1 Glycolysis continued: ATP production and NADH metabolism An
1
Glycolysis continued: ATP production and NADH metabolism
An overview of glycolysis, including compounds, enzymes, and cofactors involved at each step, the detailed structures of the compounds in each reaction (all of which were written on the board in lecture), can be found in V&V p. 446 Fig. 16-3.
I. Focus on key reactions
A. G6P ----> F6P
In the second step of glycolysis, phosphoglucose isomerase catalyzes the coversion of glucose 6-P (G6P, an aldehyde) to fructose 6-P (F6P, a ketone). This conversion is essential to the succeeding aldolase reaction (see notes for the last lecture) because it moves the carbonyl group from C1 to C2. Remember that in order to break the C3-
C4 bond and thus split the molecule symmetrically, the carbonyl group must be alpha to C3, that is, on C2. (Another way to say this is that the carbonyl must be beta to the C in the C-C bond that is part of the leaving group.)
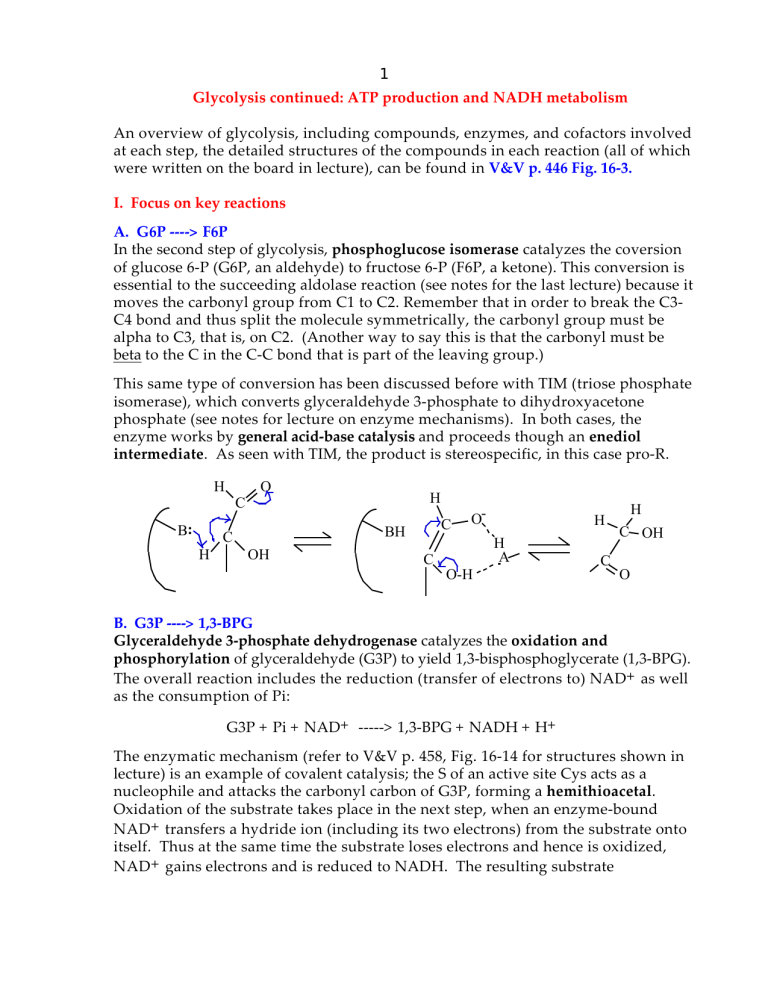
This same type of conversion has been discussed before with TIM (triose phosphate isomerase), which converts glyceraldehyde 3-phosphate to dihydroxyacetone phosphate (see notes for lecture on enzyme mechanisms). In both cases, the enzyme works by general acid-base catalysis and proceeds though an enediol
intermediate. As seen with TIM, the product is stereospecific, in this case pro-R.
H
C
O
B :
H
C
OH
B H
H
C
C
O-H
O-
H
A
H
C
C
O
H
OH
B. G3P ----> 1,3-BPG
Glyceraldehyde 3-phosphate dehydrogenase catalyzes the oxidation and
phosphorylation of glyceraldehyde (G3P) to yield 1,3-bisphosphoglycerate (1,3-BPG).
The overall reaction includes the reduction (transfer of electrons to) NAD+ as well as the consumption of Pi:
G3P + Pi + NAD+ -----> 1,3-BPG + NADH + H+
The enzymatic mechanism (refer to V&V p. 458, Fig. 16-14 for structures shown in lecture) is an example of covalent catalysis; the S of an active site Cys acts as a nucleophile and attacks the carbonyl carbon of G3P, forming a hemithioacetal.
Oxidation of the substrate takes place in the next step, when an enzyme-bound
NAD+ transfers a hydride ion (including its two electrons) from the substrate onto itself. Thus at the same time the substrate loses electrons and hence is oxidized,
NAD+ gains electrons and is reduced to NADH. The resulting substrate
2 intermediate is called a thioester and preserves the high energy from the oxidation in its high-energy S--C bond ( ∆ G of hydrolysis = - 9-10 kcal/mol).
In the final step, the S--C bond is cleaved by phosphorolysis, a reaction analogous to hydrolysis but using Pi instead of water. Like the hexokinase mechanism discussed on last time, the reaction is able to occur because the enzyme excludes water from the active site. The product, 1,3-BPG, preserves the high energy of the thioester in its phosphoanhydride bond on C1; thus 1,3-BPG is a potential phosphoryl donor.
C. 3-phosphoglycerate to 2-phosphoglycerate
Phosphoglycerate mutase catalyzes the conversion of 3PG to 2PG (see V&V p. 460 ).
A phospho-His residue is involved in this reaction. See Fig. 16-18 for details.
D. Phosphoenolpyruvate ----> enolpyruvate ----> pyruvate
As shown in V&V p. 464, Fig. 16-22, the formation of enolpyruvate from phosphoenolpyruvate produces ATP. Since one molecule of glucose produces two phosphoenolpyruvates, this step produces two molecules of ATP per glucose.
Enolpyruvate and pyruvate are tautomers - different forms of the same compound
- and can interconvert freely. In solution, pyruvate is favored by a factor of 105.
The series of reactions has walked the molecule into a high energy state
(phosphoenolpyruvate) that is stabilized by the presence of the phosphate. When the phosphate is removed, the compound decays to the favored tautomer
(pyruvate).
E. Pyruvate ----> lactate
When pyruvate forms lactate (see V&V p. 464 ), NADH is oxidized back to NAD+ so that in the overall conversion of glucose to 2 lactate, NAD+ is regenerated and no overall oxidation-reduction occurs.
II. Summary of ATP use and production
As shown in the overall reaction for glycolysis ( see notes from last time), a net total of 2 ATP is generated in the conversion of glucose to 2 lactate. Remember that
2 ATP are consumed in the two phosphorylation steps:
G --> G6P and F6P --> F1,6 bisP. The ADP that are phosphorylated in the conversion of 2 phosphoenolpyruvate to enolpyruvate recover those two initial phosphates back into the form of ATP. In addition, 2 more ATP are formed when the high energy 1,3-BPG gives up Pi to form 3-phosphoglycerate (reaction occurs in duplicate). (See V&V p, 430 Table 15-3 for the standard free energies of phosphate hydrolysis of these compounds.)
The Pi on 1,3-BPG comes from addition of new inorganic phosphate at the G3P step, which explains a requirement for Pi in the system.
3
III. Structure and function of NAD+
NAD+ (nicotinamide adenine dinucleotide) is the major electron storage compound in biology and serves as a cofactor in many oxidation-reduction reactions. The full structure, as shown in V&V p. 334 Fig. 12-2 , consists of nicotinamide, ribose, pyrophosphate, another ribose, and an adenine, but the nicotinamide ring is responsible for the oxidation-reduction work. As shown below, reduction of NAD+ to NADH consists of adding a hydride ion to C4 of the nicotinamide ring in one of two possible stereospecific positions. Each hydrogenase has its own stereospecificity depending on how it binds NAD+ in relation to the other substrates. The stereospecific pro-R position is shown in the diagram below.
The stereospecificty of glyceraldehyde dehydrogenase is pro-S.
: H O
H H
O
C
NH2
C
NH2
N N
NAD+ NADH (pro-R)
Nicotinamide itself is the vitamin commonly known as B3, or niacin, and is found in meat and fish among other sources. Niacin deficiency results in pellagra, a disease said to cause four D’s - dermatitis, diarrhea, dementia, and death.
IV. Interesting facts about glycolysis
A. Hexokinase
As discussed last lecture, hexokinase catalyzes a bisubstrate reaction involving glucose and ATP. It turns out that it is a random Bi-Bi mechanism, i.e. either glucose or ATP can bind first. However, Dan Koshland has demonstrated that hexokinase is not an ATPase, i.e no hydrolysis of ATP occurs if only ATP, but not glucose is bound to the enzyme. Again, it is the induced fit model that explains this observation. A conformational change occurs such that the residues in the active site are in the proper orientation only when glucose is present.
B. Aldolase
This enzyme catalyzes stereospecific reactions. Only the pro-S hydrogen is removed if the reaction goes in reverese. (See V&V p. 452-453 ). Also, the -OH groups attached to C3 and C4 of the di-phosphorylated hexose must be in trans for the reaction to proceed.
C. ∆ G profile for glycolysis Only a few steps of glycolysis are significantly driven by free energy changes, namely the two inital phosphorylation steps and the conversion of phosphoenolpyruvate to pyruvate. (These are also the steps where regulation of glycolysis occurs and are the steps that require other enzymes in order
4 to be run in reverse.) The steps between F1,6BP and phosphoenolpyruvate are more or less isoenergetic. Energy produced by oxidation is cancelled out by the energy consumed in producing ATP, so the main reason the reactions go forward is because the continuing consumption of each product (by the next steps) drives each reaction forward according to LeChatelier’s principle. Once pyruvate is formed a different enzyme is required for the reverse reaction to occur, so the system is saved from going backwards.
∆
G' for Glycolysis glucose
2
G, 0 kcal/mol -2
10
8
6
4
-4
-6
-8
-10
-12
-14
-8 kcal/mol - phosphorylation keeps glucose in the cell isomerization
-5.3 kcal/mol - second phosphorylation
} isoenergetic reactions (and nearly perfect enzymes!)
-4 kcal/mol (x2) pyruvate lactate
D. Concentration of enzymes vs. substrates.
The concentration of glycolytic enzymes in a cell is high - as high as 100 µ M or 1mM in a muscle cell - so in most cases nearly all available substrate is bound to enzyme.
E. Where does glycolysis occur?
Glycolysis seems to take place at the plasma membrane. Drosophila (fruit flies) that are mutant in glycerol 3-phosphate dehydrogenase exhibit a flightless phenotype. Examination of their muscle cells
(sarcomeres) compared to wild type flies show that G3P dehydrogenase is distributed in the cytoplasm in the mutant, but not in the M or Z lines as is typical of wild type cells. Since M or Z lines specify the junction between adjacent muscle cells, this suggest that these flies cannot fly because the enzymes for glycolysis are not localized in this case.








