Local Coverage Determination (LCD) for
Radiology: Nonobstetric Pelvic Ultrasound
(L30054)
Contractor Name
Cahaba Government
Benefit
Administrators®, LLC
LCD Information
Document Information
LCD ID Number
L30054
LCD Title
Radiology: Nonobstetric Pelvic
Ultrasound
Oversight Region
Region IV
Contractor's Determination
Number
AMA CPT/ADA CDT Copyright
Statement
CPT codes, descriptions and other
data only are copyright 2010
American Medical Association (or
such other date of publication of
CPT). All Rights Reserved.
Applicable FARS/DFARS Clauses
Apply. Current Dental
Terminology, (CDT) (including
procedure codes, nomenclature,
descriptors and other data
contained therein) is copyright by
the American Dental Association.
© 2002, 2004 American Dental
Association. All rights reserved.
Applicable FARS/DFARS apply.
CMS National Coverage Policy
Original Determination
Effective Date
For services performed on or after
05/04/2009
Original Determination Ending
Date
Revision Effective Date
For services performed on or after
10/01/2011
Revision Ending Date
•
Title XVIII of the Social Security Act, Section 1833 (e). This section states that no
payment shall be made to any provider for any claims that lack the necessary
information to process the claim.
•
Title XVIII of the Social Security Act, section 1862(a)(1)(A). This section allows
coverage and payment for only those services that are considered to be
reasonable and medically necessary.
•
Title XVIII of the Social Security Act, Section 1862(a)(7). This section excludes
routine physical examinations.
•
Medicare Program Integrity Manual (Pub. 100-08), Chapter 13, Local Coverage
Determinations.
Indications and Limitations of Coverage and/or Medical Necessity
Indications
1.
2.
3.
4.
5.
6.
7.
8.
9.
Pelvic pain undiagnosed by standard exam;
Dysmenorrhea;
Menorrhagia;
Metrorrhagia;
Menometrorrhagia;
Postmenopausal bleeding;
Abnormal pelvic examination;
Further evaluation of abnormality found on other imaging studies; and
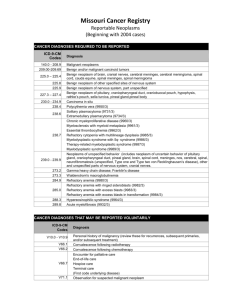
Cancer
76856 is a complete evaluation and must minimally include:
1. Female: description and measurements of the uterus and adnexal structures,
measurement of the endometrium and bladder, and a description of any pelvic
pathology.
2. Male: evaluation and measurement of the bladder, evaluation of the prostate and
seminal vesicles and any pelvic pathology.
76857 is a limited study and typically focuses on one or more elements listed under
76856 and/or the reevaluation of one or more pelvic abnormalities.
Limitations
1. Post voiding residual bladder volume is not reimbursable by CPT codes 76856
and 76857. Measurement of post voiding residual should be billed using CPT
code 51798.
2. The accuracy of ultrasonographic studies depends on the knowledge, skills and
experience of the technologist and interpreter. Consequently, the providers of
interpretations must be capable of demonstrating documented training and
experience and maintain documentation of such for possible audit. Further,
ultrasonographic studies must be either (1) performed by persons with
appropriate training that have demonstrated minimum entry level competency by
being credentialed by a nationally recognized credentialing organization in
ultrasound technology (e.g., American Registry of Radiologic Technologists
(ARRT) in sonography), (2) performed by or under the direct supervision of a
physician, or (3) performed in facilities with laboratories accredited in
ultrasonography.
Bill Type Codes:
Contractors may specify Bill Types to help providers identify those Bill Types typically
used to report this service. Absence of a Bill Type does not guarantee that the policy
does not apply to that Bill Type. Complete absence of all Bill Types indicates that
coverage is not influenced by Bill Type and the policy should be assumed to apply
equally to all claims.
999x Not Applicable
Revenue Codes:
Contractors may specify Revenue Codes to help providers identify those Revenue
Codes typically used to report this service. In most instances Revenue Codes are purely
advisory; unless specified in the policy services reported under other Revenue Codes
are equally subject to this coverage determination. Complete absence of all Revenue
Codes indicates that coverage is not influenced by Revenue Code and the policy should
be assumed to apply equally to all Revenue Codes.
99999 Not Applicable
CPT/HCPCS Codes
76856 Us exam pelvic complete
76857 Us exam pelvic limited
ICD-9 Codes that Support Medical Necessity
The correct use of an ICD-9-CM code listed in the “ICD-9 Codes that Support Medical
Necessity” section does not guarantee coverage of a service. The service must be
reasonable and necessary in the specific case and must meet the criteria specified in
this LCD.
ICD-9 codes must be coded to the highest level of specificity. Consult the ‘Official ICD-9CM Guidelines for Coding and Reporting’ in the current ICD-9-CM book for correct
coding guidelines. This LCD does not take precedence over the Correct Coding Initiative
(CCI).
158.0 158.9
MALIGNANT NEOPLASM OF RETROPERITONEUM MALIGNANT NEOPLASM OF PERITONEUM UNSPECIFIED
179
180.0 180.9
181
MALIGNANT NEOPLASM OF UTERUS-PART UNS
MALIGNANT NEOPLASM OF ENDOCERVIX - MALIGNANT
NEOPLASM OF CERVIX UTERI UNSPECIFIED SITE
MALIGNANT NEOPLASM OF PLACENTA
MALIGNANT NEOPLASM OF CORPUS UTERI EXCEPT
182.0 ISTHMUS - MALIGNANT NEOPLASM OF OTHER SPECIFIED
182.8
SITES OF BODY OF UTERUS
183.0 - MALIGNANT NEOPLASM OF OVARY - MALIGNANT NEOPLASM
183.9
OF UTERINE ADNEXA UNSPECIFIED SITE
184.0
MALIGNANT NEOPLASM OF VAGINA
MALIGNANT NEOPLASM OF FEMALE GENITAL ORGAN SITE
184.9
UNSPECIFIED
185
MALIGNANT NEOPLASM OF PROSTATE
MALIGNANT NEOPLASM OF UNDESCENDED TESTIS 186.0 MALIGNANT NEOPLASM OF OTHER AND UNSPECIFIED
186.9
TESTIS
MALIGNANT NEOPLASM OF MALE GENITAL ORGAN SITE
187.9
UNSPECIFIED
188.0 - MALIGNANT NEOPLASM OF TRIGONE OF URINARY BLADDER
188.9
- MALIGNANT NEOPLASM OF BLADDER PART UNSPECIFIED
195.3
MALIGNANT NEOPLASM OF PELVIS
218.0 - SUBMUCOUS LEIOMYOMA OF UTERUS - LEIOMYOMA OF
218.9
UTERUS UNSPECIFIED
219.0 - BENIGN NEOPLASM OF CERVIX UTERI - BENIGN NEOPLASM
219.9
OF UTERUS PART UNSPECIFIED
220
BENIGN NEOPLASM OF OVARY
BENIGN NEOPLASM OF FALLOPIAN TUBE AND UTERINE
221.0
LIGAMENTS
221.1
BENIGN NEOPLASM OF VAGINA
BENIGN NEOPLASM OF FEMALE GENITAL ORGAN SITE
221.9
UNSPECIFIED
222.0 - BENIGN NEOPLASM OF TESTIS - BENIGN NEOPLASM OF
222.9
MALE GENITAL ORGAN SITE UNSPECIFIED
233.1
CARCINOMA IN SITU OF CERVIX UTERI
CARCINOMA IN SITU OF OTHER AND UNSPECIFIED PARTS
233.2
OF UTERUS
233.30 - CARCINOMA IN SITU, UNSPECIFIED FEMALE GENITAL
233.32
ORGAN - CARCINOMA IN SITU, VULVA
233.39
CARCINOMA IN SITU, OTHER FEMALE GENITAL ORGAN
236.0
NEOPLASM OF UNCERTAIN BEHAVIOR OF UTERUS
236.2
NEOPLASM OF UNCERTAIN BEHAVIOR OF OVARY
236.3
236.5
236.7
236.99
NEOPLASM OF UNCERTAIN BEHAVIOR OF OTHER AND
UNSPECIFIED FEMALE GENITAL ORGANS
NEOPLASM OF UNCERTAIN BEHAVIOR OF PROSTATE
NEOPLASM OF UNCERTAIN BEHAVIOR OF BLADDER
NEOPLASM OF UNCERTAIN BEHAVIOR OF OTHER AND
UNSPECIFIED URINARY ORGANS
HYPERESTROGENISM - UNSPECIFIED OVARIAN
DYSFUNCTION
ANEURYSM OF ILIAC ARTERY
OTHER ANEURYSM OF UNSPECIFIED SITE
PHLEBITIS AND THROMBOPHLEBITIS OF ILIAC VEIN
ACUTE APPENDICITIS WITH GENERALIZED PERITONITIS ACUTE APPENDICITIS WITHOUT PERITONITIS
APPENDICITIS UNQUALIFIED
OTHER APPENDICITIS
REGIONAL ENTERITIS OF SMALL INTESTINE - REGIONAL
ENTERITIS OF UNSPECIFIED SITE
DIVERTICULITIS OF COLON (WITHOUT HEMORRHAGE)
HEMOPERITONEUM (NONTRAUMATIC)
OTHER SPECIFIED DISORDERS OF PERITONEUM
CALCULUS IN DIVERTICULUM OF BLADDER
OTHER CALCULUS IN BLADDER
BLADDER NECK OBSTRUCTION - UNSPECIFIED DISORDER
OF BLADDER
URINARY OBSTRUCTION, UNSPECIFIED
URINARY OBSTRUCTION, NOT ELSEWHERE CLASSIFIED
256.0 256.9
442.2
442.9
451.81
540.0 540.9
541
542
555.0 555.9
562.11
568.81
568.89
594.0
594.1
596.0 596.9
599.60
599.69
599.70 HEMATURIA, UNSPECIFIED - MICROSCOPIC HEMATURIA
599.72
601.0 ACUTE PROSTATITIS - PROSTATITIS UNSPECIFIED
601.9
ACUTE SALPINGITIS AND OOPHORITIS - UNSPECIFIED
614.0 INFLAMMATORY DISEASE OF FEMALE PELVIC ORGANS AND
614.9
TISSUES
ACUTE INFLAMMATORY DISEASES OF UTERUS EXCEPT
615.0 CERVIX - UNSPECIFIED INFLAMMATORY DISEASE OF
615.9
UTERUS
617.0 - ENDOMETRIOSIS OF UTERUS - ENDOMETRIOSIS SITE
617.9
UNSPECIFIED
OTHER SPECIFIED FISTULAS INVOLVING FEMALE GENITAL
619.8
TRACT
620.0 - FOLLICULAR CYST OF OVARY - UNSPECIFIED
620.9
NONINFLAMMATORY DISORDER OF OVARY FALLOPIAN TUBE
AND BROAD LIGAMENT
621.0 - POLYP OF CORPUS UTERI - UNSPECIFIED DISORDER OF
621.9
UTERUS
625.0
DYSPAREUNIA
625.2
MITTELSCHMERZ
625.3
DYSMENORRHEA
625.5
PELVIC CONGESTION SYNDROME
625.70
VULVODYNIA, UNSPECIFIED
625.71
VULVAR VESTIBULITIS
625.79
OTHER VULVODYNIA
OTHER SPECIFIED SYMPTOMS ASSOCIATED WITH FEMALE
625.8
GENITAL ORGANS
UNSPECIFIED SYMPTOM ASSOCIATED WITH FEMALE
625.9
GENITAL ORGANS
ABSENCE OF MENSTRUATION - UNSPECIFIED DISORDERS
626.0 OF MENSTRUATION AND OTHER ABNORMAL BLEEDING
626.9
FROM FEMALE GENITAL TRACT
627.0
PREMENOPAUSAL MENORRHAGIA
627.1
POSTMENOPAUSAL BLEEDING
752.0 - CONGENITAL ANOMALIES OF OVARIES - UNSPECIFIED
752.9
CONGENITAL ANOMALY OF GENITAL ORGANS
OTHER SPECIFIED CONGENITAL ANOMALIES OF BLADDER
753.8
AND URETHRA
789.00 - ABDOMINAL PAIN UNSPECIFIED SITE - OTHER SYMPTOMS
789.9
INVOLVING ABDOMEN AND PELVIS
998.11 - HEMORRHAGE COMPLICATING A PROCEDURE - SEROMA
998.13
COMPLICATING A PROCEDURE
998.51 - INFECTED POSTOPERATIVE SEROMA - OTHER
998.59
POSTOPERATIVE INFECTION
PERSONAL HISTORY OF MALIGNANT NEOPLASM OF
V10.40
UNSPECIFIED FEMALE GENITAL ORGAN
PERSONAL HISTORY OF MALIGNANT NEOPLASM OF CERVIX
V10.41
UTERI
PERSONAL HISTORY OF MALIGNANT NEOPLASM OF OTHER
V10.44
FEMALE GENITAL ORGANS
PERSONAL HISTORY OF OTHER GENITAL SYSTEM AND
V13.29
OBSTETRIC DISORDERS
V42.0
KIDNEY REPLACED BY TRANSPLANT
Diagnoses that Support Medical Necessity
NA
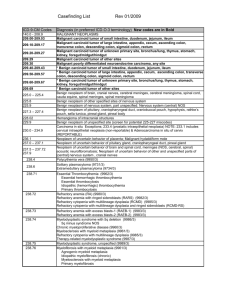
ICD-9 Codes that DO NOT Support Medical Necessity
Any ICD-9-CM code that is not listed in the “ICD-9 Codes that Support Medical
Necessity” section of this LCD.
XX000* Not Applicable
ICD-9 Codes that DO NOT Support Medical Necessity Asterisk Explanation
NA
Diagnoses that DO NOT Support Medical Necessity
Any diagnoses that are not listed in the “ICD-9 Codes that Support Medical Necessity”
section of this LCD.
Documentations Requirements
1. The medical record must contain clear documentation of medical necessity for
performing pelvic ultrasonography (e.g.: history, physical findings and/or
laboratory/imaging studies).
2. A permanent record of the sonographic examination and its interpretation
must be in the patient's record and made available to Medicare upon request.
3. Documentation must support CMS 'signature requirements' as described in
the Medicare Program Integrity Manual (Pub. 100-08), Chapter 3.
Appendices
NA
Utilization Guidelines
NA
Sources of Information and Basis for Decision
•
American College Of Radiology (ACR) Practice Guideline For "The Performance
of Pelvic Ultrasound in Females" available at www.acr.org.
•
Consultation with Cahaba GBA Part B CMDs from Alabama, Georgia and
Mississippi.
•
Consultations with the representatives to the Carrier Advisory Committee.
•
Other Medicare Carriers’ LCDs.
Advisory Committee Meeting Notes
Start Date of Comment Period
End Date of Comment Period
Start Date of Notice Period
Revision History Number
6
Revision History Explanation
Revision 6
What's New Posted Date: September 2011
Effective Date: October 1, 2011
This LCD was updated based on the 2012 ICD-9 Coding Update. ICD-9 Code 596.8 is
invalid and replaced with 596.81-596.83, 596.89.
Annual LCD Review: Added to ‘Documentation Requirements’: ‘Documentation must
support CMS 'signature requirements' as described in the Medicare Program Integrity
Manual (Pub. 100-08), Chapter 3’. (Change Request 6698).
Revision 5
What's New Posted Date: April 2011
Effective Date: May 1, 2011
The paragraph that includes qualifications of persons performing studies addressed
in this LCD is being clarified. This paragraph is being removed from the
‘Documentation Requirements Section’ and is being added to the ‘Limitation’ section.
Template language in the ‘ICD-9 Codes that Support Medical Necessity' is clarified
regarding correct coding guidelines.
Revision 4
What's New Posted Date: September 2010
Effective Date: October 1, 2010
This LCD was updated based on the 2011 ICD-9 Coding Update. ICD-9 Code 752.3
was removed and replaced with 752.31-752.36 & 752.39. ICD-9 Codes 752.43752.47 were added.
Revision 3
Posted: What's New - Part B, September 2009
Effective Date: October 1, 2009
This LCD was updated based on the 2010 ICD-9 Coding Update. The following ICD-9
codes were added: 621.34, 621.35, 789.7.
Revision 2
Start Date of Notice Period: July 14, 2009
Effective Date: August 29, 2009
As part of the J10 MAC transition, LCD effective for contractor number 10302 –
Tennessee Part B.
Revision 1
Start Date of Notice Period: June 17, 2009
Effective Date: August 1, 2009
As part of the J10 MAC transition, LCD effective for contractor number 10202 –
Georgia Part B.
Original
Start Date of Notice Period: March 20, 2009
Effective Date: May 4, 2009
As part of the J10 MAC transition, LCD effective for contractor number 10102 –
Alabama Part B.
09/06/2010 - This policy was updated by the ICD-9 2010-2011 Annual Update.
11/21/2010 - For the following CPT/HCPCS codes either the short description and/or
the long description was changed. Depending on which description is used in this
LCD, there may not be any change in how the code displays in the document:
76856 descriptor was changed in Group 1
76857 descriptor was changed in Group 1
08/27/2011 - This policy was updated by the ICD-9 2011-2012 Annual Update.
Reason for Change
CMS Requirement
ICD9 Addition/Deletion
Maintenance (annual review with new changes, formatting, etc.)
Narrative Change
Related Documents
This LCD has no Related Documents.
LCD Attachments
There are no attachments for this LCD.
All Versions
Updated on 09/16/2011 with effective dates 10/01/2011 - N/A
Updated on 04/12/2011 with effective dates 05/01/2011 - 09/30/2011
Updated on 12/10/2010 with effective dates 10/01/2010 - 04/30/2011
Updated on 11/21/2010 with effective dates 10/01/2010 - N/A
Updated on 09/17/2010 with effective dates 10/01/2010 - N/A
Updated on 12/03/2009 with effective dates 10/01/2009 - 09/30/2010
Updated on 08/28/2009 with effective dates 10/01/2009 - N/A
Updated on 06/24/2009 with effective dates 08/29/2009 - 09/30/2009
Some older versions have been archived. Please visit the MCD Archive Site to retrieve
them.
Read the LCD Disclaimer