Molecular Structure
advertisement

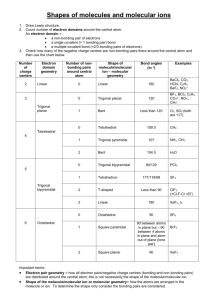
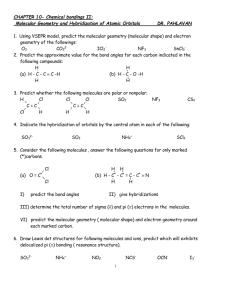
Molecular Structure Topics 3-D structure shape (location of atoms in space) Molecular Geometry Valence Bond Theory Hybrid Orbitals Multiple Bonds VSEPR (Valence Shell Electron Pair Repulsion) Valence Bond Theory Overlap of atomic orbitals – is a covalent bond that joins atoms together to form a molecule Consider each atom to donate 1 e- to the pair which makes up a bond Filled Orbitals F2 2p __ __ __ __ __ __ 2s __ __ 1s __ F __ F (http://www.hull.ac.uk/php/chsajb/concepts/ho_2.html) (http://www.ausetute.com.au/lewisstr.html) 1s2 __ 1s2 __ No empty orbitals (http://www.ausetute.com.au/lewisstr.html) Types of Bonds Sigma overlap between nuclei examples include: s and s s and p p and p Pi bond overlap above and below nuclei with parallel p orbitals Overlap of p orbitals that are perpendicular to line through nuclei Sigma ( σ ) bond Filled orbitals do not contribute to bonding but do contribute to size (http://classes.yale.edu/02-03/chem220a/studyaids.html) Pi (π ) Bond In atoms with double or triple bonds π Bond is weaker than σ since less overlap Hybridization and bond formation are simultaneous process Hybrid orbitals overlap more effectively Electron pairs of bonds are as far away from each other as possible thus there is a lower energy Lennard Jones Potential Illustrates energy that holds atoms together in a bond or molecules together in a liquid Y axis is energy and X axis is distance between atoms or molecules Lower energy when brought together but energy too high if pushed very close together Most stable position is one with lowest energy Atoms brought close together (http://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/olunderstandconfo.html) Hybrid Orbitals Element Be B C Orbitals 1s22s2 1s22s22px1 1s22s22px12py1 Bonds Expected 0 1 2 Actual Bonds 2 3 4 (http://www.platte1.k12.wy.us/Gowdy/Chemistry/chapter_13.htm) Mixing of different orbitals to form equivalent obitals is hybridization Be 2p __ __ __ 2s __ 2p __ __ -----> sp __ __ 1s __ B 1s __ 2p __ __ __ -------> 2s __ 1s __ Linear ------> sp2 __ __ __ Trigonal Planar C 2p __ __ __ ------------> 2s __ ------------> sp3 __ __ __ __ Tetrahedral 1s __ Geometry differs from either isolated orbital Energy is also different from isolated atoms To Determine structure 1) Draw Lewis structure and find number of pairs of electrons 2) Determine electron pair geometry 3) Determine Molecular Geometry Electron Pair Geometry can be different or same as molecular geometry (illustrated below) Electron Pair Geometry of CH4: Tetrahedral Molecular Geometry of CH4: Tetrahedral (http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/text_images/CH10/FG1 0_03b.JPG) Electron Pair Geometry of NH3: Tetrahedral Molecular Geometry of NH3: Pyramidal (http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/10.html) Electron Pair Geometry of H2O: Tetrahedral Molecular Geometry of H2O: Bent (http://dbhs.wvusd.k12.ca.us/webdocs/Bonding/) Molecular Geometry (shapes) (http://www.elmhurst.edu/~chm/vchembook/202linear.html) (http://www.elmhurst.edu/~chm/vchembook/202linear.html) (http://www.elmhurst.edu/~chm/vchembook/203trigplanar.html) (http://www.elmhurst.edu/~chm/vchembook/205trigpyramid.html) Square Planar (http://www.up.ac.za/academic/chem/mol_geom/planar.htm) (http://www.elmhurst.edu/~chm/vchembook/204tetrahedral.html) (http://fia.coas.unf.edu/gchm/chime.html) Octahedral (http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/10.html) Summary of Molecular Geometry No. of Electron Pair No. of Electron Geometry Pendant Molecular Pairs (Bond Angle) Atoms Geometry Click below to Formula Image show rotation. 2 linear (180o) BeH2 Show rotation s3 trigonal planar 3 (120o) trigonal planar CO32- Show rotation NO2- Show rotation 2 2 linear bent Example 4 5 6 tetrahedral (109.5o) trigonal bipyramidal (90o, 120o) octahedral (90o) 4 tetrahedral CH4 Show rotation 3 trigonal pyramidal NH3 Show rotation 2 bent H2O Show rotation 5 trigonal bipyramidal PCl5 Show rotation 4 see-saw SF4 Show rotation 3 T-shaped BrF3 Show rotation 2 linear ICl2- Show rotation 6 octahedral SF6 Show rotation 5 square pyramidal BrF5 Show rotation 4 square planar ICl4- Show rotation (http://www.molecules.org/VSEPR_table.html) Multiple Bonds Ethane (http://www-theor.ch.cam.ac.uk/people/ross/thesis/node143.html) Bond Angles ~ 109.5o Valence Carbon Electrons Ethane Carbon p __ __ __ ---> sp3 __ __ __ __ Tetrahedral s __ Ethene Carbon p __ sp2 __ __ __ Ethyne (Acetylene) Trigonal p __ __ sp __ __ Linear Hybridization in Multiple Bonds p __ __ __ p __ p __ -------> -------> sp2 __ __ __ or sp2 __ __ __ s __ Ethylene (Ethene) (http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/10.html) 5 σ bonds 1 π bond Ethyne (Acetylene) p __ __ __ p __ __ p __ __ s __ Ethyne 3-D Orbitals sp __ __ or sp __ __ (http://wps.prenhall.com/wps/media/objects/724/741576/chapter_01.html) Bond Length (A) 1.54 Carbon to Carbon single bond 1.34 Carbon to Carbon double bond 1.20 Carbon to Carbon triple bond Removal of Electrons Electrons do not come off the same order they go on Examples: Fe 3s23p63d64s2 Fe2+ 3s23p63d6 (4s2 comes off) Fe3+ 3s23p63d5 (3d off) Co [Ar] 4s23d7 Co2+ 3s23p63d7 Co3+ 3s23p63d6 Cu [Ar] 3d104s1 Cu+ 3s23p63d10 Cu2+ 3s23p63d9 VSEPR minimize electron repulsion Ammonia NH3 1s22s22px12py12pz1 (http://www.uyseg.org/greener_industry/pages/ammonia/1AmmoniaAPQ.htm) σ bond from p orbital H—N—H 107.3o Could explain by sp3 hybridization because it is close to tetrahedral angle 109.5o Water H2O 1s22s22px22py12pz1 (http://www.hrw.com/science/si-science/chemistry/atomic_structure/molecules/02mol.html) H—O—H 104.5o Could explain by sp3 hybridization because it is close to tetrahedral angle 109.5o Valence Shell Electron Pair Repulsion (VSEPR) Model Compound CH4 NH3 H2O Angle 109.5o 107.3o 104.5o Bonding Pair 4 3 2 Lone Pair 0 1 2 Bonding Pair charge is smaller Lone Pair charge cloud is larger so repulsion is greater Order of Repulsion LP—LP > LP—BP > BP—BP Polar Molecules and Electronegativity Bond Ionic metal (cation) and nonmetal (anion) Na+ClPure Covalent is with identical atoms Cl—Cl Polar Covalent is the partial transfer or uneven sharing (http://www.teachmetuition.co.uk/Chemistry/Intermolecular/dipole.htm) (http://faculty.njcu.edu/tpamer/gilbert-lessons/lesson5/gilbert6-4.htm) Dipole moment is a positive and negative side Electron pair is more toward the Cl atom Electronegativity decides what type of bonding Electronegativity is the measure of ability of an atom to pull an electron toward it Basically the strength of attraction of electrons Linus Pauling Scale (http://www.webelements.com/webelements/properties/text/image-balls/electroneg-pauling.html) (http://www.bcpl.net/~kdrews/properties/properties2.html) Electronegativity F > O > Cl ~ N > Br > I ~ C ~ S ~ Se > P H~P F is the most electronegative Cs is the least electronegative If there is more than one atom in a compound then sum up bond moments can imagine it is like a molecular tug of war with atoms pulling electron pair (http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/10.html) But with CO2 the dipole moment for the molecule results in 0 The reason is that there are equal pull from both directions (http://www.hull.ac.uk/php/chsajb/symmetry&spectroscopy/ho_2.html) These diagrams show that negative (more e-) and positive side of molecules helps in predicting reactions In the compound H—Cl when the bond breaks the electron will go with Chlorine because it is more electronegative Understand Bond Strength correlates with Electronegativity difference Compound Electronegativity Bond Energy (kJ/mol) Difference H—F 1.9 570 H—Cl 0.9 430 H—Br 0.7 360 H—I 0.4 300 Greater the Electronegativity Difference then stronger the bond Representative VSEPR Structures Orbital geometry: Describes the geometry of the orbitals, takes the nonbonding electron pairs into account because they must be in an orbital. The steric number and the hybridization will give the orbital geometry (electron-pair geometry). Therefore, there are only 5 possible orbital geometries: Octahedral (sp3d2) Trigonal Bipyrimidal (sp3d) Tetrahedral (sp3) Trigonal Planar (sp2) Linear (sp) Molecular Geometry: uses the nonbonding electron pairs to describe the geometry of the molecule (http://library.tedankara.k12.tr/chemistry/vol3/Molecular%20geometry%20and%20hybridization/z94.gif) Steps for Determining Geometry: Draw Lewis structure and find number of pairs of electron Determine electron pair geometry Determine molecular geometry Note: Electron pair geometry can be different than molecular geometry For Example: Tetrahedral Electron geometry can have 3 different molecular geometry (tetrahedral, pyramidal and bent)