PerioCol -TC - Eucare Pharmaceuticals Private Limited
advertisement

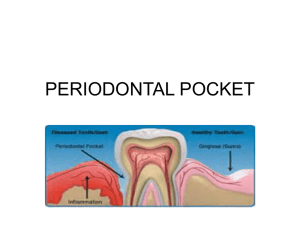
® PerioCol -TC Sterile Medicated Periodontal Collagen Fibers A cost effective solution in the management of Periodontitis & Gingivitis Introduction Periodontitis, a disease of periodontium initiated in the gingiva can be managed by several therapeutic approaches. The use of sustained release local drug delivery is used widely in various fields of medicine in the new era including periodontal therapy. The classical treatment of Periodontitis consists of surgical and non-surgical management. Surgical management is done for pockets greater than 4 mm. Non-surgical managements include supra and sub gingival professional plaque and calculus removal. It seems that local application of antimicrobials like mouthwashes and gels that are effective against periodontal pathogens, can reduce the pocket depth. However, the disadvantage of the system is that the exact concentration of antimicrobial cannot be achieved at the site of application and their access to periodontal pocket and the sub gingival flora is limited. A new approach using local delivery systems containing antimicrobial is being introduced to produce more constant and prolonged concentration profiles. The periodontal pocket provides a natural reservoir bathed by gingival crevicular fluid that is easily accessible for the insertion of a delivery device. The gingival crevicular fluid provides a leaching medium for the release of the drug from the solid dosage form and its distribution throughout the pocket. The first generation non-degradable devices have been replaced by the second-generation degradable devices possessing great advantage of requiring the patient to pay only a single visit to the therapists for insertion of the device, thus minimizes the patient visits and ensures compliance. Collagen, the biomaterial known for its excellent wound healing properties and biocompatibility has been used as a carrier device that is biodegradable. Tetracycline Hydrochloride with its proven usage in dental systems as a safe and powerful antimicrobial agent has been selected and an efficient sustained release system is being offered to contain the problematic periodontitis. Description Each PerioCol®-TC vial contains Type1, fibrillar collagen of fish origin of approximately 25 mg, impregnated with approx. 2.0mg of Tetracycline Hydrochloride IP, sterilized by gamma irradiation. Periocol-TC releases tetracycline invitro for a period of 8 to 12 days. Collagen Collagen is an extra cellular matrix protein playing a major role in connective tissue. It is the most abundant protein in human and performs multiple functions. In fish , the largest concentration of collagen is found in the skeleton, fins, skin and air bladder. The role of collagen as a vehicle for drug delivery is a well documented subject. It is biocompatible and absolutely safe for human applications. Since PerioCol ®-TC is derived from fish sources, there is no threat of BSE/TSE. ® PerioCol -TC Tetracycline Hydrochloride It is an antibiotic isolated from St. Aureofaciens. Chemically it is the hydrochloride of 4 - dimethylamino - 1, 4, 4a,5,5a,6,11,12a-octahydro3,6,10,12,12a- pentahydroxy-6- methyl - 1,11 - di oxo - 2- napthacene carboxamide. Action: It is primarily bacteriostatic and thought to exert its anti microbial effect by the inhibition of protein synthesis. It is active against a wide range of gram -ve and gram +ve organisms. It is really absorbed and bound to plasma proteins in varying degrees. This drug is contra indicated in persons who have shown hypersensitivity to any of the tetracyclines. Indications Adult periodontitis and an adjunct to scaling and root planning. For periodontal maintenance program Patients unfit for Periodontal surgical procedures due to various systemic conditions. Contraindications ® PerioCol -TC should not be used in any patient with a known sensitivity to Tetracycline Hydrochloride and collagen Not recommended in acutely abscessed periodontal pocket Application The fibers are moistened with normal saline and inserted into the periodontal pocket to closely approximate the pocket anatomy and should be in contact with the base of the pocket. To avoid dislodging of the fibers, periodontal dressing is placed. The fibers need not be removed at any time after placement since they are completely biodegradable. The treatment can be repeated once in three months in pocket measuring more than 5 mm deep. If the placed fibers accidentally fall off within a short duration, new dose of fibers should be inserted. Storage PerioCol®-TC must be stored in a dry place at between 5° C and 25° C. It is gamma sterlized with a shelf life of 2 years. Ordering Information PTCS 1001-05 SERVE THE HUMANE DESCRIPTION PerioCol®-TC, 25mg UNITS Box of 5 Manufactured by: EUCARE PHARMACEUTICALS PRIVATE LIMITED Plot No. AC 25-B, SIDCO Industrial Estate, Thirumudivakkam, Chennai - 600 044, India. Tel: +91-44-4598 9955 / 9909 / 9904 / 9908 / 9903 / 9915, Fax: +91-44-2478 2516 / 4598 9914 E-mail: eucare@vsnl.com, Website : www.eucare.in / www.eucareindia.com PerioCol®-TC - Registered Trade Mark in India. PTLA1-0613 CODE