be used for flammable storage. If the refrigerator is not explosion-proof or intrinsically safe, it shall be affixed with the approved label “NoticeDo Not Store Flammables in This Box,” available from ESD. C. Laboratory work areas Telephone numbers of emergency personnel/facilities, supervisors, and laboratory workers should be posted by a central phone. Areas where hazardous materials are stored should be posted with proper hazard warning signs. (See Appendix D for examples of recommended work area signs and posting.) A list of emergency phone numbers is included in the appendices and is on the cover of this manual. D. Primary chemical containers shall be affixed with a legible manufacturer label. E. Secondary containers containing 1. Non-hazardous chemicals shall be affixed with labels listing the identity of its contents 2. All chemicals intended for use in less than one day by a single user do not need to be labeled 3. Hazardous chemicals intended for storage and use for a period greater than one day shall be affixed with labels listing a. the identity of the hazardous chemical b. the date filled c. the hazard warning (see Appendix D for hazard warning designations and abbreviations) 4. Batches of vials or test tubes containing chemicals of the same hazard may have the hazard labels affixed to a common carrier or box. All other such secondary containers must be appropriately labeled as noted above. 5. The chemical identity given on a chemical label must be in plain English, and must list the chemical’s common name given on the MSDS or manufacturer’s label, or an accepted UGA abbreviation or acronym (see Appendix D for accepted abbreviation or acronyms). 6. The chemical’s hazard warning may be provided by use of either Section 2 - 12 the National Fire Protection Association (NFPA) hazard warning system, Hazardous Materials Identification System (HMIS) hazard warning system or the UGA hazard warning abbreviation warning system (see Appendix D for full explanation of these systems). 7. IX. If abbreviations are used on any chemical labeling all abbreviation and acronyms used must be posted in the lab (see Appendix D for appropriate postings). Spills and other laboratory accidents A. The laboratory supervisor should see that all serious injuries which require medical attention shall be reported by calling 9-911. All incidents that result in an injury or property damage are to be reported using a University Incident/Accident Report form which should be available in the offices of department or division heads. B. Chemical exposures 1. Eye contact Promptly flush eyes with water from an emergency eyewash or other suitable eye irrigation method for a prolonged period (15 minutes is recommended by hospitals) and seek medical attention. 2. Ingestion Contact the local poison control center or hospital and follow directions (see front cover of manual or Section 1.IV.B.1e. for phone numbers). 3. Skin contact Promptly flush the affected area with copious amounts of water from safety shower, sink or other appropriate source, and seek medical attention. Remove any clothing that may have chemical contamination to prevent further exposure. C. Uncontrolled release or spill 1. All laboratories that handle hazardous chemicals shall have an appropriate supply of spill cleanup kits prominently displayed or location posted. The supply must be capable of containing or cleaning up small, known chemical releases. Laboratory personnel should not attempt to clean up a spill of hazardous chemicals if appropriate spill cleanup supplies and protective Section 2 - 13 equipment are not available, or if the chemical or level of exposure hazard is unknown. In these cases, contact ESD for assistance. (See Appendix J.) 2. X. Laboratory sinks should be periodically inspected for leaks, and traps kept full of water to prevent drain vapors from entering the laboratory. Electrical safety All electrical equipment and apparatuses must be double insulated or grounded. The following instructions are mandated by the State Fire Marshal. The use of extension cords should be avoided. When extension devices (an enclosure with multiple sockets) must be temporarily used, the wire gauge of the device must be equal to or larger than the cord on the item being operated. No extension device shall be attached to building surfaces (using staples, nails, etc.) Extension devices equipped with surge protectors may be permanently used with equipment that contain microprocessors (e.g., to connect computer equipment). Surge protectors should not be used in areas subject to moisture, physical or chemical damage or flammable vapors. Surge protectors must be UL 1449 or Transient Voltage Surge Suppresser (TVSS) approved. XI. Mechanical hazards Mechanical hazards in the laboratory shall be evaluated by the supervisor and appropriate safety precautions implemented. Safety precautions shall be adopted in accordance with equipment manufacturer’s recommendations. Mechanical hazards shall be minimized by guarding exposed moving mechanisms such as belts ,pulleys, and blades, or placing equipment in areas which protect workers from moving mechanisms. If flying particles may be produced, shatter resistant safety glasses shall be provided and worn (see Section 2.VI.B). Hearing protection may also be required if 85 dB is exceeded for any 8-hour period; if so, a hearing conservation program shall be implemented (contact ESS for information). Standard operating procedures should be developed for any equipment that may represent mechanical hazards (see Appendix J for guidelines or contact ESD). XII. Synthesized chemicals If hazardous chemical substances are developed in the laboratory for in-house use, appropriate training shall be given to personnel as with any other hazardous chemical. If the chemical produced is a by-product whose composition is not known, it shall be assumed that the substance is hazardous. Synthesized chemicals and their known by-products shall be identified and stored by chemical class and shall be labeled in accordance with Section 2.VIII. Section 2 - 14 XIII. Laboratory decommissioning The following procedures shall be carried out and a Laboratory Decommissioning (Procedure for Closing a Laboratory) Form must be (see Appendix D) completed when the responsible individual leaves the University or transfers to a different laboratory. Upon completion, the laboratory closing form shall be signed by all responsible parties. For personnel leaving the University this form must be attached to the Human Resources check-out form and a copy sent to Human Resources. A. Chemicals The supervisor shall ensure that all containers of chemicals are labeled with the name of the contents. All containers are to be securely closed. Beakers, flasks, dishes, etc., shall be emptied and cleaned. (Check all refrigerators, freezers, fume hoods, and cabinets.) Determine which materials are usable and transfer the surplus to another user who is willing to take charge of them. If a user cannot be found, it shall be disposed of through the UGA waste disposal program. All fume hood surfaces and counter tops shall be washed off. The respective department head is to be notified when the laboratory has been cleaned. B. Gas cylinders If gas cylinders are not returnable, contact ESD for advice. C. Animal and human tissue If tissue is held in a liquid preservative, tissue and liquid shall be separated. Contact the University biosafety officer for instructions regarding proper disposal of human tissue. Animal tissue can be disposed of by incineration or by placing in a biohazard bag for proper treatment. Defrost and clean refrigerators and freezers if they are empty. If samples are to be saved, locate an appropriate person to take responsibility for them and notify the department head. The liquid preservative should be disposed of as a chemical waste. D. Microorganisms and cultures Decontaminate culture containers by autoclaving. Decontaminated plastic containers can be disposed of in regular trash. Clean incubators and refrigerators. If samples are to be saved, locate an appropriate person to take responsibility for them and notify the department head. If questions arise, address them to the UGA biosafety officer. E. Radioactive materials Notify the University radiation safety office of intention to leave the Section 2 - 15 University or to change laboratories at least one month in advance and follow the instructions provided by the radiation safety officer. F. Equipment If laboratory equipment is to be left for the next occupant, clean or decontaminate before departing the laboratory. XIV. Hazardous chemical and waste disposal A. XV. All hazardous chemicals and chemical waste shall be disposed of in accordance with the most current revision of the University of Georgia Hazardous Materials Program Manual. (See Appendix G for the University’s hazardous chemical waste summary procedures, and Appendix H for the University’s chemical waste minimization procedures.) The waste minimization procedures includes guidelines for bench top treatment of chemicals and procedures for surplus redistribution. Fire Safety A. Appropriate fire extinguisher(s) should be available to occupied labs and placed 75 feet apart. The fire safety office at Environmental Safety is responsible for maintaining annual inspections and monthly checks of fire extinguishers. Make sure the fire extinguisher is located near the exit and visible for use in case of emergency. The fire extinguisher should have an annual inspection tag on it. (See Appendix J) Section 2 - 16 Section 3. I. The Laboratory Facilities Minimum design provision Laboratories shall be constructed in accordance with NFPA 45 and the University System of Georgia Board of Regents’ standards. All laboratory facilities shall have the following minimum provisions: II. A. An appropriate general ventilation system with air intakes and exhausts located so as to avoid reentry of contaminated air B. Adequate chemical storage facilities having well-anchored chemicalresistant shelving, appropriate approved flammable storage and dispensing areas for the volume of flammables to be used, and approved acid and special hazard storage cabinets appropriate for the hazards present C. Laboratory fume hoods appropriate for the hazards present (see Appendix F) D. Sinks appropriate for hand washing and the cleaning of glassware and equipment E. Plumbed eyewash stations which meet the requirements of ANSI Z358.1 shall be provided in the laboratory areas in a location that provides access within ten seconds from any point in the laboratory F. Plumbed emergency showers which meet the requirements of ANSI Z358.1 shall be provided in new or newly-renovated laboratories, within the laboratory area; in existing laboratories, within a distance of no greater than 30.5 meters (100 feet) from the most remote area of the laboratory G. Break areas physically separated from contamination of laboratory and chemical storage operations H. Entrance doors to laboratories which meet fire separation requirements and shall not be used for ventilation purposes I. Vision panels which meet separation requirements and shall not exceed 100 square inches Construction and renovation review Since ESD is charged with the responsibility of inspecting all laboratories to determine if they conform to the policies set forth in this manual, it is recommended that ESD be consulted prior to construction and/or major renovation of any laboratory facility. In the event an agreement on safety issues Section 3 - 1 cannot be attained, the issues will be addressed by the Committee. The parties have a right to appeal any Committee decision to a committee consisting of the vice president for research, the vice president for academic affairs, and the vice president for business and finance, or their representatives, for a final resolution. III. General laboratory ventilation A. Purpose and use This system shall provide a source of air for breathing and for input to local ventilation devices; it should ensure that laboratory air is continually replaced, preventing increase of air concentration of toxic substances during the day; direct air flow into the laboratory from nonlaboratory areas and out to the exterior of the building; and it should not be relied on for protection from toxic substances released into the laboratory. B. Modifications Any alteration of the ventilation system should be made only if thorough testing indicates that worker protection from airborne toxic substances will continue to be adequate. C. Performance Six to twelve room air changes per hour are normally adequate general ventilation, if local exhaust systems such as fume hoods are used as the primary method of control. Doors to the laboratory opening onto corridors shall be kept closed to ensure correct air flow unless specifically designed to be kept in the open position. D. Quality General air flow should not be turbulent and should be relatively uniform throughout the laboratory, with no high velocity or stagnant air. IV. Other ventilation devices A. Questions concerning ventilated storage cabinets, canopy hoods, and snorkels should be directed to ESD (2-5801). Approved ventilated storage cabinets can be obtained from CRS. B. UGA prohibits the use of ductless fume hoods. C. Central vacuum pumps must be trapped and vented directly to the outside. Local vacuum pumps shall be trapped and appropriately filtered. Good maintenance of traps and filters is essential. Section 3 - 2 V. Exhaust stacks Chemical fume hood stacks shall extend above the building structure a minimum of seven feet and one duct diameter length above any parapet wall. Discharge velocity of hood stacks shall provide a minimum exit velocity of 2,500 fpm. These are minimum requirements. Greater heights or velocities may be required, due to building design or wind speed, to prevent reentry of chemical exhaust into the building. Section 3 - 3 Section 4. I. Particularly Hazardous Substances General requirements A. Procedures and practices 1. Definition “Particularly hazardous substances” as termed by OSHA include “select carcinogens,” reproductive toxins, and substances that have a high degree of acute toxicity. A substance of high acute toxicity is one for which acute or short-term toxicity characterizes the response (e.g., fast-acting substances, or irritants, and narcosis-producing substances). Any substances having an oral LD50 in mammals of 50 mg or less per kilogram of body weight, an inhalation LC50 in mammals of 100 parts per million (ppm), or a dermal LD50 in mammals of 50 mg or less per kilogram of body weight is considered highly toxic. (See Appendix I for lists of peroxide forming and cancer causing chemicals.) 2. Designated areas Conduct all work and transfers with these substances in a “designated area” (a restricted access fume hood, glove box, or portion of a laboratory designated for use of highly toxic substances,) for which all people with access are aware of the substances being used and necessary precautions. Use and store these substances only in areas of restricted access with special warning signs. 3. Personal protection Always avoid skin contact by wearing the proper gloves, laboratory coat, and any other appropriate apparel. Always wash hands immediately after working with these materials. 4. Prevention of spills and accidents Be prepared for accidents and spills. Assure that at least two people are present at all times if a compound in use is highly toxic or of unknown toxicity. Store breakable containers of these substances in chemical resistant trays. Work and mount apparatus above such trays, or cover work and storage surfaces with removable, absorbent, plastic backed paper. If a major spill occurs outside the fume hood, evacuate the area and contact ESD. 5. Non-contamination/decontamination Section 4 - 1 Protect vacuum pumps against contamination with scrubbers, or HEPA filters. Decontaminate vacuum pumps or other contaminated equipment, including glassware, in the fume hood before moving them from the designated area. Decontaminate the designated area before normal work is resumed. Material used during decontamination shall be considered as hazardous waste and disposed of appropriately. 6. Spills Assure that contingency plans, equipment, and materials to minimize exposures of people and property are available in case of accident. 7. Storage Store containers of these chemicals only in a ventilated, limited access area in appropriately labeled, unbreakable, chemical resistant, secondary containers. II. Standard operating procedures for particularly hazardous substances Prior to using any particularly hazardous substance (defined in Appendix I), a standard operating procedure should be developed for its safe storage, handling, and disposal. (See Appendix J for sample standard operating procedures [perchloric acid].) Section 4 - 2 Chemical and Laboratory Safety Committee Voting Members Chairman Dr. Michael Pierce, Professor, UGA Cancer Research Center Director Biochemistry & Molecular Biology, CCRC (706) 542-1702 hawkeye@uga.edu Mr. Brian Adams, Program Coordinator Hazardous Materials Program (706) 369-5706 badams@esd.uga.edu Dr. Michael Mispagel, Quality Assurance Officer, AHRC Facilities Manager Dean’s Office, Veterinary Medicine (706) 542-5729 mispagel@vet.uga.edu Dr. Carl Bergman, Assoc. Research Scientist Mr. Donald Mosser, Env. Safety Manager Complex Carbohydrate Research Center Savannah River Ecology Laboratories (706) 542-4487 (803) 725-5299 cberg@ccrc.uga.edu mosser@srel.edu Mr. Bill Favaloro, Env Safety Coordinator Dr. Ian Hardin, Professor Environmental Safety Division Textiles, Merchandise and Interiors 583-0449 (706) 542-0357 wfavaloro@esd.uga.edu ihardin@fcs.uga.edu Dr. Jaroslava Halper, Professor Dr. Larry Morris, Professor Department of Pathology School of Forest Resources (706) 542-5830 (706) 542-2532 jhalper@uga.edu lmorris@uga.edu Dr. Rob Jackson, Dept. Chair Lamar Dodd School of Art Dr. Jim Nelson, Assoc. Professor (706) 542-1657 Skidaway Institute of Oceanography jacksonr@uga.edu (912)598-2473 jim.nelson@skio.usg.edu Dr. Russell Malmberg, Assoc. Dean Arts & Sciences (706) 542-5952 malmberg@uga.edu Mr. Jerry NeSmith, Director Research Services, OVPR (706) 542-2411 nesmithj@uga.edu Dr. John Stickney, Professor, Dept. Head Chemistry, NanoSEC (706) 542-1967 Dr. Robert Maier, Professor, Eminent Scholar stickney@chem.uga.edu Microbiology Dr. Randy Walker, Director (706) 542-2323 Marine Extension Service rmaier@uga.edu (706) 542-5956 Dr. George Majetich, Professor walker@uga.edu Chemistry Dr. Zheng-Hua Ye, Assoc. Professor (706) 542-1966 Plant Biology majetich@chem.uga.edu (706) 542-1832 zhye@plantbio.uga.edu Appendix B Unsafe Laboratory Closure Policy Unsafe Laboratory Closure Policy Chemical and Laboratory Safety Committee (CLSC) I. Notice of Unacceptable Laboratory Operations A. Chronic non-compliance with established safe practices resulting in serious but not immediate risk to the health and/or safety of workers and/or students. 1. The primary researcher and department/unit head shall be given a written notice of safety violations under his/her supervision and reasonable deadlines for remedial actions. Such deadlines shall be established by the laboratory safety officer and shall be commensurate with the seriousness of the situation. 2. The researcher may request additional time from the ESD administration. 3. If sufficient action has not been taken to correct the situation, within the allotted time-frame, a second written notice shall be sent to the primary researcher, the researcher's department/unit head and dean, and the chair of the CLSC. 4. The primary researcher will be given a new compliance deadline. 5. If, following the allotted remediation time insufficient action has been taken, the laboratory operation will be reviewed by the CLSC. 6. At the next regularly scheduled quarterly meeting of the CLSC, all laboratories under review by the Committee will be discussed and appropriate action will be decided by a 2/3 Committee vote. B. Unsafe practices which pose a serious and immediate risk to workers and/or students. 1. The Environmental Safety Division specialist on the scene shall immediately notify available laboratory personnel of the safety concern, initiate evacuation of endangered areas, and put into effect emergency response procedures. 2. The specialist shall notify the Environmental Safety Division immediate supervisor and stand by for further instructions. 3. The Environmental Safety Division immediate supervisor, upon collaboration with the specialist and the associate vice president, may order the endangered area closed indefinitely per review by the CLSC. 4. If closure is mandated, the associate vice president of the Environmental Safety Division shall notify the chair of the CLSC or the committee vice-chair; the Appendix B 2 primary researcher in charge of the laboratory in question; the researcher's department/unit head and dean, the vice president of academic affairs; the vice president of research; and the vice president for business and finance. II. 5. The CLSC chair shall convene an emergency meeting of the Committee. 6. The CLSC shall discuss the laboratory closure and determine any corrective action to be taken by a 2/3 Committee vote. Corrective Actions Suggested by the CLSC: A. Action concerning non-immediate hazards 1. The CLSC shall review all serious safety citations at its regularly scheduled quarterly meeting. Based upon the citation and any additional related circumstances presented by the primary researcher, the Committee may take any of the following actions: a. b. c. 2. Dismiss the citation by permitting a waiver. Establish a new deadline for remedial actions. Issue authorization to close the laboratory until corrective actions are taken. A letter outlining actions by the committee shall be sent by the CLSC chair to the primary researcher, the researcher's department/unit head and dean, the vice president of academic affairs, the vice president for research, and the senior vice president for business and finance. B. Actions concerning conditions which pose an immediate threat to health and safety. 1. Following notification of emergency closure of a laboratory operation by the associate vice president of the Environmental Safety Division, the CLSC chair shall call an emergency meeting of the Committee. 2. The Committee shall discuss the closed laboratory operation and taken any one of the following actions: a. b. 3. Authorize the reopening of the laboratory operation under specifically expressed guidelines. Issue authorization to continue closure of the laboratory until corrective actions are taken. The CLSC chair shall prepare a written statement of committee actions. This shall be sent to the primary researcher, the researcher's department/unit head and dean, the vice president for academic affairs, the vice president for research, and senior vice president for business and finance. III. Appeal of Committee Action A. Action taken by the committee may be appealed by any directly affected employee of the University of Georgia. B. The employee shall send a written appeal to the chair of the committee. If warranted a formal hearing will be arranged between the employee and the Committee. C. The Committee chair shall respond in writing to the employee specifying a date and time for the appeal to be presented to the Committee. D. After consideration of the appeal, the Committee shall respond to the employee in writing. Appendix B 4 Appendix C Flammable, Oxidizing and Other Pressurized Gases Gas Cylinders I. Guidelines for using and storing pressurized gases The following guidelines shall be followed by all personnel using or storing pressurized gases. II. A. All personnel who will be working in areas where compressed gases are used or stored shall receive instruction regarding the safe handling of cylinders, the use of appropriate personal protective equipment, and the steps to be taken in the event of a leak or fire in an adjacent area. B. Do not remove any labels or other form of identification from any gas cylinder. C. Know how to detect the presence of leaks from any gas cylinder in your work area. Of particular importance are flammable and toxic gases. Contact ESD at 2-5801 in the event of a cylinder or valve leak. Gas cylinder storage and labeling A. When receiving a gas cylinder do not accept it until the following items are verified: 1. 2. 3. the contents are identified either by labels or stencils, it contains the appropriate DOT label, it contains a valve protection cap (if so designed). B. Store gas cylinders in a well-ventilated area away from direct heat. All cylinders must be stored in an upright position secured to a sturdy permanent structure to prevent the cylinder from falling or being knocked over. Place protective caps on those cylinders which are not in use. All cylinders should be anchored individually. C. Gases should be stored in accordance with their physical and chemical properties. See individual material safety data sheets (MSDS) for specifics with regards to this information. D. Close valves on empty gas cylinders and mark them empty. Empty cylinders should be removed from the lab as soon as possible. (See Appendix D for copies of optional tags that may be used for labeling gas cylinders.) Store empty and full gas cylinders separately. Cylinders are considered empty if their pressure is less than 25 psig. All cylinders will be considered full that are not properly identified. Appendix C-1 E. Store flammable gases away from oxidizing gases. F. Do not store gas cylinders near elevators, ventilating systems, or other openings through which gas may spread to other parts of the building should a leak occur. Do not store cylinders where there is a risk of dropping them or having heavy objects fall on them. G. Cylinders containing gases that are corrosive to cylinders or cylinder valves or that may become unstable while stored in the cylinder shall have a maximum retention period of six months, unless a shorter period is otherwise specified by the manufacturer. H. Cylinders of all gases having a health hazard rating of 3 or 4 (refer to the MSDS for rating) must be kept in a continuous mechanically ventilated storage hood or other continuous mechanically ventilated enclosure. There must be no more than three cylinders within the hood or other ventilated enclosure. Contact ESD if you have questions regarding the storage of cylinders in continuous mechanically ventilated enclosures/storage hoods. I. The maximum volume size of a cylinder of a gas with a health hazard rating of three or four stored in a laboratory work area shall be limited to 0.1 cubic feet. Cylinders of a gas with a health hazard rating of three or four stored in a laboratory work area shall be limited to no more than three maximum size cylinders or an equivalent volume (0.3 cubic feet) of smaller sized cylinders. J. The maximum volume size of a flammable or oxidizing gas cylinder stored in a laboratory work area shall be limited to two cubic feet. Cylinders of flammable and/or oxidizing gases stored in a laboratory work area shall be limited to no more than six maximum sized cylinders or an equivalent volume (12 cubic feet) of smaller sized cylinders. K. The maximum volume size of a liquefied flammable gas cylinder stored in a laboratory work area shall be limited to 0.6 cubic feet. Cylinders of liquefied flammable gas stored in a laboratory work area shall be limited to no more than three maximum sized cylinders or an equivalent volume (1.8 cubic feet) of smaller size cylinders. L. Gas cylinders shall not be retained for more than ten years. Small, disposable, empty, lecture cylinders may be discarded in the lab trash after the valve stem has been removed. Small disposable lecture cylinders that are not empty may either Appendix C-2 be reacted off to render them empty, returned to the supplier or disposed of by a licensed gas cylinder disposal company. ESD shall be consulted prior to disposing of a cylinder using the preceding methods. Non-disposable cylinders must be returned to the supplier. M. III. Cylinders and other containers stored outdoors shall be stored off the ground on a raised concrete pad and within a covered non-combustible rack. They shall not be stored where they are at risk of dropping, having heavy objects dropped on them or being struck by a vehicle. Proper handling of gas cylinders A. Always open cylinder valves slowly. Never force the valve open. If the valve cannot be opened by the wheel or small wrench provided, return the gas cylinder. To shut down a system, close the cylinder valve and relieve the pressure from the entire system through a hose that is not being used. B. Never interchange regulators and hose lines among different types of gases. C. Always turn off cylinders from the main stem valve (not the regulator). Turn off cylinders slowly. D. Suitable equipment must be available for moving cylinders and other portable containers. Hand trucks must be equipped with a clamp or chain to secure the container in place or they must be specifically designed for container handling. Never drag, roll, or slide a cylinder in an attempt to move it. E. Never drop cylinders; never permit cylinders to strike each other; and never strike cylinders with a metal instrument. F. Inspect cylinders regularly for corrosion or leaks. In case of a leak, promptly remove the cylinder to the outside (in accordance with manufacturers recommendations) and call ESD for assistance. G. Do not use cylinders without a regulator. H. Never attempt to refill a cylinder. I. Never tamper with any part of a valve such as the safety nuts or packing nuts. Appendix C-3 Appendix D Signs, Forms and Labels Laboratory Safety Signs, Forms and Labels This manual includes acceptable chemical labeling practices and acceptable common abbreviations which may be used on chemical labels, and a laboratory safety evaluation form. The other signs, forms and labels below can be obtained from the ESD web site or by calling 706-542-5801. I. Forms A. B. C. D. II. Postings A. B. C. D. E. F. G. III. UGA Laboratory Safety Survey Form CAUTION Sign Request Form Fume Hood Certification Request Form Procedures for opening (commissionin g) or closing (decommissioning) a laboratory. These procedures should be used in conjunction when adding additional laboratory space or moving from one laboratory to another laboratory. Emergency Phone Numbers (posted by lab phone) Eyewash Sign Safety Shower Sign Chemical Spill Kit Sign Chemical Storage Plan Gas Cylinder Tags Unattended Laboratory Operations Labeling Systems A. B. C. Hazardous Chemical Container Labeling Acceptable Abbreviations for Primary Hazards Acceptable Chemical Abbreviations for Chemical Secondary Container Labeling Appendix D - 1 Environmental Safety Division 542-5801 Laboratory Inspection Survey Department____________________ Principal Investigator______________________________ Building_______________________ Laboratory_____________________ Date: / / Phone and Email Address:_________________________ Lab Contact and Email: ____________________________ ESD Inspector ___________________________________ Section 1 - Laboratory Postings A. Door signs present/updated B. Refrigerators have lab use only label C. Emergency phone numbers posted in lab Section 2 - Chemical Storage A. C hem icals s tored by class/co m patibility B. Acids and b ases in sec ondary containers C. All chemicals properly labeled D. No outdated peroxide formers present E. F lam m able liquids stored pro perly F. Total flamm able volume allowed in lab OK G. V olum e ou tside flam m able cab inet O K H. Explosion proof refrigerator for flamm ables I. W aste containers properly labeled/stored J. W aste containers properly closed K. Gas cylinder properly labeled/anchored L. Lecture bottles properly labeled/stored Section 3 - Emergency Equipment A. Fire extinguishers present/inspected B. Safety shower: tested/unobstructed C. Safety shower location posted D. Eye wash: tested/unobstructed E. Eye wash location posted F. First aid kit present G. Spill kit appropriate for laboratory Section 4 - Laboratory Equipment A. Belt guarded on motors and pumps B. Equipment properly grounded C. Electrical cords not frayed D. Only UL 1449 rated power strips employed E. 1449 strips used with computers & equip. F. Outlet wiring correct G. Ex ten sion devices used only tem porarily H. Fum e hood rating (OK , Caution, Dange r) Section 5 - Laboratory Conditions A. H and washing fa cilities available B. Sink conditions OK C. Corridors and exits unobstructed D. Aisles unobstructed E. Lab doors closed to main corridor F. No eating etc aro und haza rdous chem icals G. Personal protective equip. available/used Section 6 - Laboratory Records A. RTK records and MSDS maintained B. Chemical inventory kept Additional Com m ents : Sat Unsat N/A Com me nts Sat Unsat N/A Com me nts Sat Unsat N/A Com me nts Sat Unsat N/A Com me nts Sat Unsat N/A Com me nts Sat Unsat N/A Com me nts Within two weeks, please address any items noted as unsatisfactory on this form, then contact your laboratory inspector at: @ esd .ug a.ed u. Appendix D - 2 Laboratory Inspection Survey - page 2 (Lab Safety Manual References) Section 1 - Laboratory Postings A. Door signs present/updated B. Refrigerators have lab use only label C. Emergency phone numbers posted in lab Section 2 - Chemical Storage A. Chemicals stored by class/compatibility B. Acids and bases in secondary containers C. All chemicals properly labeled D. No outdated peroxide formers present E. Flammable liquids stored properly F. Total flammable volume allowed in lab OK G. Volume outside flammable cabinet OK H. Explosion proof refrigerator for flammables I. Waste containers properly labeled/stored J. Waste containers properly closed K. Gas cylinder properly labeled/anchored L. Lecture bottles properly labeled/stored Section 3 - Emergency Equipment A. Fire extinguishers present/inspected B. Safety shower: tested/unobstructed C. Safety shower location posted D. Eye wash: tested/unobstructed E. Eye wash location posted F. First aid kit present G. Spill Kit appropriate for laboratory Section 4 - Laboratory Equipment A. Belt guarded on motors and pumps B. Equipment properly grounded C. Electrical cords not frayed D. Only UL 1449 rated power strips employed E. 1449 strips used with computers & equip. F. Outlet wiring correct G. Extension cords used temporarily H. Fume hood rating (OK, Caution, Danger) Section 5 - Laboratory Conditions A. Hand washing facilities available B. Sink conditions OK C Corridors and exits unobstructed D. Aisles unobstructed E. Lab doors closed to main corridor F. No eating etc around hazardous chemicals G. Personal protective equip. available/used Manual Reference Sec.2.VIII; App.J-6 Sec.2.VIII.B Sec.2.VIII.C; App.J-1 Manual Reference Sec.2.I.E.1; App.J-20 & 21 Sec.2.I.E.4; App.J-20 & 21 Sec.2.I.E.1; Sec.2.VIII.E.1; App.J-20 & 21 Sec.2.I.E.1; Sec.2.I.5; App.J-21; App.I-1 Sec.2.I.E.2; App.J-20 Sec.2.I.E.2; App.J-20 Sec.2.I.E.2; App.J-20 Sec.2.I.E.2; App.J-20; Sec.2.VIII.B; Sec.2.I.E.2.G App.G App.G App.C; Sec.2.I.3 App.C Manual Reference NFPA 101 Sec.2.V.C; Sec.3.I.F; App.J-17 App.J-3 Sec.2.V.C; Sec.3.I.E; App.J-16 App.J-3 Sec.2.III.P Sec.2.IX.C.1; App.J - 8 & 11; Sec.4.I.A.5 Manual Reference Sec.2.XI Sec.2.X Sec.2.X Sec.2.X Sec.2.X Sec.2.X Sec.2.X Sec.2.IV; App.J-13; App.F Manual Reference Sec.2.III.G Sec.3.I.E Sec.2.V.A; Sec.2.V.D & E Sec.2.V.A; Sec.2.V.D & E Sec.2.V.E; Sec.3.I.H Sec.2.III.D Sec.2.VI; Sec.4.I.A.3; App.J-12; Sec.2.III.K Section 6 - Laboratory Records Manual Reference A. RTK records maintained Sec.2.VII.C; App. D-4; RTK B. Chemical inventory kept Sec.2.VII.A Items marked by arrows are required by the UGA laboratory safety manual. Non marked items are suggestions for safe laboratory operations. Visit ESD on the web at: http://www.esd.uga.edu Appendix D - 3 Retain the original signed form in the employee’s personnel file. Employee On-going Chemical Specific Right to Know Training Record Employee Name: Review Period: Work Location: Job Assignment: Supervisor: Training Included:Personal Protection, Emergency Procedures, Work Area Maintenance, Detection of Chemical Release, MSDS Review, and Labels. Type of Training Annual Update Brief Description of Training Method Date New Chemical/ Hazard Product I acknowledge that I have been provided training covering the subject noted above and that I understand that training. Employee Signature and Date Appendix D - 4 222 Appendix D - 5 1222 !!!!! WARNING !!! Unattended Laboratory Operations Authorized Personnel Only In the event of an emergency contact: Name/Phone# or the Laboratory Principal Investigator: Name/Phone# or Environmental Safety Division at 542-5801 Appendix D - 6 Appendix D - 7 Appendix D - 8 Accepted Chemical Abbreviations for Chemical Secondary Container Labeling Acetic Acid Benzene Calcium Chloride Carbon Tetrachloride Chloroform Cupric Chloride Ethylene Diamine Tetraacetic Acid Ethanol Water Hydrogen Peroxide Sulfuric Acid Hydrochloric Acid Perchloric Acid Hydrofluoric Acid Nitric Acid Potassium Chloride Potassium Chlorate Potassium Nitrite Potassium Nitrate Potassium Hydroxide Potassium Phosphate Methylene Chloride Methanol Magnesium Chloride Magnesium Sulfate 4-Morpholinepropanesulfonic Acid Sodium Chloride Sodium Chlorate Sodium Nitrite Sodium Dodecyl Sulfate Sodium Nitrate Sodium Hydroxide Sodium Phosphate Trichloroethylene Tetrahydrofuran Appendix D - 9 C2H4O2 C6H6 CaC12 CC14 CHC13 CuC12 EDTA EtOH H2O H2O2 H2SO4 HCL HC1O4 HF HNO3 KC1 KC1O3 KNO2 KNO3 KOH K3PO4 MeC12 or CH2C12 MeOH MgC12 MgSO4 MOPS buffer NaC1 NaC1O3 NaNO2 SDS NaNO3 NaOH Na3PO4 TCE THF Appendix E Respiratory Protection Program RESPIRATORY PROTECTION PROGRAM SCOPE This program applies to all respirator users at the University of Georgia’s main campus and at all of its off campus facilities. POLICY The primary objective of this program is to provide a means of respiratory protection when engineering controls (e.g., ventilation, process isolation) are not feasible or adequate. The program does not diminish the supervisor’s responsibility to minimize employee exposure to air contaminants. All respirators shall be used in accordance with current applicable regulations and should be used in accordance with OSHA (29 CFR 1910.134) and ANSI Z88.2 (Practices for Respiratory Protection). In areas where respirators are necessary, employees with facial hair that prevents a proper seal between the mask and face shall not be permitted to wear respirators. Employees who are not medically capable of wearing a respirator shall also be prohibited from wearing one. RESPONSIBILITIES Environmental Safety Services shall be responsible for: 1. Assisting departments and supervisors in the identification and evaluation of hazards for which respiratory protection may be necessary. 2. Identifying engineering controls which will preclude the need for respirator use. 3. Providing guidance to the supervisor in the development and implementation of standard operating procedures for respirator use and maintenance. 4. Reviewing and maintaining a copy of all written standard operating procedures established by supervisors in accordance to this policy. 5. Assisting supervisors and users with the selection of respirators, cartridges, and related equipment for the control of specific hazards. 6. Training respirator users and supervisors in the proper use of respirators and respiratory protection devices. Appendix E 2 7. Performing qualitative respirator fit testing for personnel requiring such testing. 8. Maintaining fit test records, notifying personnel of annual retesting and advising supervisors of the records they must maintain. 9. Verifying the effectiveness of the respiratory protection program as it applies to employees and their work environment through periodic safety audits. 10. Approving the brand, model and type of respirator prior to it’s use. Supervisors are responsible for: 1. Identifying potentially hazardous operations and consulting with ESD to determine if there is a need for respirator use. 2. Establishing written standard operating procedures for each operation which requires the use of a respirator. These procedures must, as a minimum, address the following areas: a. Authorized uses and limitations b. Medical qualifications c. Training d. Fit testing e. Responsibilities of the employee as defined in this policy f. Maintenance, inspection, storage, and disinfection/decontamination g. Record keeping requirements h. Accidental exposures response and emergency procedures 3. Identifying those employees who may need to wear respirators. 4. Ensuring that such employees are physically able to wear a respirator, are trained in the proper use and maintenance of the respirator, and have been fit tested prior to the use of a respirator. 5. The procurement of respirators, cartridges, and other applicable personal protective equipment. 6. Enforcing the use of respirators when respiratory protection is needed. 7. Ensuring that respirators are used in accordance with the instructions and training provided by the manufacturer, the Environmental Safety Division, and the procedures established in the written standard operating procedure. 8. Monitoring the work area during respiratory use in case of adverse conditions and worker stress. Appendix E 3 9. Maintaining all required records pertaining to the respirators along with relevant air and personnel monitoring data as well as medical authorizations. Required records will be determined by applicable regulations and the Environmental Safety Division. 10. Acting as or appointing the person or persons responsible for maintaining the respirators according to the standard operating procedures. Respirator users are responsible for: 1. Informing their supervisors or the Environmental Safety Division of any working conditions for which they feel a respirator is needed or wanted. 2. Informing their supervisor of any personal health problems that could be aggravated when wearing a respirator or that could make wearing a respirator inadvisable. 3. Wearing and using respirators issued to them in accordance with the instructions and training provided by the Environmental Safety Division and with those procedures established in the written standard operating procedure. 4. Ensuring proper cleaning, inspection, and storage of respirators in their custody. 5. Reporting any ill fitting or malfunctioning respirator to their supervisor. 6. Reporting any adverse health effects to the supervisor, or to the Environmental Safety Division, that may have been the result of an accidental hazardous agent exposure during the performance of hazardous operations. Appendix E 4 Appendix F Fume Hood Standards Fume Hood Face Velocity Standards This standard will be applied to all existing fumehoods not covered under the Board of Regents Guidelines Design Criteria for Laboraotry Furnature and Fumehoods adopted on January 16, 1996. Certain individual fume hoods may be exempted from this standard only by the direction of the CLSC. Reference the current revision at Board of Regents Guidelines Design Criteria for Laboraotry Furnature and Fumehoods. FUME HOOD CLASSIFICATION AVG. FACE VELOCITY MAJOR STRUCTURAL CHARACTERISTICS FOR USE WITH: NOT FOR USE WITH: SASH FULL OPEN General Purpose Perchloric 100 - 120 FPM 100-120 FPM Constructed of chemical resistant material (including stack) - Hot Perchloric Acid - Highly explosive vapors Airflow construction - Medium concentration acid fumes - Mildly toxic vapors - Organic solvents Stainless steel lining and duct system; washdown system in duct work - Perchloric Acid - Nitric Acid - Organic solvents - Any combustible material - Radioactive material (< 50 mC) - Cold acid fumes - Organic solvents - Toxic vapors - Hot Perchloric Acid - Hot acid fumes - Explosive vapors Airflow construction Stainless steel lining Radiation 100-120 FPM HEPA filter in exhaust system Airflow construction Appendix F 2 Rating The Fume Hood Performance Fume hoods will be certified and rated according to average face velocity and balance. All average face velocities must exceed 100 linear feet per minute but must not exceed 120 linear feet per minute at full open sash. The variance from the average should not exceed 20% for more than 2 measurements. Any fume hood operating outside of these parameters are to be repaired. Fume hoods are to be rated as follows: Post certified sign - fume hood rated Ok on the form 1. 2. Average Airflow between 100 lfpm and 120 lfpm and; Not more than two reading are outside + 20% of the average (+10% for 18" sash hoods). Post caution sign - fume hood rated “caution” on the form 1. 2. 3. More than two readings are outside + 20% (+10% for 18" sash hoods) but no reading is less than 30 or greater than 160; or Average airflow is > 120 lfpm (on sign write: airflow too high, use caution; or Average airflow is less than 100 lfpm but greater than 80 lfpm Only use low concentrations of cold acid fumes and organic solvents until the fume hood is repaired and recertified as velocity ok. Do not use Perchloric acid or create concentrated acid fumes, toxic fumes or explosive fumes. Post danger sign - fume hood is rated “danger” on the form 1. 2. 3. 4. Average airflow is less than 80 lfpm; or any measurement is less than 30 lfpm; or Hood not working at all; or Average airflow > 160 lfpm Hazardous chemicals are not to be used until the fume hood is repaired and recertified. Appendix F 3 Appendix G Hazardous Waste Summary Procedures SUMMARY PROCEDURE FOR HAZARDOUS WASTE PICKUP Hazardous Materials Program Mr. Brian Adams Will Hunter Road, Athens, Ga. 30602 Phone: 369-5706 Fax: 369-5866 E-Mail: badams@esd.uga.edu Satellite accumulation areas (research laboratories or classrooms) may accumulate up to 55 gallons of hazardous waste or one quart of acutely hazardous waste at a location under the direct supervision of a laboratory supervisor without any restrictions on accumulation time. Before the maximum amount of waste has been accumulated, use the following procedure to arrange for pick-up. PROCEDURE: 1. Go to the http://esd.uga.edu/haz-mat and register to take the class online. Then go to Section II for the Solid and Hazardous Materials Management Manual which you can download in Adobe format and retain this manual in your lab for future reference. All necessary forms are at http://esd.uga.edu/haz-mat/online-forms and can be downloaded. 2. To prepare a container for storage of hazardous waste materials: a. b. c. d. Select a clean container, that is compatible with the hazardous waste, with a tight fitting cap or lid. Remove or deface all existing labels on the container. Label the container "Hazardous Waste." Attach the Chematix Waste Card to the container with a rubber band. On large consolidation containers, attach a glassiene sticky-backed envelope (available from HMTF) or an envelope of your own. Notes: C C C Keep containers closed except when adding hazardous waste. Do not overfill containers. Keep at least 2 inches of empty space above the liquid. Ensure that containers are in good condition (eg: free from leaks). Appendix G 2 3. When various waste mixtures from a lab or lab suite are to be added to a single container, fill out a Chematix Waste Card (https://chematix.uga.edu). Remove card as additions of chemicals are made to the container and replace with updated card. Store the card in a sticky-backed envelope also called a glassiene bag and attach to the container. Note: C Add only compatible waste to the container. If in doubt use a separate container. C To avoid unexpected chemical reactions keep the number of chemicals collected in a single container to a minimum. 4. Fill out a "Blue Tag" for each container indicating the material is waste. You may obtain more Blue Tags by calling 369-5706. a. Using a ball-point pen, press firmly to complete all copies of the "Blue Tag" as indicated. b. The principal researcher is the supervisor of the laboratory. c. Building abbreviations are acceptable. d. List all chemicals collected in the container as follows: i. Use complete chemical names. Chemical formulae or abbreviations are not acceptable. ii. Identify the chemical by the CAS number or contact 369-5706 and a CAS number can be assigned to your chemical. iii. List all components whether hazardous or not (even water). iv. List percentage of each component (best estimate). v. Total percentage must equal 100%. e. State the quantity of material in the container giving both the numeric value and the unit of measure (example: 3 gallons, 500 ml, 1 liter etc.). f. Place completed "Blue Tags" in the glassiene sticky-backed envelope and attach to each container. 5. To schedule Hazardous Waste Chemicals pick up: a. Complete the Hazardous Materials Pick-up Inventory form (see Solid and Hazardous Materials Management Manual) and forward the completed Pick up Inventory form to HMTF via fax 369-5866 or by campus mail at the above address, I. HMTF Personnel will prepare a shipping manifest. ii. The HMTF Staff will schedule with the laboratory supervisor for a suitable pick-up time. iii. Have a knowledgeable person on site at the time of pick-up to assist with the identification of containers and sign the required manifests. iv. The HMTF staff will pack individual containers into acceptable shipping containers. Appendix G 3 Note: C For specific information refer to the Hazardous Materials Manual or contact HMTF. C Containers with incomplete labels or tags are not acceptable for pick-up. C CAS numbers are available from the vendor's catalog, MSDS or the Merck Index Appendix G 4 Appendix H Waste Minimization, Bench Top Treatment and Surplus Chemical Redistribution I. Hazardous Waste Minimization Hazardous waste disposal grows increasingly more complex and costly with each passing year. The following suggestions can help hazardous waste generators to minimize the volume of waste produced in the lab, and reduce cost to the University for disposal. Scale down the size of the experiment and quantity of reagent needed to achieve the experimental goal. Consider benchtop methods, such as distillation, to recover used reagents for reuse. Investigate alternate reagents which may accomplish the experimental goals and be less hazardous. Explore benchtop treatments which may deactivate, detoxify or neutralize the material prior to disposal. Contact the Hazardous Waste Manager about questions concerning these methods. A good manual for benchtop treatment is Hazardous Laboratory Chemicals: a Disposal Guide, by M.A. Armour ( CRC Press, Boca Raton, FL. 1991). Do not buy large volumes of reagents simply because of bulk price reductions. The cost of disposal for unused material is often greater than the purchase price. A large portion of the University s waste is unused materials in the original container. Keep only a minimum supply of reagent in the lab. If regular consumption requires that larger quantities be available, arrangements can be made with Central Research Stores to order and stock the material for easy access. Date all received materials which are subject to a limited shelf life. Any chemicals which may destabilize to an explosive or highly reactive compound should be ordered in minimum quantities and used within the manufacturers recommended shelf life. Destabilized materials are usually handled with extreme precautions and resultant costs can be exorbitant. Inspect labels periodically to ensure that the label is intact and legible. Containers of unlabeled materials are considered unknown until they are identified by the user or by chemical tests. Wide spectrum analysis can be very expensive. When collecting waste, try to keep each container as homogeneous as possible. Cost differentials for various categories of waste make the segregation of waste groups very cost effective. Disposal costs escalate dramatically when different waste groups are mixed in the same container. A p p e nd i x H 2 II. Benchtop Treatment Once a hazardous material is determined to be a waste, several options exist for its disposal. Burial in a secure landfill, incineration, solidification, encapsulation in concrete or vitrification have all been practiced by various waste disposers. Disposal by such methods can be very expensive and in some cases, can be technically difficult. The problems of disposal are complicated in the university environment because of the large variety of relatively small amounts of chemicals which are disposed. The generator, normally an instructor or principal investigator, is responsible for making a waste determination. Once the chemical user has determined that the chemical has served its intended purpose and that it is to be discarded, the generator must thereafter handle the chemical as a waste. It is important, therefore, that chemical users carefully determine the goals of their processes so that maximum use can be obtained from stock before it is discarded. Universities in every state are reviewing their procedures to discover methods of minimizing their chemical utilization, and, therefore, their quantities of waste generated. Methods of recovery, recycling, and reuse make it possible for teaching and research labs to dramatically reduce the waste volume. Additional efforts at scale reduction through the use of micro-quantities and small instrumentation have assisted the conservation of valuable resources. Where waste cannot be further reduced at the source, there is a possibility for the user to treat the material at the site, in small containers to reduce or eliminate its hazardous characteristics. The on-site treatment is a final step in an experimental protocol which renders the material non-hazardous as a goal of the process. In teaching labs, these methods form part of the students education in becoming environmentally responsible. Techniques such as elementary acid/base neutralization, oxidation/reduction reactions or precipitation of insoluble solids may help to reduce the wastes which are sent to the Hazardous Materials Treatment Facility for disposal. The University of Georgia is committed to the protection of our environment through proper waste treatment. Unless the on-site treatment can be legitimately considered as part of the protocol performed on the benchtop, the process may require licensing. It is not acceptable to dilute the hazardous characteristics of a waste material to avoid proper disposal. For further specific guidance on benchtop treatments, call the Hazardous Materials Manager and refer to: Hazardous Laboratory Chemicals: Disposal Guide by M.A. Armour, 1991. CRC Press Inc. Boca Raton , Fl 33431 A p p e nd i x H 3 III. Surplus Redistribution Chemicals and products which have not been used, are not subject to waste regulations unless they have been contaminated, are off specification, or are past their recommended shelf life. If the owner makes a decision, when there is no productive use, that the chemical must be discarded, then the materials become waste. The decision to discard usable chemicals is not one that should be made lightly. Chemicals represent a large investment of capital resources for the University and a significant capital liability when they are disposed. It is not uncommon for the cost of disposal of a chemical to exceed the purchase price and, in some cases, by orders of magnitude. ( A recent quote received from a chemical waste disposal company for removal of pump oil contaminated with radioisotopes was greater than $6,000 per gallon). Chemical users may keep their stock as long as they wish. With the realization that the components of research and teaching change constantly, and that research validity is often dependant on the quality and control of the chemical constituents, the user must take responsibility for the effects of long term storage on chemical reactions. When the primary user decides to transfer chemical stock to another user, several precautions must be exercised. The chemicals must not be past the manufacturers recommended shelf life. Materials must be in a useable condition ie. powders should not be compacted into solid clumps that do not separate easily; container lids should not be crusted with residue; liquids should not be throwing a precipitate; there should be no evidence of discoloration, etc. Any material which is not acceptable to another user, may be kept by the primary user for future consumption or may be voluntarily discarded. This decision to keep or discard may be based on the probability of future use, space considerations, the value of the substance or any valid reason that the user can justify. In all cases the University maintains ownership of chemical substances and reserves the right as owner to redistribute by donation or by resale to valid users of any unused chemical surplus. Central Research Stores and the Environmental Safety Division are investigating a facility-less redistribution program which will allow users to advertise their surpluses on the list serv. The system will allow potential new users to contact the holder of the surplus directly and arrange the transport of material to the new location. The new program will help to eliminate any confusion arising from the handling of surplus materials by the staff who also remove and process hazardous waste. A p p e nd i x H 4 Appendix I Particularly Hazardous Substances Peroxide-Forming Chemicals Organic peroxides are some of the most hazardous substances handled in a laboratory. They are usually sensitive to shock, sparks, or accidental ignition. These chemicals tend to be more shock-sensitive than most primary explosives that we are familiar with such as TNT. An example of a particularly dangerous situation that may be found in a lab is an ether bottle that has evaporated to dryness. In some chemicals, inhibitors are added to extend the storage lifetime of the chemical. However, because distillation of such a stabilized liquid will remove the inhibitor, the end product must be stored with care as a potential peroxide-former. Please note: peroxide may form on the surface of alkali metals and their amides. Do not perform standard peroxide tests to these materials (alkali metals & their amides) since they are water reactive. All of these chemicals should be purchased in small quantities and used up as soon as possible. Georgia Fire Code (based on NFPA 45 (1991)) requires that all peroxide forming chemicals be dated upon opening. It is also prudent to date these chemicals upon first arrival in the facility. Unopened peroxide forming chemicals should not be used if greater than 1 year old. Types of Compounds Known to Auto oxidize to Form Peroxides: C Aldehydes C Ethers, especially cyclic ethers and those containing primary and secondary alkyl groups (never distill an ether before it has been shown to be free of peroxides). C Compounds containing benzylic hydrogens C Compounds containing allelic hydrogens (C=C!CH), including most alkenes; vinyl and vinylidene compounds C Compounds containing a tertiary C!H group (e.g., decalin and 2,5!dimethylhexane) Classes of Chemicals That Can Form Peroxides Upon Ageing: Class I: Unsaturated materials, especially those of low molecular weight, may polymerize violently and hazardously due to peroxide initiation. Acrylic acid Acrylonitrile Butadiene Chlorobutadiene (chloroprene) Chlorotrifluoroethylene Methyl methacrylate Styrene Tetrafluoroethylene Vinyl acetate Vinyl acetylene Vinyl chloride Vinyl pyridine Vinylidene chloride Class II: The following chemicals are a peroxide hazard upon concentration (distillation/evaporation). A test for peroxide should be performed if concentration is intended or suspected. A potassium iodide test strip can be used to check for peroxides after the chemical has expired or six months after openting with test results placed on the bottle. If the test is not performed, then these chemicals should be disposed of 6 months after opening. A written record of test results should be maintained. Acetal Cumene Appendix I 2 Cyclohexene Cyclooctene Cyclopentene Diacetylene Dicyclopentadiene Diethylene glycol dimethyl ether (diglyme) Diethyl ether Dioxane (p-dioxane) Ethylene glycol dimethyl ether (glyme) Furan Methyl acetylene Methyl cyclopentane Methyl-I-butyl ketone Tetrahydrofuran Tetrahydronaphthalene Vinyl ether Class III: Peroxides derived from the following compounds may explode without concentration. It is recommended that these chemicals be disposed of 3 months after opening. Organic Divinyl ether Isopropyl ether Divinyl acetylene Vinylidene chloride Inorganic Potassium metal Potassium amide Sodium amide (sodamide) (Note: Lists are illustrative but not exhaustive) ** Prudent Practices in the Laboratory: Handling and Disposal of Chemicals. National Research Council 1995 Appendix I 3 Specific Chemical Hazards Because it would be impossible to list all possible chemical hazards which might be encountered in laboratories on campus, a few of the most commonly encountered hazardous materials are listed below. Active Metals - sodium and potassium Hazards WATER REACTIVE, CORROSIVE TO SKIN, FLAMMABLE These metals react violently with water and may spontaneously ignite. Toxic vapors are given off upon combustion. Fire Extinguishing Media Class D fire extinguisher (Dry Chemical or Sodium Carbonate) Personal Protective Equipment Face shield, Splash goggles, lab coat, apron, nitrile gloves Storage Requirements Store in oil or kerosene in a cool, dry area away from water and oxidizers. Benzene Hazards CARCINOGEN, HIGHLY FLAMMABLE, VAPORS ARE TOXIC Vapors irritate the eyes. High concentrations inhaled can cause unconsciousness and death. Prolonged breathing of vapors may cause severe or fatal blood disease. Swallowing and absorption through the skin could result in major residual injury Fire Extinguishing Media Class B (Carbon Dioxide, Foam or Dry Chemical) Personal Protective Equipment Splash goggles, Certified fume hood, lab coat, Viton gloves Storage Requirements Store with flammables in an approved flammables storage cabinet. Benzoyl Peroxide Hazards EXPLOSIVE HAZARD BY SHOCK, FRICTION OR IGNITION SOURCE, CORROSIVE Benzoyl peroxide has been reported to explode spontaneously. It is an extreme fire hazard and is also a strong oxidizer. Do not get the materials in the eyes or on the skin. Fire Extinguishing Media Large volumes of water Appendix I 4 Personal Protective Equipment Splash goggles, certified fume hood, laboratory coat, apron, face shield, nitrile gloves Storage Requirements Store in a cool place away from direct sunlight. It is best stored alone separated from all other chemicals and combustible materials. Carbon Disulfide Hazards EXTREMELY FLAMMABLE! POISON, HIGHLY VOLATILE, CORROSIVE TO SKIN Carbon Disulfide is the most flammable and explosive of all common solvents. Its vapors can be ignited by contact with an ordinary lightbulb. It is toxic and major residual injury may result from overexposure in spite of prompt treatment. Mixtures of carbon disulfide in air in the presence of rust can explode. Fire Extinguishing Media Class B (Dry chemicals, foam or Carbon Dioxide). DO NOT USE WATER. Personal Protective Equipment Splash goggles, a certified fumehood, lab coat, face shield, Viton gloves Storage Requirements Store with flammable liquids in an approved flammables storage cabinet. Carbon Tetrachloride Hazards POISON, CARCINOGEN Avoid breathing the vapor. Small swallowed doses may result in death. Repeated low level exposures are likely to cause liver damage. Fire Extinguishing Media Use appropriate extinguisher for surrounding fire Personal Protective Equipment Splash goggles, face shield, lab coat, apron, certified fume hood, PVA or Viton gloves Storage Requirements Store with other Blue labeled toxins away from alkali metals, chemically active metals, Oxidizers, bases, allyl alcohol, and dimethyl formamide. Ethers See “PEROXIDE FORMING CHEMICALS” Appendix I 5 Hydrofluoric Acid Hazards EXTREMELY CORROSIVE, HIGHLY TOXIC May be fatal if swallowed. Vapors can cause severe burns. Prevent the inhalation of the vapors. Will react with water, liberating heat. Fire Extinguishing Media Use extinguishing media appropriate for the surrounding fire. If water is to be used, apply in flooding quantities form as great a distance as possible. Do not use a water stream. Personal Protective Equipment Splash goggles, face shield, lab coat, apron, certified fume hood, butyl rubber gloves Storage Requirements Store with mineral acids in an approved acids storage cabinet or in a chemical resistant tray inside a low cabinet. Do not store in glass containers. Mercury Hazards HIGHLY TOXIC, EMITS POISONOUS VAPORS. The vapor pressure of mercury at room temperature is 0.002 mm Hg which is sufficient to produce concentrations of about 200 times the permissible exposure limit (0.1 mg/m3). Although this concentration is not likely to occur with small spills in a well ventilated laboratory, every effort should be made to avoid mercury spills and to promptly clean up spills that do occur. There are specific spill kits available through CRS for mercury. Fire Extinguishing Media Use extinguishing media appropriate for the surrounding fire Personal Protective Equipment Splash goggles, lab coat, certified fume hood, nitrile gloves Storage Requirements Store in an air tight container with Blue label toxins Nitric Acid Hazards STRONG OXIDIZER, CONTACT WITH COMBUSTIBLE MATERIALS MAY CAUSE FIRE, EXTREMELY CORROSIVE, CAUSES SEVERE BURNS Nitric acid forms flammable and explosive compounds with many materials. Spills should be absorbed with inert materials such as absorbent clay. The use of paper towels to clean up spill could cause a fire. Fire Extinguishing Media Use large amounts of water. Appendix I 6 Personal Protective Equipment Splash goggles, face shield, lab coat, apron, nitrile gloves, certified fume hood Storage Requirements Store with oxidizers away from organics in a corrosives cabinet or in corrosive resistant trays. Store on lower cabinet shelves. Perchloric Acid See “Perchloric Acid S.O.P.” in Appendix J Phosphorus (White (Yellow)) Hazards SPONTANEOUSLY FLAMMABLE IN AIR, CREATES TOXIC FUMES IN AIR Phosphorus is extremely toxic and exposure via any route is likely to cause residual injury despite prompt medical attention. Fire Extinguishing Media Water Personal Protective Equipment Splash goggles, face shield, lab coat, apron, nitrile gloves, certified fume hood Storage Requirements Store in water in an air tight container. Store the container in a cool place separate from other laboratory chemicals. Picric Acid Hazards RISK OF EXPLOSION BY SHOCK, FRICTION, FIRE OR OTHER SOURCES OF IGNITION WHEN DRY, FORMS VERY SENSITIVE EXPLOSIVE METALLIC COMPOUNDS, TOXIC, CORROSIVE If the container of picric acid drys out, explosive, shock sensitive crystals will form. Dry Picric Acid must be not be handled, moved or opened. Call Environmental Safety Services immediately upon discovery of dry picric acid. Fire Extinguishing Media Water spray Personal Protective Equipment Splash goggles, face shield, lab coat, apron, nitrile gloves, certified fume hood Storage Requirements Store in a cool, dry place away from metals, salts, sparks and flames. Store with other RED label chemicals. Appendix I 7 Chemicals Suspected of or Known to Cause Cancer C HEMICAL C HEMICAL A-alpha-C(2-Amino-9H-pyrido[2,3-b]indole) Acetaldehyde AcetamideAcetochlor 2-Acetylaminofluorene Acifluorfen Acrylamide Acrylo nitrile Actinomycin D Adriamycin (Doxorubicin hydrochloride) AF-2;[2-(2-furyl)-3-(5-nitro-2-furyl)] acrylamide Aflatoxins Alachlor Alcoho lic beverages, when assoc. w/alco hol abuse Aldrin Allyl chloride 2-Aminoanthraquinone p-Aminoazobe nzene ortho-Aminoazotoluene 4-Am inobiphenyl (4-aminodiphenyl) 3-Amino-9-ethylcarbazole hydrochloride 1-Amino-2-methylanthraquinone 2-Am ino-5-(5-nitro-2-furyl)-1,3 ,4-thiadiazole Amitrole Analgesic mixtures containing phenacetin Aniline ortho-Anisidine ortho-Anisidine hydrochloride Antimony oxide (Antimony trioxide) Aram ite Arsenic (inorganic arsenic compo unds) Asbestos Auramine Azaserine Azathioprine Azacitidine Azobenzene Benz[a]anthracene Benzene Benzidine [and its salts] Benzidine-based dyes Benzo[b ]fluoranthene Benzo[j]fluorantheneBenzo[k]fluoranthene Benzofuran Benzo[a]pyrene Benzotrichloride Benzyl chloride Benzyl violet 4B Beryllium and beryllium compounds Betel quid with tobacco Bis (2-chloroethyl) ether N,N-B is(2-chloroethyl)-2-naphthylamine (Chlornapazine)Bischloroethyl nitrosourea (BCN U) (Carmustine) Bis(chloromethyl)ether Bitumens, extracts of steam-refined & air refined Bracken fern Bromo dichloromethane Bromo form 1,3-Butadiene 1,4-Butanediol dimethanesulfonate (Busulfan) Butylated hyd roxyanisole beta-Butyrolactone Cadmium and cadmium compounds Caffeic acid Captafol Captan Carbon tetrachloride Carbon -black extracts Ceramic fibers(airborne particles of respirable size) Certain combined chemotherapy for lymphomas Chlo ramb ucil Chloramphenicol Chlordane Chlordecone (Kepone) Chlordimeform Chlo rendic acid Chlorinated paraffins p-Chloroaniline (Ave rage chain length,C1 2; approx. 60% chlorine by weight) Chlorodibromo methane 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU ) (Lomustine) Chloroform Chloromethyl methyl ether (technical grade) 4-Chloro-ortho-phenylenediamine Chlo rothalonil Chrom ium (hexava lent comp ounds) C.I. Acid Red 114 Ciclosporin (Cyclosporin A; Cyclosporine) Chloroethane (Ethyl chloride) 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)1-nitrosourea (Methyl-CCNU) 3-Chloro-2-methylpropene p-Chloro-o-toluidine Chlo rozo tocin Chrysene C.I. Basic Red 9 monohydrochloride Cinna myl anthranilate Appendix I 8 Cisplatin Cobalt metal powder Coke oven em issions Creosotes Cupferron Cyclophospha mide (anh ydrous) D&C Orange No. 17 D& C Red N o. 9 Dacarbazine Dantron (Chrysazin; 1,8-Dihydroxyanthraquinone) DDD (Dichlorodiphenyldichloroethane) DDT (Dichlorodiphenyltrichloroethane) N,N'-Diacetylbenzidine 2,4-D iamino anisole sulfate 2,4-Diaminotoluene Dibenz[a,h]acridine Dibenz[a,j]acridine Dibenz[a,h]anthracene 7H -Dibenzo [c,g]carbazole Dibenzo[a,e]pyrene Dibenzo[a,h]pyrene Dibenzo[a,i]pyrene Dibenzo[a,l]pyrene 1,2-Dibromo-3-chloropropane (DBCP) 2,3-Dibromo-1-propanol p-Dichlorobenzene 3,3'-Dichlorobenzidine 1-4,-Dichloro-2-butene 3,3'-Dichloro-4-4'-diaminodiphenyl ether 1,1-Dichloroethane Dichloromethane (Methylene chloride) 1-2-Dichloropropane 1,3-Dichloropropene Dieldrin 60571 Dienestrol Diepoxybutane Diesel engine exhaust Di(2 -ethylhexyl)phtha late 1,2-Diethylhydrazine Diethyl sulfate Diethylstilbestrol Diglycidyl resorcinol ether (DGRE) Dihyd rosafro le Diisopropyl sulfate 3,3'-Dimethoxybenzidine (ortho-Dianisidine) 3-3'Dimethoxybenzidine dihydrochloride (ortho-Dianisidine dihydrochloride) Dim ethyl sulfate 4-Dimethylaminoazobenzene trans-2-[(Dimethylamino)methylimino]5-[2-(5 -nitro-2-furyl)vinyl]-1,3 , 4-oxa diazo le 7,12-Dimethylbenz(a)anthracene 3,3'-Dimethylbenzidine (ortho-Tolidine) 3,3'-Dimethylbenzidine dihydrochloride Dimethylcarbamoyl chloride 1,1-Dimethylhydrazine (UMDH) 1,2-Dimethylhydrazine Citrus R ed N o. 2 Cobalt [II] oxide Conjugated estrogens para-Cresidine Cycasin Cyclophosphamide (hydrated) D& C Red N o. 8 D&C Red No. 19 Daminozide Daunom ycin DDE (Dichlorodiphenyldichloroethylene) DD VP (Dichlorvo s) 2-4-D iamino anisole 4-4'-Diaminodiphenyl ether (4,4'-Oxydianiline) Diaminotoluene (mixed) 226368 224420 53703 194592 192654 189640 189559 191300 96128 July 1, 1987 96139 October 1, 1994 106467 January 1, 1989 91941 764410 28434868 75343 anuary 1, 1990 75092 78875 542756 January 1, 1989 84173 1464535 -117817 1615801 64675 56531 101906 94586 2973106 119904 20325400 April 1, 1993 77781 60117 55738540 57976 119937 612828 79447 57147 540738 Appendix I 9 April 1, 1992 Dimethylvinylchloride 1,6-Dinitropyrene 1,8-Dinitropyrene 2-4,Dinitrotoluene 1,4-Dioxane Diphenylhydantoin (Phenytoin) Dip henylhyd antoin (Phe nytoin), so dium salt Direct Black 38 (technical grade) Direct Blue 6 (technical grade) Direct Brown 95 (technical grade) Disperse Blue 1 513371 42397648 42397659 121142 123911 57410 630933 1937377 2602462 16071866 2475458 Epichloro hydrin Erionite 12510428 Estradiol 17 Estrone 53167 Ethinylestradiol Ethyl ac rylate Ethyl methane sulfonate Ethyl-4,4'-dichlorobenzilate Ethylene dibromide Ethylene dichloride (1,2-Dichloroethane) Ethylene oxide Ethylene thiourea Ethyleneimine 106898 Folpet Form aldehyde (gas) 2-(2-F ormylhydrazino)-4-(5-nitro -2-furyl) thiazole Furan Furazolidone Furmecyclox 133073 50000 3570750 110009 67458 60568050 Gaso line engine exhaust (co ndensates/extracts) Glasswoo l fibers(airborne particles o f respirable size)-Glu-P-1 (2-Amino-6-methyldipyrido[1,2-a:3',2'-d] imidazole) Glu-P -2 67730103 (2-Aminodipyrido [1,2-a:3',2'-d] imidazole) Glycidaldehyde Glycidol Grise ofulvin Gyromitrin (Acetaldehyde methylformylhydrazone) -- HC B lue 1 Heptachlor Heptachlor epoxide Hexachlorobe nzene Hexachlorocyclohexane (technical grade) Hexachlorodibenzodioxin Hexachloroethane Hexamethylphosphoramide Hydrazine Hyd razine sulfate Hydrazobenzene (1,2-Diphenylhydrazine) 2784943 76448 1024573 118741 -34465468 67721 680319 302012 10034932 122667 Indeno [1,2,3-cd]pyrene 193395 50282 57636 140885 62500 510156 106934 107062 75218 96457 151564 67730114 765344 556525 126078 16568028 Appendix I 10 July 1, 1987 July 1, 1987 January 1, 1989 October 1, 1993 IQ (2-Amino-3-methylimidazol[4,5-f]quinoline) Iron dextran complex Isosafrole 76180966 9004664 120581 Lactofen Lasiocarpine Lead acetate Lead and lead compounds Lead phosphate Lead subacetate Lindane and other hexachlorocyclohexane isomers 77501634 303344 301042 -7446277 1335326 -- Mancozeb Maneb Me-A-alpha-C (2-Amino-3-methyl-9H-pyrido[2,3-b] indole) Medro xypro gestero ne acetate MeIQ (2-Amino-3,4-dimethylimidazo[4,5-f]quinoline) MeIQ x 7500040 (2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline) Melphalan Merphalan Mestranol 8-Methoxypsoralen with ultraviolet A therapy 5-Methoxypsoralen with ultraviolet A therapy 2-Methylaziridine (Propyleneimine) Methylzoxymethanol Methylzoxymethanol acetate 3-Methylcholanthrene 5-Methylchrysene 4,4'-Methylene bis(2-chloroaniline) 4,4'-Methylene bis (N,N-dimethyl) benzenamine 4,4'-Methylene bis(2-methylaniline) 4,4'-Methylenedianiline 4,4'-Methylenedianiline dihydrochloride Methylhydrazine and its salts Methyl iodide Methyl methanesulfonate 2-M ethyl-1-nitro anthraquino ne (of uncertain purity) N-M ethyl-N' -nitro-N-nitrosoguanidine N-Methylolacrylamide Methylthiouracil Metiram Metronidazo le Michler's ketone Mirex Mitomycin C Mo nocrotaline 5-(Morpholinomethyl)-3[(5-nitro-furfurylidene)-amino]-2-oxalolidi none Mustard Gas 8018017 12427382 68006837 Nafenop in 1-Naphthylamine 2-Naphthylamine Nickel and certain nickel compounds 3771195 134327 91598 -- 71589 7094112 January 1, 1989 October 1, 1992 October 1, 1994 October 1, 1994 148823 531760 72333 298817 484208 75558 590965 592621 56495 3697243 101144 101611 838880 101779 13552448 -74884 66273 129157 70257 924425 56042 9006422 443481 90948 2385855 50077 315220 139913 505602 Appendix I 11 July 1, 1987 July 1, 1992 Nickel carbon yl Nickel refinery dust from pyrom etallurgical process Nickel subsulfide Nirid azole Nitrilotriacetic acid Nitrilotriacetic acid, trisodium salt monohyd rate 5-Nitroacenaphthene 5-Nitro-o-anisidine o-Nitroaniso le 4-Nitrobip henyl 6-Nitrochrysene Nitrofen (technical grade) 2-Nitrofluorene Nitrofurazone 1-[(5-Nitrofurfurylidene)-amino]-2-imidazolidinone N-[4-(5-Nitro-2-furyl)-2-thiazoly]acetamide Nitrogen mustard (Mechlorethamine) Nitrogen mustard hydrochloride (Mechlorethamine hydrochloride) Nitrogen mustard N-oxide Nitrogen mustard N-oxide hydrochloride 2-Nitropropane 1-Nitropyrene 4-Nitropyrene N-Nitrosodi-n-butylamine N-Nitrosodiethanolamine N-Nitrosodiethylamine N-Nitrosodimethylamine p-Nitrosodiphenylamine N-Nitrosodiphenylamine N-Nitrosodi-n-propylamine N-Nitroso-N-ethylurea 3-(N -Nitrosomethylamino) propionitrile 4-(N-Nitrosomethylamino)-1-(3-pyridyl)1-butanone N-Nitrosomethylethylamine N-Nitroso-N-methylurea N-Nitroso-N-methylurethane N-Nitrosomethylvinylamine N-Nitrosomorpho line N-Nitrosonornicotine N-Nitrosopiperidine N-Nitrosopyrrolidine N-Nitrososarcosine Norethisterone (Norethindrone) 13463393 -12035722 61574 139139 18662538 602879 99592 91236 92933 7496028 1836755 607578 59870 555840 531828 51752 55867 Ochratoxin A Oil Orange SS Oral contraceptives, combined Oral contraceptives, sequential Oxadiazon Oxymetholone Oxazepam 303479 2646175 --19666309 434071 604751 Panfuran S Pentachlorophenol Phe nacetin Phenazop yridine -87865 62442 94780 April 1, 1989 October 1, 1992 126852 302705 79469 5522430 57835924 924163 1116547 55185 62759 156105 86306 621647 759739 60153493 64091914 10595956 684935 615532 4549400 59892 16543558 100754 930552 13256229 68224 Appendix I 12 October 1, 1994 Phenazopyridine hydrochloride 136403 Phe nesterin 3546109 Phenobarbital 50066 Phenoxybenzam ine 59961 Phenoxybenzamine hydrochloride 63923 Phenyl glycidyl ether 122601 Phe nylhydrazine and its salts -o-Phenylphenate, sodium 132274 PhiP 105650235 (2-Amino-1-methyl-6-phenylimidozol[4,5-b]pyridine) Polybrominated bip henyls -Polychlorinated b iphenyls -Polychlorinated biphe nyls -(containing > 60% chlorine by molecu lar weight) Polychlorinated dibenzo-p-dioxins -Polychlorinated dibenzofurans -Polygeenan 53973981 P on ce au MX 3761533 Ponceau 3R 3564098 Potassium bromate 7758012 Procarbazine 671169 Procarbazine hydrochloride 366701 Procymidone 32809168 Progesterone 57830 1,3-Propane sultone 1120714 Pro pargite 2312358 beta-Propiolactone 57578 Propylene oxide 75569 Pro pylthiouracil 51525 Radionuclides Reserpine Resid ual (heavy) fuel oils -50555 -- Saccharin Saccharin, sodium Safro le Selenium sulfide Shale-oils Silica, crystalline (airborne particles of respirable size) 81072 128449 94597 7446346 68308349 -- July 1, 1992 October 1, 1994 October 1, 1992 October 1, 1992 October 1, 1994 October 1, 1994 Soots, tars, and mineral oils -(untreated and mildly treated oils and used engine oils) Sterigm atocystin 10048132 Strep tozotocin 18883664 Styrene oxide 96093 Sulfallate 95067 Talc containing asbestiform fibers Terrazo le Testosterone and its esters 2,3,7,8-Tetrachlorodibenzo-para-dioxin (TCDD) 1,1,2,2-Tetrachloroethane Tetrachloroethylene (Perchloroethylene) p- , , -Tetrachlorotoluene Tetranitromethane -2593159 58220 1746016 79345 127184 5216251 509148 Appendix I 13 October 1, 1994 Thioacetamide 4,4'-Thiodianiline Thiourea Thorium dioxide To bacco, oral use o f smokeless products Tob acco, smoke To luene d iisocyan ate ortho-Toluidine ortho-Toluidine hydrochloride para-Toluidine To xaphene (Polychorinated camphenes) Treosulfan Trichlormethine (Trimustine hydrochloride) 2,4,6-Trichlorophenol Triphenyltin hydroxide Trichloroethylene Tris (aziridinyl)-para-benzoquinone (Triaziquone) Tris(1-aziridinyl)phosphine sulfide (Thiotepa) Tris(2-chloroethyl)ph osphate Tris(2,3-dibro mop ropyl)pho sphate Trp-P-1 (Tryptophan-P-1) Trp-P-2 (Tryptophan-P-2) Trypan blue (commercial grade) 62555 139651 62566 1314201 --26471625 95534 636215 106490 8001352 299752 817094 88062 76879 79016 68768 52244 115968 126727 62450060 62450071 72571 Unleaded gasoline (wholly vaporized) Uracil mustard Urethane (Ethyl carbamate) -66751 51796 Vinyl bromide Vinyl chloride 4-Vinyl-1-cyclohexene diepoxide (Vinyl cyclohexene dioxide) Vinyl trichloride (1,1,2-Trichloroethane) 2,6-Xylidine (2,6-Dimethylaniline) 593602 75014 106876 Zineb 12122677 79005 87627 Appendix I 14 January 1, 1992 July 1, 1992 April 1, 1992 Chemicals Known to Cause Reproductive Toxicity Developmental Toxicity Chemical Chemical Acetohydroxamic ac id Dicumarol Actinomycin D Diethylstilbestrol (DES) All-trans re tinoic ac id Dinocap Alprazolam Dinoseb Amikacin sulfate Diphenylhydantoin (Phenytoin) Aminoglutethimide Doxycycline (internal use) Aminoglycosides Doxycycline hyclate (internal use) Aminopterin Doxycycline monohydrate (internal use) Angiotensin converting (ACE) inhibitors Ergotam ine tartrate Anisindione Ethyl alcohol in alcoholic beverages Aspirin (NO TE : It is especially impo rtant not to use aspirin during the last three m onths o f pregn ancy, unless specifically directed to do so by a physician because it may cause problems in the unborn child or complications during delivery.) Barbiturates Benom yl Benzphetamine hydrochloride Benzodiazepines Ethylene thiourea Bischlorethyl nitrosourea (BCN U) (Carmustine) Bro mox ynil Butabarbital sodium 1,4-Butanediol dimethylsufonate (Busulfan) Carbon disulfide Carbon monoxide Carb oplatin Chenodiol Chlorcyclizine hydrochloride Chlo ramb ucil Chlordecone (Kepone) Chlordiazepoxide Chlordiazepoxide hydrochloride 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU ) (Lomustine) Clom iphene citrate Clorazepate dipotassium Cocaine 50362 Colchicine Conjugated estrogens Cyanazine Cycloheximide Cyclophospha mide (anh ydrous) Cyclophosphamide (hydrated) Cyhexatin Cytarabine Danazol 17230885 Daunorubicin hydrochloride Demeclocycline hydrochloride (internal use) Diazepam Ethylene glycol monoethyl ether Ethylene glycol monomethyl ether Ethylene glyco l monoethyl ether acetate Ethylene glyco l monome thyl ether acetate Etoposide Etretinate Fluoroura cil Fluoxymesterone Flurazepam hydrochloride Flutamide Halazepam Hexachlorobe nzene Ifosfamide Iodine-131 Isotretinoin Lead Lithium carbonate Lithium citrate Lorazepam Lovastatin Medro xypro gestero ne acetate Megestro l acetate Melphalan Meno tropins Mepro bam ate Mercap topurine Mercury and mercury compounds Methacycline hydrochloride Methimazole Methotre xate Methotrexate sodium Methyl bromide as a structural fumigant Methyl mercury Methyltestosterone Midazolam hydrochloride Minocycline hydrochloride (internal use) Riba virin Appendix I 15 Misoprostol Secobarbital sodium Mitoxantrone hydrochloride Strep tomyc in sulfate Nafarelin acetate Tamox ifen citrate Niomycin sulfate (internal use) Temazepam Netilmicin sulfate Testostero ne cyp ionate Nicotine Testostero ne ena nthate Nitrogen mustard (Mechlorethamine) 2,3,7,8-Tetrachlorodibenzo-para-dioxin (TCDD) Nitrogen mustard hydrochloride Tetracyclines (internal use) (Mechlorethamine hydrochloride) Tetracycline (internal use) Norethisterone (Norethindrone) Tetracycline hydrochloride (internal use) Norethisterone acetate (Norethindrone acetate) Thalidomide Norethisterone (Norethindrone)/Ethinyl estradiol Thioguanine Norethisterone (Norethindrone)/Mestranol To bacco sm oke (primary) Norgestrel To bram ycin sulfate Oxaxepam Toluene Oxytetracycline (internal use) Triazolam Oxytetracycline hydrochloride (internal use) Trilostane Paramethadione Trimethadione Penicillamine Uracil mustard Pentobarbital sodium Urethane Phenacemide Uro follitropin Phenprocoumon Valproate (Valproic acid) Pipobroman Vinb lastine sulfate Plicamycin Vinc ristine sulfate Polybrominated bip henyls W arfarin Polychlorinated b iphenyls Procarbazine hydrochloride Pro pylthiouracil Retinol/retinyl esters, when in daily dosages in excess of 10,000 IU, or 3,000 retinol equivalents. (NOTE : Retinol/retinyl esters are required and essential for maintenance of normal reproductive function. The recommende d daily level during pregnancy is 8,00 0 IU .) Appendix I 16 Female Reproductive Toxicity Chemical CAS # Date Aminopterin Anabolic steroids Aspirin 50782 (NO TE : It is especially impo rtant not to use aspirin during the last three m onths o f pregn ancy, unless specifically directed to do so by a physician because it may cause problems in the unborn child or complications during delivery.) Carbon disulfide Cocaine 50362 Cyclophospha mide (anh ydrous) Cyclophosphamide (hydrated) Ethylene oxide Lead To bacco sm oke (primary) Male Reproductive Toxicity Chemical Anabolic steroids Benom yl Carbon disulfide Colchicine Cyclophospha mide (anh ydrous) Cyclophosphamide (hydrated) 1,2-Dibromo-3-chloropropane (DBCP) m-Dinitrobenzene o-Dinitrobenzene p-Dinitrobenzene Dinoseb 88857 Ethylene glycol Ethylene glycol Ethylene glyco l Ethylene glyco l monoethyl ether monomethyl ether monoethyl ether acetate monome thyl ether acetate 110805 109864 111159 110496 January 1, 1993 January 1, 1993 Hexamethylphosphoramide 680319 October 1, 1994 Lead -- Nitro furantoin 67209 To bacco sm oke (primary) -- Uracil mustard 66751 Date: October 1, 1994 Appendix I 17 April 1, 1991 January 1, 1992 Appendix J Recommended Laboratory Standard Operating Procedures And Other Resource Information Suggested Outline for Your Laboratory-Specific Chemical Care and Handling Plan Procedures and protocols for each individual laboratory or group of laboratories should be developed to handle potential emergency situations. Standard operating procedures (SOP) for using specific chemicals or apparatus that could cause injury should also be developed. This appendix will provide guidance as to what procedures or protocols should be developed and what information should be contained within the procedures or protocols. The SOPs or protocols should be brief and to the point. If it is too lengthy, it will not be read. Example: procedures and protocols provided in this appendix can be modified specifically for your facility or used directly as written. I. Safety procedures and protocols A. Notification protocol 1. This procedure should include: a. b. c. d. e. f. 2. B. names of all personnel to be notified in the event of an emergency. how these people are to be notified (i.e., telephone, loud speaker, etc.) a list of all of areas in the building for which you are responsible. a system for notifying people who might be in remote locations in the building (i.e., cold rooms, environmental chambers, darkrooms, etc.) in the event that the entire building is being evacuated. information on the types of signs that should be posted. where they will be posted in the event there is an emergency in a particular area and to keep people from returning to the area. Refer to the notification procedure example given at the end of this Appendix. Chemical spill clean-up procedure 1. This procedure should include: a. b. c. 2. a detailed description of the storage location and the contents of your labs chemical spill clean-up kit. instructions on the clean-up and disposal of any type of chemical spill that might occur in the lab. instructions of what to do when a spill is beyond the capabilities of the laboratory personnel. Refer to the chemical spill procedure and chemical spill clean-up kit examples given at the end of this Appendix. Appendix J - 1 C. Personal protective equipment (PPE) safety 1. This procedure should include: a. b. c. d. e. f. 2. D. Refer to the eye wear procedure example given at the end of this Appendix. Fume hood use 1. This protocol should include: a. b. c. d. e. 2. E. the type(s) of fume hoods present in the laboratory proper use of the hood(s). materials that are acceptable for use within the hood(s). acceptable and unacceptable operations that may be performed in the hood. specific, step by step procedures for the use of perchloric acid hoods and wash down systems. Refer to the chemical fume hood operating procedure example given at the end of this Appendix. Fire Procedures 1. This protocol should include: a. b. c. d. 2. II. the specific types of PPE that should be utilized for each group of chemicals in the laboratory. the specific types of PPE that should be utilized for individual chemicals that are being used in bulk or that have particularly hazardous properties. proper use, cleaning and decontamination of PPE. proper storage of the PPE. inspecting PPE. procuring new PPE. what to do if you discover a fire. what to do in the event of a fire alarm. how and when to use a fire extinguisher. how to exit the building from a given laboratory. Refer to the portable fire extinguisher operating procedure and fire protocol examples given at the end of this Appendix. Personal injury procedures A. Eye injury (eye wash station) Appendix J - 2 1. This procedure should include: a. b. c. d. e. f. 2. B. Refer to the emergency eyewash standard operating procedure example given at the end of this Appendix. Skin injury (safety shower) 1. This procedure should include: a. b. c. d. e. f. 2. C. where the safety shower is located. how to use the safety shower. emergency notifications. first aid procedures. reporting the incident. special considerations concerning chemicals of extreme hazard. Refer to the safety shower standard operating procedure example given at the end of this Appendix. Respiratory injury 1. This procedure should include: a. b. c. d. e. 2. D. where the eyewash is located. how to use the eyewash. emergency notifications. first aid procedures. reporting the incident. special considerations concerning chemicals of extreme hazard. immediate actions to be taken (i.e., first aid). emergency notifications. procedures for medical transportation. reporting the incident. special considerations concerning chemicals of extreme hazard. Refer to the respiratory injury procedure example given at the end of this Appendix. Ingestion of hazardous chemicals 1. This procedure should include: a. b. c. d. immediate actions to be taken (i.e., first aid). emergency notifications. procedures for medical transportation. reporting the incident. Appendix J - 3 e. 2. III. Refer to the ingestion procedure example given at the end of this Appendix. Chemical storage plan A. The chemical storage plan should address classes of chemicals and specific, high hazard chemicals used in the laboratory. The storage plan should include: 1. 2. 3. 4. 5. 6. B. IV. special considerations concerning chemicals of extreme hazard. storage locations of chemicals by group. color coding on labels used to identify compatible storage. alphabetizing chemicals within compatible groups. protection of shelving and storage cabinets. special storage considerations regarding high hazard chemicals. storage location for chemical waste. Refer to the chemical storage plan for laboratories example given at the end of this Appendix. Chemical waste disposal Refer to Appendix G V. Lab apparatus protocols and operating procedures Make manufacturers equipment manuals available to employees. VI. Particularly hazardous substances Information regarding the step by step procedures for handling particular hazardous substances can be obtained from the manufacturer, the MSDS, or reference literature. The perchloric acid protocol given on the next page is provided as an example. Appendix J - 4 WORK AREA NOTIFICATION PROTOCOL In the event of an emergency that results in serious injury to laboratory staff, damage to property, or serious disruption of laboratory operations, follow emergency notifications and response, and initiate notification of laboratory personnel and management. Emergency Notifications: principal researcher office home laboratory manager office home department head office home Notification of personnel outside the laboratory: Post a sign on all entrance doors to the laboratory where the spill occurred indicating: Do Not Enter - Hazardous Chemical Spill For more information call at your name phone # you will be at In the event of a building evacuation: Notify all personnel in rooms of the ev acua tion on your w ay ou t. ) at ( Con tact ( principal researcher Con tact ( ) or ( office ) at ( laboratory manager ) home ) or ( office Appendix J - 5 ) home HOW TO PROPERLY COMPLETE A CAUTION SIGN In order to bring greater uniformity to safety signs throughout the University and to reduce clutter on laboratory doors and hallways, the University of Georgia provides all laboratories with door caution signs. All standard safety warnings are concentrated on one 8.5 x 11 inch yellow and black caution placard. These placards should be posted on all laboratory entrances and in lab service areas where hazardous materials are used or stored. Lab door caution signs may be requested free of charge from ESD at 2-5801. MARKING THE SIGN The caution signs will be laminated when received. Use a fine point permanent marker, such as a Sharpie, to mark hazards, degree of hazard, quantities, contact information, date posted, etc. When the information on the sign needs updating, use isopropyl alcohol to erase the old information. DO NOT destroy or dispose of the sign. These placards are meant to be reused. If the lab is to be closed, please return the sign to ESD for reissue to another lab. HAZARDS SECTION The Hazards section of the door caution sign is divided into Primary and Specific Hazards and is used to indicate that a chemical hazard with a degree of hazard 1 through 4 (see definitions below under the NFPA Diamond section) is present in the laboratory. Each hazard is listed by type (health, flammable, reactive or biohazard). Place a dark check mark in the appropriate box to the left of the hazard symbol to indicate that a hazard is present. Next, indicate the quantity of the hazard present by listing the approximate amount in the space provided to the right of the hazard symbol. An exact amount is not required and quantities may be estimated. For example, acetone is used in the lab and is ordered in a 20 liter container. Acetone is a flammable hazard 3, so a check mark is placed in the box to the left of the flammable symbol. In the space to the right of the symbol place the quantity normally found in the lab; i.e., 20 liters. The degree of hazard for many commonly used lab chemicals can be found on the manufacturer’s label, on the material safety data sheet (MSDS), in the manufacturer’s catalog or at http://esd.uga.edu/chemical-lab-safety/right-know/msds-access. Each substance is rated on a scale of 0 (non-hazardous) to 4 (extremely hazardous) for each category: • • • Health Hazard - the danger or toxic effect of a substance if inhaled, ingested or absorbed. Flammable Hazard - the tendency of the substance to burn. Reactive Hazard - the potential of a substance to explode or react violently with air, water or other substances. If your laboratory employs biohazard materials, the appropriate safety level must be placed in the space provided to the right of the biohazard symbol. Please call the biosafety office at 2-7265 to have the biosafety level in your laboratory assessed. • Biohazard - the biosafety level assigned by the biosafety officer/committee. Check the box to the left of each Specific Hazard (contact, compressed gas cylinder, Appendix J - 6 air/water reactive or ultraviolet light) and indicate the quantity of the hazard present. The contact hazard box would be checked if the substance presents a danger when exposed to skin, eyes or mucous membranes. If compressed gas cylinders are present, check the box to the left and indicate the number of cylinders by product in the space to the right of the hazard symbol. For example, three cylinders of carbon dioxide would be written as CO 2 - 3. If your laboratory employs radioisotopes, all radioisotopes listed on the laboratory license must also be listed in the space entitled “Other Hazards” on the caution sign. Additionally, a rad sticker must be placed on the door sign in the space provided to the right of the white hazard boxes. Please call ESD at 542-5801 to obtain a rad sticker. THE NFPA DIAMOND The NFPA (National Fire Protection Association) diamond, located on the right hand side of the door caution sign, is used to record the Degree of Hazard (0 - 4)of all hazardous substances in the lab. The diamond gives a quick visual determination of the highest level of hazards present in a given laboratory. The NFPA diamond is divided into four sections with the following designations: Blue Red Yellow White - Health rating - Flammability rating - Reactivity rating - Special warnings such as air or water reactive substances. Each of the first three sections should be filled in with a number from 0 to 4 to indicate the highest level of hazard found in your lab. For instance, if the most flammable substance in your laboratory has an NFPA flammability rating of 3, a large 3 should be placed in the red box of the NFPA diamond. If the most reactive substance in your lab has a rating of 2, a large 2 should be placed in the yellow reactivity box. Many reagent bottles labels contain NFPA diamonds indicating the associated hazards. In this instance, NFPA ratings are easily determined. If the ratings are not on the bottle, consult material safety data sheets (MSDS) or NFPA rating charts to get the appropriate ratings. Also, see http://esd.uga.edu/chemical-lab-safety/right-know/msds-access for NFPA listings of many chemicals commonly found in the laboratory. The level of hazard associated with each numerical rating is found below: Health (Blue) Flammability (Red) Reactivity (Yellow) 0 - Normal Material 0 - Will not burn 0 - Stable 1 - Slightly Hazardous 1 - Flash Point above 200 F 1 - Unstable if heated 2 - Hazardous 2 - FP between 100 & 200 F 2 - Violent change 3 - Extreme danger 3 - FP below 100 F 3 - Shock and heat may detonate 4 - Deadly 4 - FP below 73 F 4 - May detonate The white section or “Special Warnings” would contain the symbol A, W or OX indicating that air or water reactive or oxidizing chemicals are present in the laboratory. Appendix J - 7 CONTACT INFORMATION In this section, list two people that may be contacted in case of an emergency in the laboratory. The first name recorded should be that of the professor who is the primary researcher for the laboratory. His/her department, office room number, office phone number and home phone number should be recorded. A second name (usually the laboratory supervisor) should be listed in the same manner in the event that the primary researcher cannot be contacted during an emergency. The second person listed should be someone who regularly works in the laboratory and can make responsible decisions in the event of an emergency. DO NOT record the telephone number of ESD, UGA Police Department or 9-911 in this space. DATE POSTED Place the month followed by the year that the sign is posted to the right of this field. The placard and its contents should be reviewed annually. If any changes are made during the year, the sign should be updated to indicate current laboratory conditions. The date that the sign was updated should be indicated in the date posted section by placing the corresponding month followed by the year. All information contained on the caution sign is helpful to emergency personnel responding to a reported fire, spill or injury in the lab. An example of a caution sign that is properly filled out is given below. If you have any questions concerning your caution sign, please call ESD at 2-5801. Appendix J - 8 Chemical Spill Procedure • Do not attempt to clean up any spill if • the appropriate PPE is not available. • appropriate spill clean-up materials are unavailable. • the chemical or level of exposure hazard is unknown. • you have not been appropriately trained in chemical spill clean up. • you do not have and/or have not been trained to use respiratory protection devices. • the chemical is of extreme hazard. (NFPA 49 in any section) If you need assistance contact ESD at 2-5801 • • General procedures • Get away. Avoid contact with the chemical(s). Evacuate the area if there is an immediate risk to occupants. Turn on exhaust ventilation (fume hood/emergency exhaust) and, if flammables are involved, eliminate sources of ignition and flames. • Identify the chemical. Know the chemical name(s), state and concentrations. • Get help. Call ESD at 2-5801. If the emergency involves fire, also call 9-911. • Seal off the area and alert others. Notify anyone in surrounding areas who may be affected by the spill. Keep anyone from entering the affected area. • Look for injuries. If any injury involves chemical contact, immediately disrobe the affected areas and wash continuously (follow safety shower or eyewash SOP). Notify the University Police at 2-2200 of the need for emergency personnel. Await instructions from emergency response personnel. • Initiate work area notification procedures. Solvent spills • Don protective gloves (list gloves appropriate for solvents in the laboratory), respirator (if available in the laboratory and if personnel are trained in its use, see Appendix E ), safety goggles, and a lab coat. Appendix J - 9 • • • • Absorb the spill onto universal absorbent pads. If the spill is small enough absorbent paper (note: this does not mean paper towels) may be used. Place absorbent material onto a fiber, glass, or metal tray and place it in the nearest functioning fume hood. Contact HMTF at 9-369-5706 for disposal instructions. Acid or base spills • Don protective gloves (list glove(s) appropriate for acid available in the laboratory), respirator (if personnel are trained in its use, see Appendix E) , safety goggles, and a lab coat. • Use universal absorbent pads to absorb the spill. Place the pad in a container and dispose of through the hazardous material program. • If universal absorbent pads are unavailable cover acid contaminated surfaces with sodium bicarbonate or spill kit acid neutralizing material or cover basic spills with a dilute acid solution (vinegar or citric acid) or spill kit base neutralizing material. • If universal pads are NOT used, test the spill with pH paper to ensure that it has been completely neutralized. Use available non-combustible absorbent material to absorb the spill (i.e., kitty litter, vermiculite, etc.) • Contact HMTF (9-369-5706) to see if the material needs to be disposed of as hazardous waste. Wash the spill area thoroughly. Solid spills • Don nitrile rubber gloves, safety goggles, and a lab coat. Respiratory protection will be necessary in the event of a large spill release in a confined area, or spill under conditions of higher than normal temperatures. ( see Appendix E for respirator use) • Sweep into a chemical resistant dust pan or onto paper. Place into a plastic bag or other sealable resistant container. Contact HMTF at 9-369-5706 for disposal instructions. Mercury spills • Don disposable rubber gloves, safety glasses or goggles, a lab coat and a respirator (if necessary and personnel are trained in its use, see Appendix E). • Collect all droplets and pools at once using a commercial mercury spill kit (available through CRS or ESS) or a small aspirator with a capillary tube and connected to a pump that can be used for collecting droplets. • Cover fine droplets in non-accessible cracks with calcium polysulfide and excess sulfur. Combine all contaminated mercury in tightly stoppered bottle. Recycle, or dispose of through HMTF. Appendix J - 10 • Following the spillage of mercury onto a carpeted floor, the area is to be decontaminated using a mercury spill kit. Occupants are not to be allowed onto the contaminated area and the floor is not to be vacuumed until the extent of the spill can be assessed by ESD. In the event of extensive contamination the carpet will have to be removed. • No mercury or mercury contaminated items shall be discarded into the sewer system or trash. All waste mercury or mercury contaminated items shall be given to HMTF for recycling or disposal. Appendix J - 11 Developing a General Laboratory Spill Kit Listed below are the materials necessary to develop a fairly inexpensive general spill kit for your laboratory. Purchasing the bulk of these materials through a hardware or discount store is suggested since it will lessen the expense of the kit. This kit should be able to handle < 1 liter of most acids, bases, and solvents found in a typical laboratory. Do not attempt to clean up a spill of greater volume. Materials: Large plastic tub to hold the contents of the kit (3-5 gallons) Plastic dust pan and brush (non-sparking) Chemical safety goggles and face shield Appropriate chemical resistant gloves (ex. neoprene) 3-5 waste disposal bags that can be sealed/closed 4-5 absorbent pads note: brown paper towels and other combustible tissue may ignite when brought into contact with certain chemicals pH paper Bleach (if biohazards are present) Kitty litter for liquid spills Sodium bicarbonate for acids Citric acid for bases “Powersorb Universal Absorbent Pads” (3M) or other “universal” pads note: we recommend these pads. They may be used for most acids, bases, and solvents found in the lab. They absorb more liquid than kitty liter and therefore produce less waste (~ 5 pads for 1 liter). Place litter or pads in a chemical resistant container and dispose of through HMTF. Specific Hazardous Chemicals: This general chemical spill kit is not meant for use with mercury, hydrofluoric acid, sodium metal, cytotoxic drugs, and numerous other chemicals. It is the responsibility of all laboratory personnel to evaluate a potential spill and develop spill response procedures for the specific hazards present. This kit can be modified to meet the needs of your laboratory. Refer to the MSDSs for the chemicals handled in the lab to identify if special spill materials are needed. Contact ESD if you have any questions regarding spill materials for your lab. Any major spill should be reported to ESD at 2-5801. Appendix J - 12 STANDARD OPERATING PROCEDURES FOR THE SELECTION AND USE OF PERSONAL PROTECTIVE EQUIPMENT EYE PROTECTION Industrial grade safety eye wear must be worn at all times when working or observing work procedures in the laboratory. Safety eye wear which is appropriate to the hazard or operation is to be selected using the following criteria: Safety Glasses to be used when: - working with, or observing operations involving, small quantities of noncorrosive, low eye hazard chemicals working with, or observing operations involving, low hazard chemicals (no greater than a 2 in any of the NFPA designations) working with, or observing operations involving, ultraviolet light working with, or observing operations involving, possible flying particles or projectiles. Safety Goggles to be used when: - working with, or observing operations involving, chemicals capable of damaging the eyes working with, or observing operations involving, larger quantities of low hazard chemicals (1 gallon or more) working with, or observing operations involving, chemicals with an NFPA hazard rating of 3 or 4 in any of the designations. Face Shields to be used: - in place of safety glasses for a larger protection area in conjunction with safety goggles when working with large volumes (1 gallon or greater) of corrosive or skin absorbable chemicals. Protective eye wear is to be near the entrance of the laboratory in an easily accessible location. Inspect all eye wear prior to use. If the eye wear is damaged in any way, bring it to the attention of the supervisor for immediate replacement. Decontaminate and clean eye wear prior to storage after each use. Appendix J - 13 Chemical Fume Hood Standard Operating Procedure General Purpose Hoods Use this type of hood only for the removal of vapors released or generated by chemical reactions involving: - mildly toxic materials acids (not heated) organic solvents < 10 milliCurie of radioactive materials In this type of hood, do not use: - hot perchloric acid hot concentrated acids highly toxic materials unstable chemicals or explosives > 10 milliCuries of radioisotopes perchloric acid if used routinely If a CAUTION or DANGER sign is posted, do not work in the hood until the face velocity has been adjusted - Do not use large pieces of equipment in the hood. Close doors and windows when hoods are in operation. Avoid foot traffic and rapid arm/body movement. Place chemical sources and equipment at least 6 inches behind the face of the hood. Do not extend your head inside of the hood while experiments are being performed Perform work with the sash height as low as possible (at most 10-12 inches). Keep fume hoods and adjacent work areas clean since solid debris can enter the hood’s exhaust duct work. Protect spark sources from flammable vapors. Permanent electrical receptacles are not permitted in the hood. Do not cut holes into the hood or its duct work. Do not store chemicals in a fume hood unless storage is the sole use of the hood. Only those chemicals necessary to perform the experiment should be left in the hood. Do not use hood evaporation as a means of chemical disposal. Appendix J - 14 IN CASE OF FIRE If you discover a fire: 1) Activate the fire alarm pull station. 2) Evacuate the building using stairwells and corridors. Close as many doors between you and the fire. 3) From a safe location, call 9-911 and report the fire. Then call 2-2200 (University Police) and report the fire. Initiate your work area notification protocol. 4) Do not reenter the building until the all clear is given by the fire department. A silenced alarm does not indicate an all clear. If you hear a fire alarm: 1) Proceed to the nearest exit. Evacuate the building using stairwells and corridors. Do not use elevators. If the door is closed in the room you are in, do not open the door until you have felt the knob and upper door for heat. If the door or knob are hot, do not open the door. Stuff the door cracks with towels, lab coats, throw rugs, etc. If the window is clear of smoke or flames, open it and hang a lab coat or some other material as a signal to firefighters that you are in the room. If a phone is available and working, call 9-911. 2) If you encounter excessive smoke while evacuating, get as low as possible and crawl to the nearest exit. If possible, cover your mouth and nose with a wet cloth. 3) Once outside, move to a safe location. Do not reenter the building until the all clear is given by the fire department. A silenced alarm does not indicate an all clear. Appendix J - 15 Portable Fire Extinguisher Standard Operating Procedure Portable extinguishers are intended as a first line of defense to cope with fires of limited size. The selection, installation, inspection and maintenance are important parameters for acceptable extinguishing equipment. ALL FIRES MUST BE REPORTED TO ESD WITHIN 24 HOURS OF THE FIRE. SELECTION The selection of extinguishers for a given situation shall be determined by the character of the anticipated fire. Class A Extinguishers: Class A fires are ordinary combustible materials such as wood, cloth and paper. Extinguishers for protecting class A hazards shall be selected from water type and multipurpose dry chemical. Class B Extinguishers: Class B fires are in flammable liquids, oils, greases, tars and flammable gases. Extinguishers for the protection of class B hazards shall be selected from aqueous film forming foam, film forming fluoroprotein foam, carbon dioxide, dry chemical types and halogenated agent types. Class C Extinguishers: Class C fires involve energized electrical equipment where the electrical nonconductivity of the extinguishing media is of importance. Extinguishers for protection of class C hazards shall be selected from carbon dioxide and dry chemical types. Class D Extinguishers: Class D fires involve combustible metals such as magnesium, titanium, zirconium, sodium, lithium and potassium. Extinguishers for the protection of class D hazards shall be of the types approved for the specific combustible metal hazard. OPERATION Extinguishers vary in operation and instructions are required to be in plain view on the front of the extinguisher. The basic steps for actuation should apply to all extinguishers and are as follows: 1. 2. 3. Remove fire extinguisher from its mount. Remove locking device: Extinguishers have a locking safeguard to prevent accidental actuation. These usually consist of lock pins or ring pins and must be removed before actuation. Discharge: These may require one or more of several actions including pushing, pulling, turning, or squeezing a valve handle or lever. Direct the stream of the extinguishing agent toward the base of the fire. Nameplate information has advisory notes regarding the application of the agent to different types of fires. If there are any additional questions consult the codes and standards of the National Fire Protection Association. If you would like Fire Extinguisher Training, please contact Fire Safety at 369-5706. Appendix J - 16 Emergency Eyewash Station Standard Operating Procedure LOCATION(S): TO USE THE EMERGENCY EYEWASH STATION: 1. 2. 3. 4. 5. 6. 7. 8. Do not panic. Shout out for help to allow co-workers to assist you. Get to the eyewash station and turn the eyewash on. Someone should be calling for EMS. From campus, dial 9-911. Rinse both eyes with copious amounts of water for a minimum of 15 minutes. Keep your eyelids open by using your hands to ensure adequate flushing of the eyes. Continue rinsing eyes until emergency medical personnel arrive to assist. Contact ESD at 2-5801 in the event of an emergency. Please note: The emergency eyewash station is only for first aid. It is not medical treatment for chemical exposures. Make certain that you seek proper medical attention. ! ! ! ! ! ! ! THINGS TO REMEMBER Keep the eyewash and safety shower free of obstructions at all times. Keep the “pressure pop-off” covers (protective caps) on the eyewash stations. Test eyewash stations for approximately five minutes every week. Be sure that there are no electrical wires or outlets in the surrounding vicinity and the eyewash station should be easy to reach. All personnel should be able to identify the location of the eyewash station before any work begins. Know the location of the eyewash station with your eyes closed. Be ready to assist co-workers in the event of an accident. Turn off hot water from the main or completely remove the hot water knob for eyewash stations that are mounted to a faucet. Appendix J - 17 Safety Shower Standard Operating Procedure LOCATION(S): TO USE AN EMERGENCY SAFETY SHOWER: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Do not panic. Shout out for help. Allow co-workers to assist you. Someone should be calling for emergency medical assistance. On campus this may be done by dialing 9-911. Get to the safety shower and pull the shower handle. Begin removing all articles of clothing and jewelry. Modesty is not an issue in a life threatening situation. Rinse with copious amounts of water for a minimum of 15 minutes. Co-workers, assist. The victim may faint, go into shock, or may not wish to stay under the shower due to water coldness or fatigue. Allow emergency medical personnel to help with further assistance. Seek immediate medical attention. Additional Measures to Consider: ! ! ! ! ! ! ! ! The safety shower should remain free from obstructions at all times. There should be no electrical sockets in the surrounding vicinity and the safety shower should be easy to reach. Have all personnel identify the location of the safety shower before any work begins. You should know the location with your eyes closed. Be ready to assist co-workers in the event of an accident. Decontaminate helpers. Discard any clothing that may be contaminated as hazardous waste. Contain water flow from showers with absorbent material to prevent the spread of contamination. Contact ESD at 2-5801 anytime the safety shower is used. Appendix J - 18 RESPIRATORY INJURY PROTOCOL In the event that toxic vapors or large quantities of moderately hazardous vapors are inhaled: - S - Remove from exposure area to fresh air immediately. If breathing has stopped, give artificial respiration. Keep affected person warm and at rest. Call University Police at 2-2200 or 9-911 to get assistance. Contact ESD at 2-5801 to get an MSDS. Remain with the affected person until emergency responders arrive. Have a copy of the MSDS sent with emergency responders or to the hospital. Fill out two UGA Accident/Incident report forms and two Employer’s First Report of Injury forms; forward to human resources. Initiate laboratory work area notification procedures. In the event small quantities of moderately hazardous vapors are inhaled: - Remove from exposure area to fresh air immediately. Contact ESD at 2-5801 to get assistance. In a timely manner, seek medical attention. Fill out two UGA Accident/Incident report forms and two Employer’s First Report of Injury forms; forw ard two copies to human resources. Appendix J - 19 HAZARDOUS CHEMICAL INGESTION PROTOCOL In the event that toxic, corrosive, or moderately hazardous chemicals are ingested: - Keep affected person warm and at rest. - Call University Police at 2-2200 or 9-911 to get assistance. - Contact ESD at 2-5801 to get chemical specific ingestion protocols and an MSDS. - Remain with the affected person until emergency responders arrive. - Have the MSDS sent with emergency responders or to the hospital. S Fill out two UGA Accident/Incident Report and two Employer’s First Report of Injury or Occupational Disease forms. Forward to human resources. - Initiate laboratory work area notification procedures. Appendix J - 20 Chemical Storage Plan For Laboratories C C C C C C C C C C Chemicals should be stored according to hazard class (i.e., flammables, oxidizers, toxics, corrosives, etc.) Incompatible chemicals should be physically separated from each other during storage. Store chemicals away from direct sunlight or localized heat. Containers of corrosive chemicals (acids/bases) should be stored in chemical-resistant catch trays large enough to contain any spill or leakage. All chemical containers must be labeled in accordance with the Chemical and Laboratory Safety Manual. Store hazardous chemicals at a safe reachable height for all workers in the laboratory. Shelves should be made of chemical resistant materials and/or covered with a chemical resistant coating. Shelves should be secure and strong enough to hold chemicals being stored on them. Do not overload shelves. Personnel should be aware of the hazards associated with all hazardous materials. Separate solids from liquids. The following are examples of groups of chemicals that can be categorized for chemical storage. Use these groups as examples when separating your chemicals by compatibility. Please note that reactive chemicals must be more closely evaluated since they have a greater potential for reacting with chemicals in their same group. See Appendix D or chemical storage based on color code system. This posting may be posted in storage areas to aid workers in chemical storage. Contact laboratory safety if you have any questions concerning chemical storage. Acids: C C C C Make sure that all acids are stored by compatibility. Store concentrated acids on lower shelves in chemical-resistant catch trays or in a corrosives cabinet. This will temporarily contain spills or leaks. Separate acids from bases and active metals such as sodium, magnesium, and potassium. Acids should be separated from chemicals which can generate toxic gases when combined (i.e., sodium cyanide and iron sulfide). Bases: C C C Make sure that all bases are stored by compatibility. Store bases away from acids. Store concentrated bases on lower shelves in chemical-resistant catch trays or in a corrosives cabinet. This will temporarily contain spills or leaks. Flammables: C Make sure that all flammables are stored by compatibility. C You may store 10 gallons of flammable liquids per 100 sq.ft. of flammable liquids in non-fire separated lab areas (NFPA 30 & 45). Lab areas that are properly fire separated or are sprinkled may store 20 gallons of flammable liquids per 100 sq.ft. in the area. The maximum allowable quantity for flammable liquid storage in any size lab is not to exceed 120 gallons. C Approved flammable storage cabinets should be used for flammable storage. Appendix J - 21 C C Regarding flammable liquid storage outside of approved flammable storage cabinets, there may be a maximum of 10 gallons of flammable liquids in original containers and an additional 25 gallons in approved safety cans not to exceed 2 gallon size (NFPA 45). Use only explosion-proof or intrinsically safe refrigerators and freezers for storing flammable liquids. University of Georgia guidelines for flammable storage follow NFPA 30 & 45. Peroxide-Forming Chemicals: C Make sure that all peroxide-forming chemicals are stored by compatibility. C Peroxide-forming chemicals should be stored in airtight containers in a dark, cool, and dry place. C Unstable chemicals such as peroxide-formers must always be labeled with date received, date opened, and disposal/expiration date. C Peroxide-forming chemicals should be properly disposed of before the date of expected peroxide formation (typically 6 months after opening). C Suspicion of peroxide contamination should be immediately investigated. Contact ESD at 2-5801 for procedures. Water-Reactive Chemicals: C Make sure that all water-reactive chemicals are stored by compatibility. C Water-reactive chemicals should be stored in a cool, dry place. Do not store waterreactive chemicals under sinks or near water baths. C Class D fire extinguishers for the specific water-reactive chemical being stored should be made available. Oxidizers: C Make sure that all oxidizers are stored by compatibility. C Store oxidizers away from flammables, combustibles, and reducing agents. Toxics: C C C Make sure that all toxics are stored by compatibility. Toxic compounds should be stored according to the nature of the chemical, with appropriate security employed when necessary. A poison control network telephone number should be posted in the laboratory where toxics are stored. Appendix J - 22 Perchloric Acid Standard Operating Procedure The following is a sample SOP for perchloric acid. This SOP should be placed in the “Particularly Hazardous Substances” section of your Laboratory-Specific Chemical Care & Handling Plan. Please note: this SOP is a general procedure for the use of perchloric acid. It does not address specific issues for each unique operation that may be performed in a lab. Please modify this procedure to meet the needs of your particular situation. • Procedures and practices • Persons working with perchloric acid should be thoroughly familiar with general guidelines for the safe handling of hazardous chemicals supplemented by additional precautions particular for this chemical. Section 2 of the Laboratory Safety Manual covers SOP for general laboratory practices. General safety guidelines include use of the PPE, laboratory apparel, etc. • Storage and handling. • Perchloric acid should be used only in standard analytical procedures from well recognized analytical texts. Work with > 85% perchloric acid requires special precautions and should be carried out only by specially trained personnel. • As a minimum, splash goggles, nitrile gloves, and a lab coat should be worn when handling perchloric acid. • Always transfer perchloric acid over a chemical resistant catch tray in order to catch any spills and afford a ready means of disposal. • Precautions should be taken to prevent the build up of explosive perchlorates. Light, mechanical shock, heat and certain catalysts can be initiators of explosive reactions with the perchlorates that may be formed from perchloric acid. Anhydrous acid which may be formed with strong dehydrating agents decomposes at ordinary temperatures and explodes on contact with most organic materials. Perchloric acid containers should be kept open no longer than 15 minutes per experiment. • Perchloric acid should be stored in well-ventilated location separated from organic substances and other combustible materials. Do not store perchloric acid in a refrigerator or other dehydrating atmosphere. • Keep incompatible chemicals away from perchloric acid and the area in which perchloric acid will be used. Those chemicals that are incompatible with perchloric acid include oxidizable organic compounds such as alcohols, ketones, aldehydes, ethers, and dialkyl sulfoxides; strong acids such as sulfuric acid; dehydrating agents; anhydrous phosphorous pentoxide; formaldehyde; antimony or bismuth; and reducing agents. Seventy percent perchloric acid may react with cellulose materials such as wood, paper, and cotton. Preventing contact with Appendix J - 23 incompatible chemicals during storage may be accomplished by placing perchloric acid containers in nonbreakable, chemical resistant containers which are capable of holding the entire contents of the container. • Fume hoods • General purpose fume hoods Heating of perchloric acid or perchloric acid reactions that involve heat shall not be conducted in a general purpose fume hood. Use of perchloric acid (<72%) at ambient temperature may be conducted in a general purpose fume hood if the following procedures are followed: • • • Only small quantities are used on an infrequent basis. Easily accessible areas in the fume hood, which are exposed to perchloric acid, are immediately wet wiped or rinsed with a squirt bottle of distilled water after use. This procedure prevents the buildup of explosive perchlorates. Periodic methylene blue tests should be conducted after each perchloric acid use for the presence of any perchlorates. Perchloric acid fume hoods: Special precautions involving heated perchloric acid • • Anhydrous perchloric acid is a powerful oxidizer that may explode if it comes in contact with organic materials. Anhydrous perchloric acid can be produced when heating perchloric acid, during the evaporation of perchloric acid, or during reactions involving dehydrating agents. Chemicals that are incompatible with anhydrous or hot concentrated perchloric acid include acetic anhydride, acetic acid, aniline, carbon (wood charcoal & carbon black), paper, wood fiber, or sawdust. • • • Procedures involving heated perchloric acid, reactions involving dehydrating agents, or routine use of perchloric acid must be conducted in a perchloric acid fume hood equipped with a water wash-down system. The wash down system should be turned on immediately after perchloric acid has been heated in the hood or after general use of the fume hood. Step by step instructions should be written on how to operate the wash-down for perchloric acid hoods. Tests shall be conducted for explosive perchlorates before any inspection, cleaning, maintenance, or other work performed on the exhaust system or hood interior. Perchloric acid hoods are specifically designed for the use of perchloric acid and other material that can deposit shock sensitive crystalline materials in the hood and Appendix J - 24 exhaust system. Only those chemicals for which the hood is specifically designed should be used in a perchloric acid hood. • • • In the event of exposure • In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. Seek medical attention immediately. In case of eye contact, promptly wash with copious amounts of water for a minimum of 15 minutes (lifting upper and lower lids occasionally) and obtain medical attention. If perchloric acid is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once. • In the event of any type of exposure to this chemical, contact Public Safety Dispatch at 2-2200 who will call an EMT and contact Environmental Safety Division. After any exposure, an incident/accident form should be filled out with Environmental Safety Division. Spills • Spill control materials should be available to control the release of perchloric acid. Appropriate protective equipment for clean-up should be worn (i.e., lab coats, protective gloves, protective rubber boots). Clean-up the spill according to established SOP. • Perchloric acid spilled on the floor or bench top represents a hazard since the evaporation of the spill may lead to the formation of more dangerous concentrations of the acid. It should not be mopped up, nor should it be soaked up with dry combustibles. • Remove all combustibles from the surrounding area (i.e., wood, paper, oils). A water spray may be used to help reduce vapors and keep the area wet. Measures should be taken to keep the material or spill area from drying. Neutralize the spill with a dilute solution of sodium hydroxide and then use absorbent material such as universal pads or absorbent clay to absorb it. Place the material in closed flammable waste disposal can. • The area of the spill should be thoroughly rinsed once again and tested for the presence of perchlorates. You may want to neutralize this area also. Hazardous waste disposal Excess perchloric acid and waste material containing perchloric acid should be placed in a glass reagent container and disposed of according to those procedures. Appendix J - 25 • Test procedure for perchlorates • • • • • Collect approximately 10-20 ml of wash water flushed from duct work of the perchloric acid hood and contaminated surfaces. Add approximately 2-5 drops of 0.1% solution methylene blue in water. If a violet precipitate forms perchlorates are present. If perchlorates are present, flush the duct work with water until the test is negative. Contact ESD if you have any questions. Appendix J - 26