© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 1
7
INJURIES TO THE LIVER, BILIARY
TRACT, SPLEEN, AND DIAPHRAGM
Jon M. Burch, M.D., F.A.C.S., and Ernest E. Moore, M.D., F.A.C.S.
Injuries to the Liver
ASSESSMENT
The initial step in the management of penetrating abdominal
injuries and of blunt abdominal injuries in cases when nonoperative treatment is contraindicated or has failed is exploratory laparotomy [see 7:6 Operative Exposure of Abdominal Injuries and Closure
of the Abdomen].
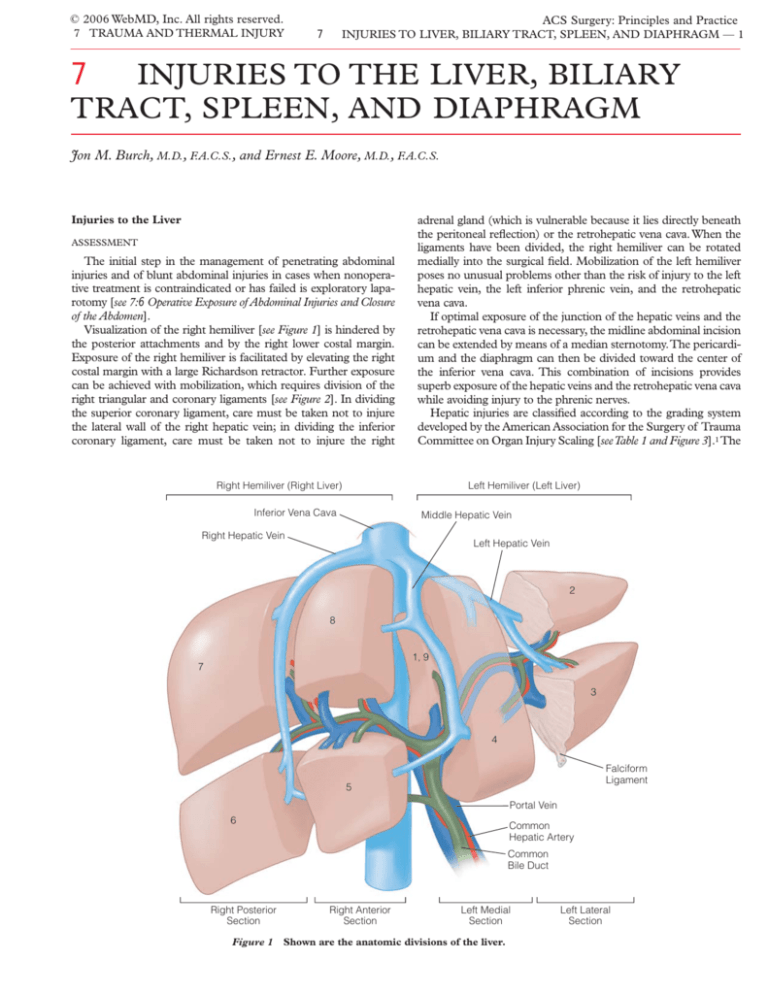
Visualization of the right hemiliver [see Figure 1] is hindered by
the posterior attachments and by the right lower costal margin.
Exposure of the right hemiliver is facilitated by elevating the right
costal margin with a large Richardson retractor. Further exposure
can be achieved with mobilization, which requires division of the
right triangular and coronary ligaments [see Figure 2]. In dividing
the superior coronary ligament, care must be taken not to injure
the lateral wall of the right hepatic vein; in dividing the inferior
coronary ligament, care must be taken not to injure the right
adrenal gland (which is vulnerable because it lies directly beneath
the peritoneal reflection) or the retrohepatic vena cava. When the
ligaments have been divided, the right hemiliver can be rotated
medially into the surgical field. Mobilization of the left hemiliver
poses no unusual problems other than the risk of injury to the left
hepatic vein, the left inferior phrenic vein, and the retrohepatic
vena cava.
If optimal exposure of the junction of the hepatic veins and the
retrohepatic vena cava is necessary, the midline abdominal incision
can be extended by means of a median sternotomy.The pericardium and the diaphragm can then be divided toward the center of
the inferior vena cava. This combination of incisions provides
superb exposure of the hepatic veins and the retrohepatic vena cava
while avoiding injury to the phrenic nerves.
Hepatic injuries are classified according to the grading system
developed by the American Association for the Surgery of Trauma
Committee on Organ Injury Scaling [see Table 1 and Figure 3].1 The
Right Hemiliver (Right Liver)
Left Hemiliver (Left Liver)
Inferior Vena Cava
Middle Hepatic Vein
Right Hepatic Vein
Left Hepatic Vein
2
8
1, 9
7
3
4
Falciform
Ligament
5
Portal Vein
6
Common
Hepatic Artery
Common
Bile Duct
Right Posterior
Section
Figure 1
Right Anterior
Section
Left Medial
Section
Shown are the anatomic divisions of the liver.
Left Lateral
Section
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
Right
Hepatic Vein
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 2
Inferior
Vena Cava
Right Triangular
Ligament
Middle
Hepatic Vein
Coronary
Ligament
Left
Hepatic Vein
Left Triangular
Ligament
Left Branch of Portal Vein
Falciform Ligament
Right Branch
of Portal Vein
Portal Vein
grading scale ranges from I to VI, with I representing superficial
lacerations and small subcapsular hematomas and VI representing
avulsion of the liver from the vena cava. Isolated injuries that are
not extensive (grades I to III) often require little or no treatment;
however, extensive parenchymal injuries and those involving the
juxtahepatic veins (grades IV and V) may require complex maneuvers for successful treatment, and hepatic avulsion (grade VI) is
lethal.
Clamping of the hepatic pedicle—the Pringle maneuver—is
helpful for evaluating grade IV and V hepatic injuries [see Figure 4].
This maneuver allows one to distinguish between hemorrhage
from branches of the hepatic artery or the portal vein, which ceases when the clamp is applied, and hemorrhage from the hepatic
veins or the retrohepatic vena cava, which does not. When performing the Pringle maneuver, we prefer to tear open the lesser
omentum manually and place the clamp from the patient’s left
side while guiding the posterior blade of the clamp through the
foramen of Winslow with the aid of the left index finger. The
advantages of this approach are the avoidance of injury to the
structures within the hepatic pedicle, the assurance that the clamp
will be properly placed the first time, and the inclusion of a replacing or accessory left hepatic artery between the blades of the
clamp.
MANAGEMENT OF INJURIES
Techniques for Temporary Control of Hemorrhage
Temporary control of hemorrhage is essential for two reasons.
First, during treatment of a major hepatic injury, ongoing hemorrhage may pose an immediate threat to the patient’s life, and temporary control gives the anesthesiologist time to restore the circulating volume before further blood loss occurs. Second, multiple
bleeding sites are common with both blunt and penetrating trau-
Figure 2 Depicted are the
venous drainage and suspensory attachments of the liver.
Ligamentum
Teres
ma, and if the liver is not the highest priority, temporary control
of hepatic bleeding allows repair of other injuries without unnecessary blood loss. The most useful techniques for the temporary
control of hepatic hemorrhage are manual compression, perihepatic packing, and the Pringle maneuver.
Periodic manual compression with the addition of laparotomy
pads is useful in the treatment of complex hepatic injuries to provide time for resuscitation [see Figure 5].2-4 Hands and pads
should be positioned to realign the liver in its normal anatomic
position. Perihepatic packing with carefully placed laparotomy
pads is capable of controlling hemorrhage from almost all hepatic injuries.5-9 The right costal margin is elevated, and the pads are
strategically placed over and around the bleeding site [see Figure
6]. Additional pads may be placed between the liver and the
diaphragm and between the liver and the anterior chest wall until
the bleeding has been controlled.Ten to 15 pads may be required
to control the hemorrhage from an extensive right lobar injury.
Packing is not as effective for injuries to the left hemiliver, because
with the abdomen open, there is insufficient abdominal and thoracic wall anterior to the left hemiliver to provide adequate compression. Fortunately, hemorrhage from the left hemiliver can be
controlled by dividing the left triangular and coronary ligaments
and compressing the hemiliver between the hands. Two complications may be encountered with the packing of hepatic injuries.
First, tight packing compresses the inferior vena cava, decreases
venous return, and reduces left ventricular filling; hypovolemic
patients may not tolerate the resultant decrease in cardiac output.
Second, perihepatic packing forces the right diaphragm superiorly and impairs its motion; this may lead to increased airway pressures and decreased tidal volume. Careful consideration of the
patient’s condition is necessary to determine whether the risk of
these complications outweighs the risk of additional blood loss.
The Pringle maneuver is often used as an adjunct to packing
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
Table 1
Injured Structure
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 3
AAST Organ Injury Scales for Liver, Biliary Tract, Diaphragm, and Spleen
AAST Grade
I
II
III
Liver*
IV
V
VI
I
II
III
Extrahepatic biliary tree*
IV
Characteristics of Injury
Hematoma: subcapsular, nonexpanding, < 10% surface area
2
Laceration: capsular tear, nonbleeding, < 1 cm parenchymal depth
2
Hematoma: subcapsular, nonexpanding, 10%–50% surface area; intraparenchymal,
nonexpanding, < 10 cm in diameter
2
Laceration: capsular tear, active bleeding, 1–3 cm parenchymal depth, < 10 cm in length
2
Hematoma: subcapsular, > 50% surface area, expanding; ruptured subcapsular hematoma with
active bleeding; intraparenchymal, > 10 cm or expanding
3
Laceration: > 3 cm parenchymal depth
3
Hematoma: ruptured intraparenchymal hematoma with active bleeding
4
Laceration: parenchymal disruption involving 25%–75% of hepatic lobe or 1–3 Couinaud’s
segments within a single lobe
4
Laceration: parenchymal disruption involving > 75% of hepatic lobe or > 3 Couinaud’s segments
within a single lobe
5
Vascular: juxtahepatic venous injuries (i.e., injuries to retrohepatic vena cava or central major
hepatic veins)
5
Vascular: hepatic avulsion
5
Gallbladder contusion/hematoma
2
Portal triad contusion
2
Partial gallbladder avulsion from liver bed; cystic duct intact
2
Laceration or perforation of gallbladder
2
Complete gallbladder avulsion from liver bed
3
Cystic duct laceration
3
Partial or complete right or left hepatic duct laceration
3
Partial common hepatic duct or common bile duct laceration (< 50%)
3
> 50% transection of common hepatic duct or common bile duct
V
Diaphragm†
3–4
Combined right and left hepatic duct injuries
3–4
Intraduodenal or intrapancreatic bile duct injuries
3–4
I
Contusion
2
II
Laceration < 2 cm
3
III
Laceration 2–10 cm
3
IV
Laceration > 10 cm, with tissue loss < 25 cm2
3
V
Laceration with tissue loss > 25 cm2
3
Hematoma: subcapsular, nonexpanding, < 10% surface area
2
Laceration: capsular tear, nonbleeding, < 1 cm parenchymal depth
2
Hematoma: subcapsular, nonexpanding, 10%–50% surface area; intraparenchymal,
nonexpanding, < 5 cm in diameter
2
Laceration: capsular tear, active bleeding, 1–3 cm parenchymal depth, not involving a trabecular
vessel
2
Hematoma: subcapsular, > 50% surface area or expanding; ruptured subcapsular hematoma with
active bleeding; intraparenchymal, > 5 cm or expanding
3
Laceration: > 3 cm parenchymal depth or involving trabecular vessels
3
Hematoma: ruptured intraparenchymal hematoma with active bleeding
4
Laceration: laceration involving segmental or hilar vessels producing major devascularization
(> 25% of spleen)
4
I
II
Spleen*
AIS-90 Score
III
IV
V
Laceration: completely shattered spleen
5
Vascular: hilar vascular injury that devascularizes spleen
5
*Advance one grade for multiple injuries, up to grade III.
†Advance one grade for bilateral injuries, up to grade III.
AAST—American Association for the Surgery of Trauma
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 4
Bleeding is coming from right upper quadrant
Take down falciform ligament.
Inspect and palpate liver.
Temporarily control bleeding with packing or Pringle
maneuver, as needed.
Make initial assessment of grade of liver injury.
Minor injury (grade I or II)
Apply topical agents.
Do not drain.
Close abdomen.
Bleeding is
controlled
Close abdomen
without drains.
Moderate to severe injury (grade III, IV, or V);
bleeding is controlled with Pringle maneuver
Moderate to severe injury (grade III, IV, or V);
bleeding is not controlled with Pringle maneuver
Divide coronary and triangular ligaments and
open liver parenchyma as needed to expose injuries.
Apply topical agents to areas with minimal injury.
For superficial injuries, ligate individual bleeding
vessels or close parenchyma with sutures.
Divide coronary and triangular ligaments as needed to
gain exposure.
Use topical agents and buttressed sutures as indicated.
If bleeding persists, use packs, potentially as definitive
treatment.
Bleeding continues
(mostly low pressure
before Pringle maneuver)
Suture bleeding vessels,
even those deep in the
parenchyma.
Pack abdomen if necessary.
Drain as indicated; close
abdomen.
Abdomen is not packed
Bleeding continues (mostly
high pressure before Pringle
maneuver)
Suture bleeding vessels, even
those deep in the parenchyma.
If necessary, ligate right
or left hepatic artery.
Drain as indicated; close
abdomen.
Bleeding is
controlled
Close abdomen
without drains.
Remove packs in
1 or 2 days.
Bleeding continues
Gain exposure as needed with
extension of midline celiotomy into
median sternotomy.
Control bleeding with intrahepatic
balloon tamponade, atriocaval
shunt, or vascular isolation, as
necessary.
Repair injury to hepatic vein or
vena cava.
Drain as indicated; close abdomen.
Abdomen is packed
Remove packs in 1 or 2 days.
Follow for postinjury complications (bleeding, abscess, hemobilia, etc.).
Evaluate and treat with arteriography, embolization, imaging, and drainage, as indicated.
Figure 3
Shown is an algorithm for the treatment of hepatic injuries.
for the temporary control of hemorrhage.3 Over the years, the
length of time for which surgeons believe a Pringle maneuver can
be maintained without causing irreversible ischemic damage to
the liver has increased. Several authors have documented the
maintenance of a Pringle maneuver for longer than 1 hour in
patients with complex injuries, without appreciable hepatic damage.4,10 When a life-threatening hepatic injury is encountered on
entry into the abdomen, the Pringle maneuver should be performed immediately and perihepatic packs placed. Persistent
bleeding in the face of effective inflow occlusion implies that either
the retrohepatic vena cava or hepatic vein has been injured.
Perihepatic packing is more likely to control bleeding from the
retrohepatic vena cava.
Another technique for temporary control of hepatic hemorrhage is the application of a tourniquet or a liver clamp.11 Once the
bleeding hemiliver is mobilized, a 2.5 cm Penrose drain is wrapped
around the liver near the anatomic division between the left
hemiliver and the right. The drain is stretched until hemorrhage
ceases, and tension is maintained by clamping the drain.
Unfortunately, tourniquets are difficult to use: they tend to slip off
or tear through the parenchyma if placed over an injured area. An
alternative is the use of a liver clamp; however, the application of
such devices is hindered by the variability in the size and shape of
the liver. We have not had consistent success with either of these
methods.
Juxtahepatic venous injuries are technically challenging, difficult to control with packing, and often lethal. Complex procedures
may be required for temporary control of these large veins. Of
these procedures, the most important are hepatic vascular isolation with clamps, placement of the atriocaval shunt, and use of the
Moore-Pilcher balloon.
Hepatic vascular isolation is accomplished by executing a
Pringle maneuver, clamping the aorta at the diaphragm, and
clamping the suprarenal and suprahepatic vena cava.12 In patients
scheduled for elective procedures, this technique has enjoyed nearly uniform success, but in trauma patients, the results have been
disappointing. The relative ineffectiveness of hepatic vascular isolation with clamps in this setting is presumably due to the inability of a patient in shock to tolerate an acute reduction in left ventricular filling pressure; on occasion, sudden death has occurred
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 5
Figure 4 The Pringle maneuver controls arterial and portal vein
hemorrhage from the liver. Any hemorrhage that continues must
come from the hepatic veins.
on placement of the venous clamps.13 If, however, a trauma
patient requiring hepatic vascular isolation has been maintained in
a relatively normal physiologic condition, it is reasonable to consider this method.
An alternative approach to exposure of the retrohepatic vena
cava and the hepatic veins has been developed in which vascular
isolation of the liver is achieved by means of clamping and the
suprahepatic vena cava is divided between vascular clamps [see
Figure 7].14 The liver and the suprahepatic vena cava are then
Figure 5 Manual compression of large hepatic injuries temporarily controls blood loss in hypovolemic patients until the circulating blood volume can be restored.
rotated anteriorly to provide direct access to the posterior aspect
of the retrohepatic vena cava. Anterior injuries of the large veins
are repaired through an incision in the posterior aspect of the
retrohepatic vena cava.
The atriocaval shunt was designed to achieve hepatic vascular
isolation while still permitting some venous blood from below the
diaphragm to flow through the shunt into the right atrium.4 After
a few early successes, the initial enthusiasm for the atriocaval shunt
declined as high mortalities associated with its use began to be
reported.15-20 Surgeons’ lack of familiarity with the technique; the
manipulation of a cold, acidotic heart; and poor patient selection
have all contributed to the poor overall results.13 A variation on the
original atriocaval shunt has been described in which a 9 mm
endotracheal tube is substituted for the usual large chest tube [see
Figure 8].21 The balloon of the endotracheal tube makes it unnecessary to surround the suprarenal vena cava with an umbilical
tape. This minor change eliminates one of the most difficult
maneuvers required for the original shunt procedure: because
hemorrhage must be controlled by posterior pressure on the liver
during the insertion of the shunt, access to the suprarenal vena
cava is severely restricted, and thus, surrounding this vessel with
an umbilical tape is almost impossible. A side hole must be cut in
the tube to allow blood to enter the right atrium. Care must be
taken to avoid damage to the integral inflation channel for the
balloon.
An alternative to the atriocaval shunt is the Moore-Pilcher balloon.21 This device is inserted through the femoral vein and
advanced into the retrohepatic vena cava. When the balloon is
properly positioned and inflated, it occludes the hepatic veins and
the vena cava, thus achieving vascular isolation.The catheter itself
is hollow, and appropriately placed holes below the balloon permit
Figure 6 Perihepatic packing is often effective in managing
extensive parenchymal injuries. It has also been successfully
employed for grade V juxtahepatic venous injuries.
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 6
Techniques for Definitive Management of Injuries
Techniques available for the definitive management of hepatic
injuries range from manual compression to hepatic transplantation. Grade I or II lacerations of the hepatic parenchyma can generally be controlled with manual compression. If these injuries do
not respond to manual compression, they can often be controlled
with topical hemostatic measures.
The simplest of these measures is electrocauterization, which
can often control small bleeding vessels near the surface of the
liver (though the machine’s power output may have to be
increased). Bleeding from raw surfaces of the liver that does not
respond to the electrocautery may respond to the argon beam
coagulator. This device imparts less heat to the surrounding
hepatic tissue and creates a more consistent eschar, which
enhances hemostasis. Also useful in similar situations is microcrystalline collagen in the powdered form. The powder is placed
on a clean 10 × 10 cm sponge and applied directly to the oozing
surface, with pressure maintained on the sponge for 5 to 10 minutes. Thrombin can also be applied topically to minor bleeding
injuries by saturating either a gelatin foam sponge or a microcrystalline collagen pad and pressing it to the bleeding site.
In previous years, there was interest in the use of “bathtub” fi-
Azygos Vein
Inferior
Vena Cava
Adrenal Vein
Renal Vein
Figure 7 With hepatic vascular isolation accomplished, the
suprahepatic vena cava is divided between clamps, and the liver
and the suprahepatic vena cava are rotated anteriorly to afford
access to the posterior aspect of the retrohepatic vena cava.
blood to flow into the right atrium, in much the same way as the
atriocaval shunt. At present, the survival rate for patients with juxtahepatic venous injuries who are treated with this device is similar to that for patients treated with the atriocaval shunt.18
Surgeons who attempt hepatic vascular isolation should be
aware that none of these techniques provide complete hemostasis.
Drainage from the right adrenal vein and the inferior phrenic
veins and persistent hepatopetal flow resulting from unrecognized
replacing or accessory left hepatic arteries contribute to this problem. The relatively small volume of blood that continues to flow
after vascular isolation is readily removed by means of suction.
An adjunct to vascular isolation with clamps is venovenous
bypass. This technique provides vascular decompression for the
small bowel and maintains high cardiac filling pressures, which are
often necessary. Venovenous bypass is accomplished by placing a
catheter in the inferior vena cava via the femoral vein and a second catheter in the superior mesenteric vein [see Figure 9].22 A
centrifugal pump withdraws blood from these veins and pumps it
into the superior vena cava through a third catheter placed in the
internal jugular vein.
Figure 8 Shown is a method of achieving hepatic vascular isolation with a 9 mm endotracheal tube.
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 7
To Internal Jugular Vein
Suprahepatic
IVC Clamp
Pump
Figure 9 Shown is venovenous bypass. Catheters are
placed into the inferior vena
cava (IVC) and the superior
mesenteric vein (SMV), and a
centrifugal pump withdraws
blood from these veins and
pumps it into the superior
vena cava via a third catheter
placed into the internal jugular vein.
Pringle
Maneuver
Suprarenal
IVC Clamp
To SMV via IMV
To IVC via Femoral Vein or
Greater Saphenous Vein
brin glue (made by mixing concentrated human fibrinogen with a
solution containing bovine thrombin and calcium) to treat hepatic lacerations.23,24 This substance has now been rendered obsolete
by the commercial availability of numerous glues and sealants [see
Table 2].
Another relatively new hemostatic adjunct that can be highly
useful in the setting of hepatic injury is recombinant activated factor VII (NovoSeven; Novo Nordisk, Copenhagen), which works by
promoting coagulation at the lacerated edges of blood vessels.
Many trauma surgeons have personally witnessed the abrupt cessation of hemorrhage when factor VII has been administered after
other materials have failed. Although this agent seems at times to
have an almost magical effect, it does not always work, and it is
extremely expensive; furthermore, the only prospective study to
date that addressed the use of factor VII in trauma patients reported only a modest decrease in total blood use and failed to demonstrate a survival advantage.25 For these reasons, many institutions,
including ours (University of Colorado Health Sciences Center),
have created protocols for the use of factor VII. At our institution,
for factor VII to be used, (1) the patient must be salvageable; (2) the
patient must have received at least 10 units of packed red blood
cells (PRBCs) plus clotting factors; (3) surgical control of hemorrhage must be achieved; and (4) the patient must still be experiencing diffuse hemorrhage.The usual dose is 60 to 90 µg/kg, which
may be repeated once. It should be kept in mind that factor VII is
not a substitute for fresh frozen plasma and platelets and that adequate amounts of fibrin and platelets must be present for it to work.
Although some grade III and IV lacerations respond to topical
measures, many do not. In these instances, one option is to suture
the hepatic parenchyma. Although this hemostatic technique has
been maligned as a cause of hepatic necrosis, it still is frequently
used.3,4,10,17,26,27 Suturing of the hepatic parenchyma is often
employed to control persistently bleeding lacerations less than 3
cm in depth; it is also an appropriate alternative for deeper lacerations if the patient cannot tolerate the further hemorrhage associated with hepatotomy and selective ligation. If, however, the capsule of the liver has been stripped away by the injury, this technique is far less effective.
The preferred suture material is 0 or 2-0 chromic catgut
attached to a large, blunt-tipped, curved needle; the large diameter prevents the suture from pulling through Glisson’s capsule. For
shallow lacerations, a simple continuous suture may be used to
approximate the edges of the laceration. For deeper lacerations,
interrupted horizontal mattress sutures may be placed parallel to
the edges.When tying sutures, one may be sure that adequate tension has been achieved when hemorrhage ceases or the liver
blanches around the suture.
Most sources of venous hemorrhage can be managed with
parenchymal sutures. Even injuries to the retrohepatic vena cava
and the hepatic veins have been successfully tamponaded by closing the hepatic parenchyma over the bleeding vessels.13,28 Venous
hemorrhage caused by penetrating wounds traversing the central
portion of the liver may be managed by closing the entrance and
exit wounds with interrupted horizontal mattress sutures.
© 2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
7
7 TRAUMA AND THERMAL INJURY
Table 2
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 8
Characteristics of Selected Commercially Available Tissue Glues and Sealants
Tisseel VH*
FloSeal*
BioGlue†
CoSeal*
Contents
Human fibrinogen and thrombin; calcium chloride;
bovine aprotinin
Bovine gelatin and thrombin
Polyethylene glycol
Glutaraldehyde; bovine
albumin
Method of
absorption
(time)
Fibrinolysis (10–14 days)
Cell-mediated inflammation
(6 wk)
Hydrolysis (30 days)
Not absorbed
Physical
properties
Flexible and elastic
Granular; conforms to irregular
surfaces
Clear hydrogel; flexible and elastic
Ridged and inelastic
Preparation
time
7–15 min
1–2 min
1–2 min
1–2 min
General
applications
Tissue sealing and adherence; hemostasis in venous
oozing
Hemostasis in wet fields up to arterial pressure
Tissue sealing in dry fields
Tissue sealing in dry fields
Specific
applications
Venous oozing; sealing of
staple lines; decortication;
pleurodesis
Active bleeding
Sealing of small vessels and synthetic
grafts; prevention of adhesion in pediatric cardiac surgical patients; sealing
of large vessels
Sealing of large vessels
Means of
application
Cannula; spray; minimally invasive surgery
Cannula; minimally invasive
surgery; 8 cm or 10 cm bulb tip
Flexible cannula; spray; minimally invasive surgery
Cannula
Limiting factors
Arterial pressure
Does not seal
Wet field
Wet field, nerves
Set times
3 min
2 min
1 min
30 sec
Stability
4 hr
2 hr
2 hr
—
*Baxter
International, Deerfield, Illinois.
Inc., Kennesaw, Georgia.
†CryoLife,
Although this measure may lead to the formation of intrahepatic
hematomas that may then become infected, the risk is reasonable
compared with the risks posed by an intracaval shunt or a deep
hepatotomy. Still, suturing of the hepatic parenchyma is not always
successful in controlling hemorrhage, particularly hemorrhage
from the larger branches of the hepatic artery. If it fails, one must
acknowledge the failure promptly and remove the sutures so that
the wound can be explored.
Hepatotomy with selective ligation of bleeding vessels is an
important technique that is usually reserved for deep or transhepatic penetrating wounds. Most authorities prefer it to parenchymal suturing3,4,10,29,30; some even favor it over placement of an atriocaval shunt for exposure and repair of juxtahepatic venous
injuries.20 The finger-fracture technique is used to extend the
length and depth of a laceration or a missile tract until the bleeding vessels can be identified and controlled [see Figure 10]. It
should be remembered that considerable blood loss may be
incurred with the division of viable hepatic tissue in the pursuit of
bleeding from deep penetrating wounds. As an alternative to finger
fracture, we have begun to use the LigaSure vessel sealing system
(Valleylab, Boulder, Colorado) and have observed significant
decreases in blood loss with this device.
An adjunct to parenchymal suturing or hepatotomy is the use of
the omentum to fill large defects in the liver and to buttress hepatic sutures.The rationale for this use of the omentum is that it provides an excellent source for macrophages and fills a potential dead
space with viable tissue.31 In addition, the omentum can provide a
little extra support for parenchymal sutures, often enough to prevent them from cutting through Glisson’s capsule.
Hepatic arterial ligation may be appropriate for patients with
arterial hemorrhage from deep within the liver32; however, it plays
only a limited role in the overall treatment of hepatic injuries,
because it does not stop hemorrhage from the portal and hepatic
venous systems.33 Its primary role is in the management of deep
injuries when application of the Pringle maneuver results in the cessation of arterial hemorrhage. If the bleeding from the wound stops
once the left or right hepatic artery is isolated and clamped, hepatic arterial ligation is a reasonable alternative to deep hepatotomy. Generally, ligation of the right or left hepatic artery is well
tolerated; however, ligation of the proper hepatic artery (distal to the
origin of the gastroduodenal artery) may produce hepatic necrosis.
An alternative to suturing the entrance and exit wounds of a
transhepatic penetrating injury or to performing an extensive hepatotomy is the use of an intrahepatic balloon.34 These devices are
hand-crafted by the surgeon in the operating room. One method
of fashioning such a device is to tie a 2.5 cm Penrose drain to a hollow catheter [see Figure 11]. The balloon is then inserted into the
bleeding wound and inflated with a soluble contrast agent. If the
hemorrhage is controlled, a stopcock or clamp is used to occlude
the catheter and maintain the inflation. (It should be noted that the
balloon catheter may not be able to generate sufficient intraparenchymal pressure to tamponade major arterial hemorrhage.)
The balloon is left in the abdomen and removed at a subsequent
operation after 24 to 48 hours. The hemorrhage may recur when
the balloon is deflated.
Resectional debridement is indicated for peripheral portions of
nonviable hepatic parenchyma. Except in rare circumstances, the
amount of tissue removed should not exceed 25% of the liver.
Resectional debridement is performed by means of the finger-fracture technique and is appropriate for selected patients with grade
III to grade V lacerations. Because additional blood loss occurs
during removal of nonviable tissue, this procedure should be
reserved for patients who are in sound physiologic condition and
can tolerate additional blood loss.
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 9
Figure 10 Hepatotomy with selective ligation is an important
technique for controlling hemorrhage from deep (usually penetrating) lacerations. This technique includes finger fracture to
extend the length and depth of the wound until vessels or ducts are
encountered and controlled.
Perihepatic packing is the most significant advance in the treatment of hepatic injuries to occur in the past 25 years.The practice
of packing hepatic injuries is not a new one, but the concepts and
techniques associated with it have changed. In the past, liver lacerations were packed with yards of gauze, and one end of the gauze
strip was brought out of the abdomen through a separate stab
wound35; the remainder of the gauze was then teased out of the
wound over a period of days. Unfortunately, this approach often
led to abdominal infection and failed to control the hemorrhage,
and as a result, it eventually fell from favor. The current approach
is not to place packing material in the laceration itself but rather to
place it over and around the injury to compress the wound by
compressing the liver between the anterior chest wall, the
diaphragm, and the retroperitoneum.5-9 The abdomen is closed,
and the patient is taken to the surgical intensive care unit for resuscitation and correction of metabolic derangements. Within 24
hours, the patient is returned to the OR for removal of the packs.
Perihepatic packing is indicated for grade IV and V lacerations and
for less severe injuries in patients who have a coagulopathy caused
by associated injuries.
A technique that may be attempted if packing fails is to wrap the
injured portion of the liver with a fine porous material (e.g., polyglycolic acid mesh) after the injured hemiliver has been mobilized.36,37 Using a continuous suture or a linear stapler, the surgeon
constructs a tight-fitting stocking that encloses the injured hemiliver. Blood clots beneath the mesh, which results in tamponade of
the hepatic injury. Although this technique is intuitively attractive,
to date it has achieved only limited success.
The final alternative for patients with extensive injuries to one
hemiliver is anatomic hepatic resection. In elective circumstances,
anatomic hemihepatectomies can be performed with excellent
results; however, in the setting of trauma, the mortality associated
with this procedure exceeds 50% in most series.26,27,29,38-40
Consequently, hepatic resection is rarely performed in trauma
patients, having been largely replaced by perihepatic packing,
resectional debridement, and hepatotomy with selective ligation.
Nonetheless, there are two circumstances in which anatomic resection may still be a reasonable choice. The first is prompt resection
in patients with extensive injuries of the left lateral section of the
liver; because hemorrhage from the left hemiliver is easily controlled with bimanual compression, the risk of uncontrolled blood
loss is not as high as it is with left or right anatomic hemihepatectomies. The second is delayed anatomic hemihepatectomy in
patients whose hemorrhage has been controlled but whose left or
right hemiliver is nonviable as a result of ligation or thrombosis of
essential blood vessels. Because of the large mass of necrotic liver
tissue, there is a high risk of subsequent infection or persistent
hyperinflammation, setting the stage for the multiple organ dysfunction syndrome (MODS). The necrotic hemiliver should be
removed as soon as the patient’s condition permits.
Hepatic transplantation has been successful in several trauma
patients with devastating hepatic injuries who required total hepatectomy.41-44 In each of these five patients, the mean anhepatic
period was approximately 24 hours. All five survived the transplantation, though two died of disseminated viral infections within
2 months of the procedure.Two others were alive and well 16 and
17 months after the procedure; no follow-up was reported for the
fifth patient. Hepatic transplantation represents the ultimate
expression of aggressive trauma care. All other injuries must be
well delineated (particularly injuries to the CNS), and the patient
must have an excellent chance of survival aside from the hepatic
injury. High cost and limited availability of donors restrict the performance of hepatic transplantation for trauma, but it seems probable that this procedure will continue to be performed in extraordinary circumstances.
Figure 11 A handmade balloon from a Robinson catheter and a
Penrose drain may effectively control hemorrhage from a transhepatic penetrating wound.
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 10
b
a
Figure 12 (a) The first step in mobilizing the
spleen is to make an incision in the peritoneum
and the endoabdominal fascia, beginning at the
inferior pole and continuing posteriorly and
superiorly. (b) The correct plane of dissection is
between the pancreas and Gerota’s fascia.
Subcapsular Hematoma
An uncommon but troublesome hepatic injury is subcapsular
hematoma, which arises when the parenchyma of the liver is disrupted by blunt trauma but Glisson’s capsule remains intact.
Subcapsular hematomas range in severity from minor blisters on
the surface of the liver to ruptured central hematomas accompanied by severe hemorrhage [see Table 1]. They may be recognized
either at the time of the operation or in the course of CT scanning.
Regardless of how the lesion is diagnosed, subsequent decision
making is often difficult. If a grade I or II subcapsular
hematoma—that is, a hematoma involving less than 50% of the
surface of the liver that is not expanding and is not ruptured—is
discovered during an exploratory laparotomy, it should be left
alone. If the hematoma is explored, hepatotomy with selective ligation may be required to control bleeding vessels. Even if hepatotomy with ligation is effective, one must still contend with diffuse hemorrhage from the large denuded surface, and packing
may also be required. A hematoma that is expanding during operation (grade III) may have to be explored. Such lesions are often
the result of uncontrolled arterial hemorrhage, and packing alone
may not be successful. An alternative strategy is to pack the liver
to control venous hemorrhage, close the abdomen, and transport
the patient to the interventional radiology suite for hepatic arteriography and embolization of the bleeding vessels. Ruptured
grades III and IV hematomas are treated with exploration and
selective ligation, with or without packing.
Perihepatic Drainage
For years, all hepatic injuries were drained via Penrose drains
brought out laterally or through the bed of the resected 12th rib;
recently, the use of large sump drains and closed suction drains
has become increasingly popular. Several prospective and retrospective studies have demonstrated that the use of either Penrose
or sump drains carries a higher risk of intra-abdominal infection
than the use of either closed suction drains or no drains at all.45-47
It is clear that if drains are to be used, closed suction devices are
preferred. What remains unclear, however, is whether closed suction drains are better or worse than no drains, particularly in view
of the advent of percutaneous catheter drainage. Patients who are
initially treated with perihepatic packing may also require
drainage; however, drainage is not indicated at the initial procedure, given that the patient will be returned to the OR within the
next 48 hours.
MORTALITY AND COMPLICATIONS
Overall mortality for patients with hepatic injuries is approximately 10%.The most common cause of death is exsanguination,
followed by MODS and intracranial injury. Three generalizations
may be made regarding the risk of death and complications: (1)
both increase in proportion to the injury grade and to the complexity of repair; (2) hepatic injuries caused by blunt trauma carry
a higher mortality than those caused by penetrating trauma; and
(3) infectious complications occur more often with penetrating
trauma.48
Postoperative hemorrhage occurs in a small percentage of
patients with hepatic injuries. The source may be either a coagulopathy or a missed vascular injury (usually to an artery). In most
instances of persistent postoperative hemorrhage, the patient is
best served by being returned to the OR. Arteriography with
embolization may be considered in selected patients. If coagula-
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 11
tion studies indicate that a coagulopathy is the likely cause of postoperative hemorrhage, there is little to be gained by reoperation
until the coagulopathy is corrected.
Perihepatic infections occur in fewer than 5% of patients with
significant hepatic injuries. They develop more often in patients
with penetrating injuries than in patients with blunt injuries, presumably because of the greater frequency of enteric contamination. An elevated temperature and a higher than normal white
blood cell count after postoperative day 3 or 4 should prompt a
search for intra-abdominal infection. In the absence of pneumonia,
an infected line, or urinary tract infection, an abdominal CT scan
with intravenous and upper gastrointestinal contrast should be
obtained. Many perihepatic infections can be treated with CTguided drainage; however, infected hematomas and infected
necrotic liver tissue cannot be expected to respond to percutaneous drainage. Right 12th rib resection remains an excellent
approach for posterior infections and provides superior drainage in
refractory cases.
Bilomas are loculated collections of bile that may or may not be
infected. If a biloma is infected, it is essentially an abscess and
should be treated as such; if it is sterile, it will eventually be
resorbed. Biliary ascites is caused by disruption of a major bile
duct. Reoperation after the establishment of appropriate drainage
is the prudent course. Even if the source of the leaking bile can be
identified, primary repair of the injured duct is unlikely to be successful. It is best to wait until a firm fistulous communication is
established with adequate drainage.
Biliary fistulas occur in approximately 3% of patients with major
hepatic injuries.40 They are usually of little consequence and generally close without specific treatment. In rare instances, a fistulous
communication with intrathoracic structures forms in patients
with associated diaphragmatic injuries, resulting in a bronchobiliary or pleurobiliary fistula. Because of the pressure differential
between the biliary tract and the thoracic cavity, most of these fistulas must be closed operatively; however, we know of one pleurobiliary fistula that closed spontaneously after endoscopic sphincterotomy and stent placement.
Hemorrhage from hepatic injuries is often treated without identifying and controlling each bleeding vessel individually, and arterial pseudoaneurysms may develop as a consequence. As the
pseudoaneurysm enlarges, it may rupture into the parenchyma of
the liver, into a bile duct, or into an adjacent branch of the portal
vein. Rupture into a bile duct results in hemobilia, which is characterized by intermittent episodes of right upper quadrant pain,
upper GI hemorrhage, and jaundice; rupture into a portal vein
may result in portal vein hypertension with bleeding varices. Both
of these complications are exceedingly rare and are best managed
with hepatic arteriography and embolization.
Injuries to the Bile Ducts and Gallbladder
Injuries to the extrahepatic bile ducts [see Table 1] can be caused
by either penetrating or blunt trauma; however, they are rare in
either case.49-53
The diagnosis is usually made by noting the accumulation of
bile in the upper quadrant during laparotomy for treatment of
associated injuries.Treatment of common bile duct (CBD) injuries
after external trauma is complicated by the small size and thin wall
of the normal duct, which render primary repair almost impossible except when the laceration is small and there is no tissue loss.
When there is tissue loss or the laceration is larger than 25% to
50% of the diameter of the duct, the best treatment option is a
Roux-en-Y choledochojejunostomy [see 5:22 Procedures for Benign
and Malignant Biliary Tract Disease].54-57 Treatment of injuries to
the left or right hepatic duct is even more difficult—so much so
that we question whether repair should even be attempted under
emergency conditions. If only one hepatic duct is injured, a reasonable approach is to ligate it and deal with any infections or atrophy of the hemiliver rather than to attempt repair.58 If both ducts
are injured, each should be intubated with a small catheter brought
through the abdominal wall. Once the patient has recovered sufficiently, delayed repair is performed under elective conditions.
Injuries to the intrapancreatic portion of the CBD are treated by
dividing the duct at the superior border of the pancreas, ligating
the distal portion, and performing a Roux-en-Y choledochojejunostomy.
The Roux-en-Y choledochojejunostomy is done in a single layer
with interrupted 5-0 absorbable monofilament sutures.To prevent
ischemia and possible stricture, no circumferential dissection of the
duct is performed. A round patch of approximately the same diameter as the CBD is removed from the seromuscular layer of the
small bowel, but the mucosa and submucosa are only perforated,
not resected. The posterior row of sutures is placed first, with fullthickness bites taken through both the duct and the small bowel.
The anterior row is then completed. Finally, three or four 3-0
polypropylene sutures are placed to secure the small bowel around
the anastomosis to the connective tissue of the porta hepatis. The
only purpose for these sutures is to spare the fragile anastomosis
any potential tension. No T tubes or stents are employed. Closed
suction drainage is added in the case of injuries to the intrapancreatic portion of the duct or at the surgeon’s discretion.
Injuries to the gallbladder [see Table 1] are treated by means of
either lateral repair with absorbable sutures or cholecystectomy [see
5:21 Cholecystectomy and Common Bile Duct Exploration]; the decision between the two approaches depends on which is easier in a
given situation. Cholecystostomy is rarely, if ever, indicated.
Injuries to the Spleen
Splenic injuries [see Table 1] are treated operatively by means of
splenic repair (splenorrhaphy), partial splenectomy, or resection,
depending on the extent of the injury and the condition of the
patient.57,58 The continued enthusiasm for nonoperative management of splenic injuries is driven, in part, by concern about the rare
but often fatal complication known as overwhelming postsplenectomy infection (OPSI). OPSI is caused by encapsulated bacteria
(e.g., Streptococcus pneumoniae, Haemophilus influenzae, and
Neisseria meningitidis) and is very resistant to treatment: mortality
may exceed 50%. OPSI occurs most often in young children and
immunocompromised adults and is uncommon in otherwise
healthy adults. For this reason, splenic salvage is attempted more
vigorously in pediatric patients than in adult ones [see Discussion,
Nonoperative Management of Blunt Hepatic and Splenic Injuries,
below].
To ensure safe removal or repair, the spleen should be mobilized
to the point where it can be brought to the surface of the abdominal wall without tension. To this end, the soft tissue attachments
between the spleen and the splenic flexure of the colon must be
divided. Next, an incision is made in the peritoneum and the
endoabdominal fascia, beginning at the inferior pole, 1 to 2 cm lateral to the posterior peritoneal reflection of the spleen, and continuing posteriorly and superiorly until the esophagus is encountered [see Figure 12a]. Care must be taken not to pull on the spleen,
so that it will not tear at the posterior peritoneal reflection, causing
significant hemorrhage. Instead, the spleen should be rotated
counterclockwise, with posterior pressure applied to expose the
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 12
peritoneal reflection. It is often helpful to rotate the operating table
20° to the patient’s right so that the weight of the abdominal viscera facilitates their retraction. A plane is thus established between
the spleen and pancreas and Gerota’s fascia that can be extended
to the aorta [see Figure 12b]. With this step, mobilization is complete, and the spleen can be repaired or removed without any need
to struggle to achieve adequate exposure.
Splenectomy [see 5:25 Splenectomy] is the usual treatment for
hilar injuries or a pulverized splenic parenchyma. It is also indicated for lesser splenic injuries in patients who have multiple
abdominal injuries and a coagulopathy, and it is frequently necessary in patients in whom splenic salvage attempts have failed.
Partial splenectomy is suitable for patients in whom only a portion
of the spleen (usually the superior or inferior half) has been
destroyed. Once the damaged portion has been removed, the same
methods used to control hemorrhage from hepatic parenchyma
can be used to control hemorrhage from splenic parenchyma [see
Figure 13]. When horizontal mattress sutures are placed across a
raw edge, gentle compression of the parenchyma by an assistant
facilitates hemostasis; when the sutures are tied and compression
is released, the spleen will expand slightly and tighten the sutures
further. Drains are never used after completion of the repair or
resection.
If splenectomy is performed, vaccines effective against the
encapsulated bacteria are administered. The pneumococcal vaccine is routinely given, and vaccines effective against H. influenzae
and N. meningitidis should also be given if available.
Injuries to the Diaphragm
In cases of blunt trauma to the diaphragm, the injury is on the
left side 75% of the time, presumably because the liver diffuses
some of the energy on the right side. With both blunt and penetrating injuries [see Table 1], the diagnosis is suggested by an abnormality of the diaphragmatic shadow on chest x-ray. Many of these
abnormalities are subtle, particularly with penetrating injuries, and
further diagnostic evaluation may be warranted.The typical injury
from blunt trauma is a tear in the central tendon; often, the tear is
quite large. Regardless of the cause, acute injuries are repaired
Figure 13 Methods for controlling hemorrhage from the splenic
parenchyma are similar to those for controlling hemorrhage from
the hepatic parenchyma. Shown are interrupted mattress sutures
across a raw edge of the spleen.
through an abdominal incision. Because of the concave shape of
the diaphragm and the overlying anterior ribs, anterior diaphragmatic injuries may be difficult to suture. Repair is greatly facilitated by using a long Allis clamp to grasp part of the injury and evert
the diaphragm. Lacerations are repaired with continuous No. 1
monofilament nonabsorbable sutures. Occasionally, with large
avulsions or gunshot wounds accompanied by extensive tissue loss,
polypropylene mesh is required to bridge the defect.
The explosive growth of laparoscopic procedures has led to the
application of this technology for both diagnostic and therapeutic
purposes in trauma patients. In a number of patients with low
anterior thoracic stab wounds who otherwise were not candidates
for a laparotomy, small diaphragmatic lacerations have been identified and repaired with laparoscopy and stapling.
Discussion
Nonoperative Treatment of Blunt Hepatic and Splenic
Injury
Only a few years ago, blunt and penetrating hepatic and splenic
injuries were managed in a similar fashion on the basis of a positive diagnostic peritoneal lavage or the probability of peritoneal
penetration: a laparotomy was performed, and the injured organs
were identified and treated. Currently, although penetrating
abdominal injuries are still treated in the same way, nearly all children and 50% to 80% of adults with blunt hepatic and splenic
injuries are treated without laparotomy.59-68 This remarkable
change was made possible by the development of the high-speed
helical CT scanner, the replacement of diagnostic peritoneal
lavage by ultrasonography, and the growth of interventional
radiology.
The diagnosis of blunt abdominal trauma is suspected on the
basis of the mechanism of injury and the presence of associated
injuries (e.g., right or left lower rib fractures). Ultrasonographic
examination of the abdomen may reveal a fluid stripe in
Morrison’s pouch, the left upper quadrant, or the pelvis, which
suggests a hemoperitoneum.This observation prompts a CT scan
of the abdomen, which establishes the presence or absence of
injuries to the liver or the spleen and, to some degree, serves as a
means of grading the severity of organ injury. Patients may be
observed either in the SICU or on the ward, depending on the
apparent severity of the parenchymal injury on the CT scan, the
presence and extent of any associated injuries, and the overall
hemodynamic status.69,70
The primary requirement for nonoperative therapy is hemodynamic stability.63-72 To confirm stability, frequent assessment of
vital signs and monitoring of the hematocrit are necessary.
Continued hemorrhage occurs in 1% to 4% of patients.65,66,68-73
Hypotension may develop, usually within the first 24 hours after
hepatic injury but sometimes several days later, especially when
splenic injury is present.71,72 It is often an indication that operative intervention is necessary. A persistently falling hematocrit
should be treated with PRBC transfusions. If the hematocrit con-
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 13
tinues to fall after two or three units of PRBCs, embolization of
the liver in the interventional radiology suite should be considered.66 Overall, nonoperative treatment obviates laparotomy in
more than 95% of cases.59-65
Out of concern over the risk of delayed hemorrhage or other
complications, follow-up CT scans have often been recommended; unfortunately, there is no consensus as to when or even
whether they should be obtained. Given that patients with grade I
or II hepatic or splenic injuries rarely show progression of the
lesion or other complications on routine follow-up CT scans, it is
reasonable to omit such scans if patients’ hematocrits remain stable and they are otherwise well. Patients with more extensive
injuries often have a less predictable course, and CT scanning
may be necessary to evaluate possible complications. Routine
scanning before discharge, however, is unwarranted. On the other
hand, patients who participate in vigorous or contact sports
should have CT documentation of virtually complete healing
before resuming those activities.
A more convenient and less expensive alternative to follow-up
CT scanning is ultrasonographic monitoring of lesions.
Ultrasonographic monitoring is particularly useful for following
up splenic injuries; however, it may not be useful for following up
hepatic injuries, because the technology currently available is incapable of reliably imaging the entire liver.
Other complications of nonoperative therapy for blunt hepatic
and splenic injuries occur in 2% to 5% of patients; these include
missed abdominal injuries, parenchymal infarction, infection, and
bile leakage (a complication associated solely with hepatic
injuries).59,62-64 Aseptic infarcts, infected hematomas, and bile collections are suspected on the basis of a clinical picture suggestive of
infection and confirmed by CT-guided aspiration. Aseptic infarction usually does not necessitate operative intervention. Fluid collections are drained, with the method depending on the viscosity of
the fluid: CT-guided drainage may be effective in treating thin collections, but operative intervention is required for thicker collections, those with solid components, and those for which percutaneous drainage was attempted without success. Extrahepatic bile
collections should be treated with percutaneous drainage under
CT guidance. Most biliary fistulas close spontaneously; endoscopic stent placement may hasten closure in recalcitrant cases.74
Intrahepatic collections of blood and bile are managed expectantly. Complete absorption of large intrahepatic collections may take
several months. If a collection becomes infected, CT-guided aspiration is performed and drainage obtained as described.
Missed enteric and retroperitoneal injuries are another cause of
failed nonoperative treatment. Such injuries are present in 1%
to 4% of patients in whom nonoperative treatment is attempted.59,61-64 High-quality images and expert interpretation minimize
the number of missed injuries on CT scans but cannot eliminate
them entirely. Therefore, patients must be watched carefully for
the development of peritoneal irritation and other signs of intraabdominal pathology.
References
1. Moore EE, Cogbill TH, Jurkovich GJ, et al:
Organ injury scaling: spleen and liver (1994 revision). J Trauma 38:323, 1995
2. Hepatic trauma revisited. Feliciano DV, Pachter
HL, Eds. Curr Probl Surg 26, 1986
3. Moore EE: Critical decisions in the management of hepatic trauma. Am J Surg 148:712,
1984
4. Feliciano DV, Mattox KL, Jordan GL, et al:
Management of 1000 consecutive cases of
hepatic trauma (1979–1984). Ann Surg
204:438, 1986
5. Feliciano DV, Mattox KL, Burch JM, et al:
Packing for control of hepatic hemorrhage. J
Trauma 26:738, 1986
6. Ivantury RR, Nallathambi M, Gunduz Y, et al:
Liver packing for uncontrolled hemorrhage: a
reappraisal. J Trauma 26:744, 1986
caval shunt: facts and fiction. Ann Surg 207:555,
1988
14. Buechter KJ, Gomez GA, Zeppa R: A new technique for exposure of injuries at the confluence
of the retrohepatic veins and the retrohepatic
vena cava. J Trauma 30:328, 1990
15. Schrock T, Blaisdell FW, Matthewson C Jr:
Management of blunt trauma to the liver and
hepatic veins. Arch Surg 96:698, 1968
16. Bricker DL, Morton JR, Okies JE, et al: Surgical
management of injuries to the vena cava: changing patterns of injury and newer techniques of
repair. J Trauma 11:725, 1971
17. Yellin AE, Chaffee CB, Donovan AJ: Vascular
isolation in treatment of juxtahepatic venous
injuries. Arch Surg 102:566, 1971
18. Walt AJ: The mythology of hepatic trauma: or
Babel revisited. Am J Surg 125:12, 1978
7. Carmona RH, Peck DZ, Lim RC: The role of
packing and planned reoperation in severe
hepatic trauma. J Trauma 24:779, 1984
19. Millikan JS, Moore EE, Cogbill TH, et al:
Inferior vena cava injuries: a continuing challenge. J Trauma 23:207, 1983
8. Cue JI, Cryer HG, Miller FB, et al: Packing and
planned reexploration for hepatic and retroperitoneal hemorrhage: critical refinements of a useful technique. J Trauma 30:1007, 1990
20. Pachter HL, Spencer FC, Hofstetter SR, et al:
The management of juxtahepatic venous injuries
without an atriocaval shunt. Surgery 99:569,
1986
9. Beal SL: Fatal hepatic hemorrhage: an unresolved problem in the management of complex
liver injuries. J Trauma 30:163, 1990
21. Pilcher DB, Harman PK, Moore EE:
Retrohepatic vena cava balloon shunt introduced via the sapheno-femoral junction. J
Trauma 17:837, 1977
10. Pachter HL, Spencer FC, Hofstetter SR, et al:
Significant trends in the treatment of hepatic
trauma: experience with 411 injuries. Ann Surg
215:492, 1992
11. Murray DH Jr, Borge JD, Pouteau GG:
Tourniquet control of liver bleeding. J Trauma
18:771, 1978
12. Heaney JP, Stanton WR, Halbert DS, et al: An
improved technic for vascular isolation of the
liver. Ann Surg 163:237, 1966
13. Burch JM, Feliciano DV, Mattox KL: The atrio-
22. Biffl WL, Moore EE, Franciose RJ: Venovenous
bypass and hepatic vascular isolation as adjuncts
in the repair of destructive wounds to the retrohepatic inferior vena cava. J Trauma 45:400,
1998
patic wounds: case reports. J Trauma 31:408, 1991
25. Boffard KD, Riou B, Warren B, et el:
Recombinant factor VIIa as adjunctive therapy
for bleeding control in severely injured trauma
patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma
59:8, 2005
26. Ochsner MG, Maniscalco-Theberge ME,
Champion HR: Fibrin glue as a hemostatic
agent in hepatic and splenic trauma. J Trauma
30:884, 1990
27. Trunkey DD, Shires GT, McClelland R:
Management of liver trauma in 811 consecutive
patients. Ann Surg 179:722, 1974
28. Levin A, Gover P, Nance FC: Surgical restraint
in the management of hepatic injury: a review of
Charity Hospital experience. J Trauma 18:399,
1978
29. Lucas CE, Ledgerwood AM: Prospective evaluation of hemostatic techniques for liver injuries.
J Trauma 16:442, 1976
30. Camona RH, Lim RC Jr, Clark GC: Morbidity
and mortality in hepatic trauma: a 5 year study.
Am J Surg 144:88, 1982
31. Moore FA, Moore EE, Seagrave A:
Nonresectional management of major hepatic
trauma: an evolving concept. Am J Surg
150:725, 1985
32. Stone HH, Lamb JM: Use of pedicled omentum
as an autogenous pack for control of hemorrhage in major injuries of the liver. Surg Gynecol
Obstet 141:92, 1975
33. Mays ET: Lobar dearterialization for exsanguinating wounds of the liver. J Trauma 12:397,
1972
23. Kram HB, Nathan RC, Stafford FJ, et al: Fibrin
glue achieves hemostasis in patients with coagulation disorders. Arch Surg 124:385, 1989
34. Flint LM, Polk HC: Selective hepatic artery ligation: limitations and failures. J Trauma
19:319, 1979
24. Berguer R, Staerkel RL, Moore EE, et al:Warning:
fatal reaction to the use of fibrin glue in deep he-
35. Poggetti RS, Moore EE, Moore FA, et al:
Balloon tamponade for bilobar transfixing
© 2006 WebMD, Inc. All rights reserved.
7 TRAUMA AND THERMAL INJURY
hepatic gunshot wounds. J Trauma 33:694, 1992
ACS Surgery: Principles and Practice
7
INJURIES TO LIVER, BILIARY TRACT, SPLEEN, AND DIAPHRAGM — 14
drains in liver trauma. J Trauma 28:337, 1988
ma: identification of patterns of injury. J Trauma
39:344, 1995
36. Madding GF, Lawrence KB, Kennedy PA: War
wounds of the liver. Tex State J Med 42:267,
1946
51. Fabian TC, Croce MA, Stanford GG, et al:
Factors affecting morbidity after hepatic trauma.
Ann Surg 213:540, 1991
37. Reed RL, Merrell RC, Meyers WC, et al:
Continuing evolution in the approach to severe
liver trauma. Ann Surg 216:524, 1992
52. Posner MC, Moore EE: Extrahepatic biliary
tract injury: operative management plan. J
Trauma 25:833, 1985
38. Jacobson LE, Kirton OC, Gomez GA:The use of
an absorbable mesh wrap in the management of
major liver injuries. Surgery 111:455, 1992
53. Ivatury RR, Rohman M, Nallathami M, et al:
The morbidity of injuries of the extra-hepatic biliary system. J Trauma 25:967, 1985
67. Powell M, Courcoulas A, Gardner M, et al:
Management of blunt splenic trauma: significant
differences between adults and children. Surgery
122:654, 1997
39. Lim RC Jr, Knudson J, Steele M: Liver trauma:
current method of management. Arch Surg
104:544, 1972
54. Sheldon GF, Lim RC,Yee ES, et al: Management
of injuries to the porta hepatis. Ann Surg
202:539, 1985
68. Richardson JD: Changes in the management of
injuries to the liver and spleen. J Am Coll Surg
200:648, 2005
40. Donovan AJ, Michaelian MJ, Yellin AE:
Anatomical hepatic lobectomy in trauma to the
liver. Surgery 73:833, 1973
55. Feliciano DV, Bitondo CG, Burch JM, et al:
Management of traumatic injuries to the extrahepatic biliary ducts. Am J Surg 150:705, 1985
41. Defore WW, Mattox KL, Jordan GL, et al:
Management of 1590 consecutive cases of liver
trauma. Arch Surg 111:493, 1976
56. Bade PG, Thomson SR, Hirshberg A, et al:
Surgical options in traumatic injury to the extrahepatic biliary tract. Br J Surg 76:256, 1989
69. Sclafani SJA, Shaftan GW, Scalea TM, et al:
Nonoperative salvage of computed tomography–diagnosed splenic injuries: utilization of
angiography for triage and embolization for
hemostasis. J Trauma 39:818, 1995
42. Esquivel CO, Bernardos A, Makowka L, et al:
Liver replacement after massive hepatic trauma.
J Trauma 27:800, 1987
57. Csendes A, Diaz JC, Burdiles P, et al: Late results
of immediate primary end to end repair in accidental section of the common bile duct. Surg
Gynecol Obstet 168:125, 1989
43. Angstadt J, Jarrell B, Moritz M, et al: Surgical
management of severe liver trauma: a role for
liver transplantation. J Trauma 29:606, 1989
44. Ringe B, Pichlmayr R, Ziegler H, et al:
Management of severe hepatic trauma by twostage total hepatectomy and subsequent liver
transplantation. Surgery 109:792, 1991
45. Jeng LB, Hsu C, Wang C, et al: Emergent liver
transplantation to salvage a hepatic avulsion
injury with a disrupted suprahepatic vena cava.
Arch Surg 128:1075, 1993
46. Fischer RP, O’Farrell KA, Perry JF Jr: The value
of peritoneal drains in the treatment of liver
injuries. J Trauma 18:393, 1978
47. Noyes LD, Doyle DJ, McSwain NE: Septic complications associated with the use of peritoneal
drains in liver trauma. J Trauma 28:337, 1988
48. Kozar RA, Moore FA, Cothren CC, et al:
Predicting hepatic-related morbidity associated
with nonoperative management of complex
blunt hepatic injuries: a multicenter trial. Arch
Surg (in press)
58. Howdieshell TR, Hawkins ML, Osler TM, et al:
Management of blunt hepatic duct transection
by ligation. South Med J 83:579, 1990
59. Barrett J, Sheaff C, Abuabara S, et al: Splenic
preservation in adults after blunt and penetrating
trauma. Am J Surg 145:313, 1983
60. Feliciano DV, Spjut-Patrinely V, Burch JM, et al:
Splenorrhaphy: the alternative. Ann Surg
211:569 1990
61. Cogbill TH, Moore EE, Jurkovich JJ, et al:
Nonoperative management of blunt septic trauma: a multicenter experience. J Trauma 29:1312,
1989
62. Meredith JW, Young JS, Bowling J, et al:
Nonoperative management of blunt hepatic trauma: the exception or the rule? J Trauma 36:529,
1994
63. Pachter HL, Hofstetter ST: The current status of
nonoperative management of adult blunt hepatic
injuries. Am J Surg 169:442, 1995
49. Jurkovich GJ, Hoyt DB, Moore FA, et al: Portal
triad injuries: a multi-institutional study. J
Trauma 39:426, 1995.
64. Croce MA, Fabian TC, Menke PG, et al:
Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Ann Surg 221:744, 1995
50. Noyes LD, Doyle DJ, McSwain NE: Septic complications associated with the use of peritoneal
65. Boone DC, Federle M, Billiar TR, et al:
Evolution of management of major hepatic trau-
66. Pachter HL, Knudson MM, Esrig B, et al: Status
of nonoperative management of blunt hepatic
injuries in 1995: a multicenter experience with
404 patients. J Trauma 40:31, 1996
70. Malhotra AK, Fabian TC, Crou MA, et al: Blunt
hepatic injury: a paradigm shift from operative to
nonoperative management in the 1990’s. Ann
Surg 231:804, 2000
71. Sutyak JP, Chiu WC, D’Amelio LF, et al:
Computed tomography is inaccurate in estimating the severity of adult splenic injury. J Trauma
39:514, 1995
72. Croce MA, Fabian TC, Kudsk KA, et al: AAST
organ injury scale: correlation of CT-graded liver
injuries and operative findings. J Trauma 31:806,
1991
73. Gates JD: Delayed hemorrhage with free rupture
complicating the nonsurgical management of
blunt hepatic trauma: a case report and review of
the literature. J Trauma 36:572, 1994
74. Sugimoto K, Asari Y, Sakaguchi T, et al:
Endoscopic retrograde cholangiography in the
nonsurgical management of blunt liver injury. J
Trauma 35:192, 1993
Acknowledgments
Figure 1 Tom Moore.
Figures 2, 7, and 9 Thom Graves.
Figure 3 Marcia Kammerer.
Figures 4 through 6, 8, and 10 through 13 Susan
Brust, C.M.I.
Table 2 Information provided by Baxter International,
Deerfield, Illinois.