PREP Workshop #31 - The Feinstein Institute for Medical Research
advertisement

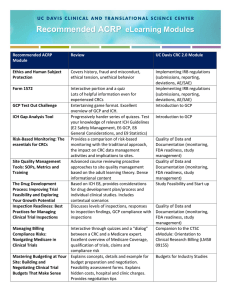
PREP Workshop #31: Preparing for Certification as a Clinical Research Coordinator Presented by: Susan Ray, MS, CCRC Supervisor Research Coordination Clinical Research Service CME Disclosure Statement • The North Shore LIJ Health System adheres to the ACCME’s new Standards for Commercial Support. Any individuals in a position to control the content of a CME activity, including faculty, planners, and managers, are required to disclose all financial relationships with commercial interests. All identified potential conflicts of interest are thoroughly vetted by the North Shore-LIJ for fair balance and scientific objectivity and to ensure appropriateness of patient care recommendations. • Course Director and Course Planner, Kevin Tracey, MD and Tina Chuck, MPH have nothing to disclose. • Susan Ray has nothing to disclose. Objective: Review requirements and possible preparation options for becoming certified as a clinical research coordinator Topics: Discuss SoCRA and ACRP requirements for taking the certification exams Review the Alliance Coordinator Award Program Discuss preparation tools, courses and options Test current knowledge – study review questions What is covered in the exam(s)? SoCRA • Ethical Principles / Informed Consent / Safety: 20% - 25% • Institutional Review Board / Institutional Ethics Committee (IRB/IEC) Roles and Responsibilities: 7% - 11% • Clinical Trial Protocol and Protocol Amendments: 4% - 8% • Investigator Roles and Responsibilities: 28% - 32% • Sponsor Roles and Responsibilities: 31% 35% See Exam Outline •FDA regulations. You will not be tested on FDA guidance. However, some of the guidance documents published by the FDA may be helpful in explaining concepts. ACRP General knowledge of: • Laboratory terminology, tests, and procedures • Basic math, including adding, subtracting, multiplying, dividing, and calculating percentages Proficiency in the areas of: • Investigational Product Management • Protocol • Safety • Trial Management • Trial Oversight See Detailed Content Outline (DCO) • ICH GCP Guidelines E6, E2A, E8, E9 • Declaration of Helsinki http://www.socra.org/certification/ccrp-certification-exam/exam-outline http://www.acrpnet.org/PDF/CRC_Handbook.pdf Regulations and Guidance Documents Nuremberg Code Declaration of Helsinki Belmont Report DHHS 45 CFR Part 46 Subpart A = Common Rule * FDA 21 CFR 50 * 56 * • Department of Health and Human Services, Title 45 Public Welfare, Code of Federal Regulations , Part 46 Protection of Human Subjects • Protection of Human Subjects (ICF) • Institutional Review Boards (IRB) 11 • Electronic Records, Electronic Signatures (EMR) 54 • Financial Disclosure by Clinical Investigators (COI) 312 • Investigational New Drug Application (IND) 812 • Investigational Device Exemptions (IDE) ICH GCP Guidelines E6 • Good Clinical Practice (GCP) E2A • Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E8 • General Considerations for Clinical Trials E9 • Statistical Principles for Clinical Trials Review of SoCRA’s Exam • CRP Certified: “CCRP” • Exam dates: NY area (Hartford, CT) exam will be September 25, 2015 Applications due: August 13, 2015 • Exam dates: NY area (Philadelphia, PA) exam will be October 10, 2015 Applications due: August 28, 2015 Fees: examination application fee $195 + $75 membership fee Duration: 4 hrs •135 multiple choice and true/false questions •Test questions are designed to be straightforward and easily understood. •Case studies that relate to clinical research practice •Current cut score is 80% and requires that the candidate correctly completes 108 out of the 135 examination questions. Period of Certification: 3 years SoCRA Eligibility Requirements 1. Member of SoCRA 1. Working with Good Clinical Practice (GCP) guidelines under IRB approved protocols 2. One of the following : Category 1: 2 yrs FT Clinical Research Professional (or have 3,500 hours part-time) within 5 years Category 2: degree in "Clinical Research" + 1 yr. year FT (or 1,750 hours part-time) within 2 years in Clinical Research Professional Category 3: Hold an Undergraduate or Graduate Certificate in “Clinical Research” with a curriculum of no less than 12 semester (credit) hours or totaling a minimum of 144 credit hours from an academic institution of higher learning (community college, college or university) AND hold an Associate’s or Bachelor’s Degree in a science, health science, pharmacy or related field AND have completed a minimum of one year of full-time experience (or 1750 hours parttime) during the past two years as a Clinical Research Professional. http://www.socra.org/certification/ccrp-certification-exam/candidate-eligibility Review of ACRP’s Exam • CRC Certified: “CCRC” • Fall 2015 Exam dates: September 9 – October 3, 2015 Applications Open: May 1, 2015 Applications Due: August 15, 2015 Fees: $450 for ACRP members and nonmembers by July 1 2015 $525 for nonmembers July 2 through August 15 2015 • Spring 2016 Exam dates: February 25 – March 21, 2016 Applications Open: October 1, 2015 Applications Due: February 1, 2016 Fees: $450 for ACRP members and nonmembers by December 1 2015 $525 for nonmembers December 2, 2015 through February 1 2016 http://www.acrpnet.org/PDF/CRC_Handbook.pdf Duration: 3 hrs •125 multiple choice questions •Practice based exam with hypothetical scenarios, no trick questions •Approximately 74% of candidates are successful on their first exam attempt. •Scaled score of 600 (800 max.) required to pass indicates that, while a different number of correct answers may be required from one administration to the next, the passing point for all examinations represents the same level of knowledge. Period of Certification: 2 years ACRP’s Eligibility Requirements One of the following: Option 1: RN, Associates, or Bachelor’s or higher, 3000 hrs, documentation of performed essential duties (CV and Job Description) Option 2: Other such as LPN, LVN, Med. Assistant, Lab Technician OR HS diploma, 4500 hrs, documentation of performed essential duties (CV and Job Description) http://www.acrpnet.org/PDF/CRC_Handbook.pdf Alliance Coordinator Award Program Send it to the CRS; SRay@nshs.edu Preparation Tools 1. 2. 3. 4. 5. ACRP Certification Exam Preparation Package (http://www.acrpnet.org/prepareforyourexam) – Exam Practice Exercise – ACRP's new Certification Exam Preparation eLearning Course – Exam Prep Packages RAN Institute Flash Cards: http://www.raninstitute.com/flashcards.php www.mobilizepress.com - 1-2 hour reviews on other ICH documents. Tip: You can purchase these and have unlimited access to use to prepare for the exams. www.raninstitute.com ICH Guidelines: www.ich.org (Go to Work Products/ICH Guidelines/Efficacy Guidelines) Declaration of Helsinki: www.wma.net/en/20activities/10ethics/10helsinki/index.html Preparation Tools Pharmaschool (free): http://www.pharmaschool.co/resources.asp. Under the general resources there is a “jargonbuster” with key definitions “GCP and Clinical Research Tests” there are various multiple choice quizzes Registered Users can go to “My GCP Zone” to score your quizzes and tell you how you compare to the average user. *The website is based out of the UK there are some specific questions regarding EU regulations, but overall was found to be extremely helpful. 15 Preparation Ideas • STEP 1: Assess your own professional experience • STEP 2: Start early and plan ahead. • STEP 3: Schedule your study time. The key is not to memorize what you read, but to understand concepts behind ICH/GCP and best practices in each knowledge category area to supplement your experience in answering questions on the exam. • STEP 4: Assemble your study notes in a binder. • STEP 5: Choose the methods that are right for YOUR study plan. Choose a mentor or colleague who has more experience in the areas in which you are less familiar and ask him/her to review concepts with you. • STEP 6: Stick to your study group’s plan. • STEP 7: Don’t panic! Follow the excellent pre-exam advice that the Academy provides, and come to the exam well-rested and prepared. http://www.acrpnet.org/PDF/CRC_Handbook.pdf Exam Preparation – CRS Study Groups? • Additional prep sessions using RAN Institute Flash Cards • Contact your Regional CRS • Electronic GCP • Hard-copy box of flash cards 17 Test Current Knowledge Pre-Exam Assessment Test current knowledge by completing the questionnaires Code of Federal Regulation – Slides on screen CFR questions -Broader Good Clinical Practice – The RAN Institute's On Line Training Campus <http://raninstitute.trainingcampus.net> GCP -More specific Final Tips Certification Prep from Coordinators: • When you apply to take the certification exam: be prepared to literally justify why and how you meet all of the qualification requirements – detail your experience and make sure it adds up to the specific requirements indicated. They will reject your application if you do not show how you meet eligibility. • Concentrate on the big picture: ICH guidelines E6, E8, Declaration of Helsinki. • Make sure you understand the difference between your local guidelines/requirements and the international/ federal regulations for clinical research • Noticed emphasis on “phases of clinical drug development” and “statistical principles for clinical trials” • Take the practice exam: dissect each question as there's some truth or reason to all the answers listed. • Be able to critically asses various situations that could happen in research - especially those you are not familiar with because you will most likely come across that and slow you down during the exam • Be prepared for math/calculation questions for drug accountability/subject compliance, etc. • Understand the process of the testing center and taking a test on a computer Special Thanks Contributors Division/Department/Facility This presentation was primarily created by Cerdi Beltre, CIP, CCRP. Clinical Research Service, Feinstein Institute for Medical Research, NSLIJHS