Exp4_Acetaminophen SynthesisF15
advertisement

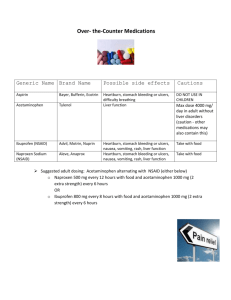
Page 1 of 7 College of San Mateo Experiment 4 – Acetaminophen Synthesis CHEMISTRY BACKGROUND After aspirin was successfully introduced to the pharmaceutical market, the race was on to find other pain-relief medication. One of the early compounds to show promise as another wonder drug was 4-amino-phenol, which did have antipyretic (fever reduction) properties. However, it is also highly toxic. Phenacetin (acetophenetidin) is another antipyretic compound that is also analgesic (pain-relieve) which is less toxic than 4-aminophenol. Phenacetin soon became a commonly used ingredient of analgesic-antipyretic medications until later on, in 1949, when it was found that phenacetin is converted in the body to an even less toxic metabolite, acetaminophen. From then on, acetaminophen gradually replaced phenacetin as a popular analgesic. It is now widely available as an ingredient in many non-prescription drugs to relieve migraines and tension headaches. It is also known as paracetamol and the most popular brand name include Tylenol. O H2N N H OH OH 4-aminophenol acetaminophen O N O H Acetaminophen does not have anti-inflammatory activity and it does not affect blood clotting phenacetin (homeostasis). It is therefore not a NSAID compound (see background in previous experiment) its pain relieving ability is about equal to that of aspirin and it is preferred over aspirin when the homeostatic side effects of aspirin must be avoided. Acetaminophen is fast acting: within an hour of oral administration, acetaminophen is completely absorbed from the gastrointestinal tract. The biologic half-life of acetaminophen in normal adults is about 2-3 hours. Acetaminophen Synthesis YML 231 Experiment 04 Page 2 of 7 College of San Mateo EXPERIMENTAL AND LEARNING OBJECTIVES You must prepare acetaminophen by reaction between 4-aminophenol and acetic anhydride. The reaction is analogous to aspirin synthesis in experiment 03 and it is another synthesis reaction. You should be able to identify the four stages of synthesis in this experiment: reaction, isolation, purification and characterization. H NH2 O H3C + O O N CH3 heat O HO + OH HO 4-aminophenol O Acetic anhydride Acetic acid Acetaminophen In addition, you have the opportunity to review operations we’ve learned so far including recrystallization, vacuum filtration, recrystallization, m.pt. determination, use of IR spectrometer. Acetaminophen is prepared by the reaction of an amine with acetic anhydride. Your crude product may be highly colored. There are two major sources of color contaminants in this reaction: old samples of 4-aminophenol contains traces of air-oxidized products which are intense-colored dyes of unknown structure. These may be carried through the experiment and end up in your crude product. In addition, oxidation during reaction is a possible side reaction, which also produces these colored dyes. An extra decolorization step may be necessary between isolation and purification. PHYSICAL DATA Acetaminophen 4-Aminophenol Acetic Anhydride Molar Mass g/mol 151.2 109.1 102.1 m.pt oC 170 189 - Density g/mL 1.08 DIRECTIONS • Instructor will have a heating set up on display. SAFETY: All chemicals are toxic and/or corrosive. Wear gloves and goggles. Acetaminophen Synthesis YML 231 Experiment 04 Page 3 of 7 College of San Mateo PROCEDURE A) Reaction 1. Weigh between 0.550g and 0.650 g of 4-aminophenol (MW = 109.1 g/mol) and place in a 10 mL RB flask. Record exact mass used. 2. Add 5mL of water followed by 700 µL acetic anhydride (MW = 102.1 g/mol and d = 1.08 g/mL) to the reaction flask. Add a stir vane – pointed end down - and attach an air condenser. 3. Place RB flask in an aluminum block, place on heater. Stir mixture gently and set heat control to medium. Allow reaction to heat at temperature for 30 minutes (Alum block temperature should be approximately 120 °C. Make sure the mixture is hot when you begin timing the reaction). Towards end of reaction time, prepare ice-bath and also chill approx. 5 mL water ready for filtration. 4. When reaction is complete, remove RB flask from heat. As soon as flask is cool enough to touch, detach condenser, remove stir bar and rinse it with 2 or 3 drops of warm water and allow it to drain back into the round bottom flask. Allow reaction mixture to cool to room temp slowly. B) Isolation 5. If necessary, induce recrystallization by scratching or seeding. Complete crystallization by chilling RB flask in an ice bath for 15 – 20 minutes. 6. Collect crude crystals by vacuum filtration using Hirsch funnel. 7. Wash crude products with chilled water (2 portions of approx. 2 mL each). Continue vacuum for another 30 seconds. 8. Transfer solid to a small preweighed container (25mL Erlenmeyer or 30 mL beaker). 9. Water has low evaporation and it would take several hours to air dry. However, the next stage does not require completely dry product. Therefore, record a crude yield now (noting in your lab book product is not dry) and carry on to next stage. 10. If your crude product is highly colored, you need to decolorize it before recrystallization. If your crude product is off white or only very slightly yellow, you can skip the decolorization section. (check with instructor) 11. Set aside a melting point sample of your crude product. Acetaminophen Synthesis YML 231 Experiment 04 Page 4 of 7 College of San Mateo C) Purification 12. Set a hot plate on medium heat (the alum block should be approximately 100 °C). Warm 10 ml of a 50:50 mixture of water and methanol (5ml methanol: 5ml water). The solubility of acetaminophen in this hot solvent system is approximately 0.2g/ml – use this as a guide only 13. Add a small amount of the warm methanol: water solvent mixture to the crude acetaminophen saved from part B and warm on the hot plate. 14. Add the minimum amount of the methanol: water mixture to fully dissolve the solid. 15. Remove solution from hotplate and allow it to cool to room temperature. 16. When crystals begin to form. Chill mixture in an ice-bath. After crystals form, collect the crystals using vacuum filtration. Wash the collected crystals with ice –cold water. Continue to draw air through filter flask for about 2 minutes. 17. Transfer crystals to a preweighed small container (such as 10mL beaker). Determine and record mass of crystals (the product is still wet at this stage so the mass you record now is not the true mass. You will need to reweigh again after crystals have air-dried until next session to obtain its true mass and yield). 18. Write your name, date and name of compound on the container and allow it to dry until next session. D) Characterization 19. Next session: reweigh your dry, pure sample. Record mass and determine yield of product. 20. Obtain an FT-IR of your product and compare with reference IR spectrum. (Instructor will assist). 21. Determine melting point ranges of your crude and pure acetaminophen crystals. Disposal & Cleaning • • • Rinse all used glassware with acetone in your fumehood before washing in water. Transfer waste acetone wash to waste station. Dispose of waste in organic waste station. Wipe fumehood with damp paper towels. Acetaminophen Synthesis YML 231 Experiment 04 Page 5 of 7 College of San Mateo Working Notes • • • • Your notes should have your name and date the work was done, a title indicating scenario and/or experimental objective(s). Include any relevant data and information such as chemical structures or reaction equation. Report and interpret relevant evidence – including observations, data and measurements and calculations. Calculations of yields must be clearly laid out. State your conclusion, and explain how your evidence supports this. Acetaminophen Synthesis YML 231 Experiment 04 Page 6 of 7 College of San Mateo Name: ___________________________ Prelab Questions: Acetaminophen Synthesis 1. What are the pharmaceutical properties of acetaminophen? 2. Name one popular brand name for acetaminophen. 3. In both aspirin synthesis and acetaminophen synthesis, acetic anhydride acts as an “acylation” agent. Explain. (Hint: consider the functional group changes from salicylic acid to aspirin and from 4-aminophenol to acetaminophen). 4. State the functional groups that you will identify in the IR spectra of your pure sample. 5. What difference will you expect to see in the IR spectra when you compare the starting material to the final product? (What peaks will disappear and what peaks will form?) Acetaminophen Synthesis YML 231 Experiment 04 Page 7 of 7 College of San Mateo Name: ___________________________ Postlab Questions: Acetaminophen Synthesis 1. Calculate the % yield of the acetaminophen. Show all working clearly. 2. Quote your melting point of your pure sample and compare it to the literature value. Comment on the purity of the sample. 3. Include a fully labeled IR spectrum in your lab notebook. Acetaminophen Synthesis YML 231 Experiment 04