The Fine Structure of Synapses
advertisement

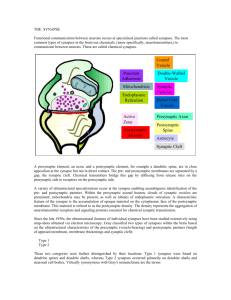
12/15/12 Ev ernote Web The Fine Structure of Synapses Saturday, December 15 2012, 12:12 PM The Fine Structure of Synapses Citation: Peters, A (2008) The Fine Structure of Synapses IBRO History of Neuroscience [http://www.ibro.info/Pub/Pub_Main_Display.asp?LC_Docs_ID=3313] Accessed: date Alan Peters The word “synapse” was coined by Sir Charles Sherrington in 1897 to denote the normal anatomical relations between contiguous neurons (see Foster and Sherrington, 1897). The origin of the word is explained in a footnote in Fulton’s Physiology of the Nervous System (Fulton, 1938, p. 55), and in the literature is it is quite clear that Sherrington recognized the synapse as a discontinuous intercellular junction. Although most scientists in the 1930s and afterwards accepted the neuron doctrine of Cajal, which postulates that each cell in the nervous system is a discrete entity that has no cytoplasmic continuity with other cells, it was not until synapses were examined by electron microscopists in the 1950s that the last doubt about the existence of cytoplasmic continuity between cells was laid to rest. In the 1950s it became evident that in both the invertebrate (Robertson, 1953) and vertebrate (Palade and Palay, 1954; Palay, 1956) nervous systems the pre- and postsynaptic elements of a synapse are separated by a cleft. It also became evident that the presynaptic elements contain vesicles, which were soon postulated to contain the chemicals released to allow conduction. From a morphological point of view, a chemical synapse can be described in term of its parts (Figure 1). https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 1/12 12/15/12 Ev ernote Web Figure 1: Asymmetric synapses (S1 and S2) between two axon terminals (At1 and At2), which contain spherical vesicles, and the smooth surfaced dendrite (Den) of a non-pyramidal cell in cerebral cortex. At the synaptic junctions the pre- and postsynaptic membranes are separated by a wide cleft and the postsynaptic membrane has a prominent dense coating on its cytoplasmic surface. Rat auditory cortex. x100, 000. The presynaptic component, which is most commonly an axon terminal, is characterized by its content of synaptic vesicles that often mingle with mitochondria; in the central nervous system the apposing postsynaptic element can have the features of any part of a neuron, and at a neuromuscular junction the postsynaptic component is a muscle cell (see Figure 2); the synaptic cleft is the intercellular space between the pre- and postsynaptic components and is usually between 20 and 30nm wide; the synaptic junction is formed by the plasma membranes of the preand postsynaptic components, the synaptic cleft, and the densities that occur both on the cytoplasmic faces of the pre- and postsynaptic components and within the synaptic cleft; at https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 2/12 12/15/12 Ev ernote Web some synaptic junctions vesicles accumulate against the presynaptic membrane at specific vesicles release sites, referred to as active zones (see Figure 2). https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 3/12 Figure 2: A motor end plate. Passing down the middle of the field is the basal lamina (B), which occupies the synaptic cleft separating the membranes of the axon terminal (At) and the striated muscle cell on the left. The axon terminal contains synaptic vesicles (sv) that are concentrated at the active zones (*) of the axolemma. Although no coated vesicles are evident in the axon terminal, the empty “shells” or “baskets” that form the coats of such vesicles are apparent in the axon terminal (arrowheads). Rat diaphragm. x 75,000. The following is a brief account of the different types of synapses found in the nervous system, with references to when some of the first descriptions of these synapses were made. Types of chemical synapses The study that had most influence on how chemical synapses are classified was that of Gray (1959). In tissue from cerebral cortex fixed by immersion in osmic acid, Gray (1959) encountered two types of synapses, which he called type I and type II. In his account he states that type I synapses, which involve dendritic spines and shafts, are formed by axon terminals that contain https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 4/12 12/15/12 Ev ernote Web round synaptic vesicles These synapses have a synaptic cleft that is abut 20nm wide and there is a prominent density on the cytoplasmic face of the postsynaptic membrane. Type II synapses on the other hand involve neuronal perikarya and dendritic shafts, have a narrower synaptic cleft of about 12nm, and a less prominent density beneath the postsynaptic membrane. In addition, the synaptic vesicles are smaller in the axon terminals forming type II synapses. Similar synapses have been found to occur in other parts of the central nervous system, and in the ventral cochlear nucleus Lenn and Reese (1966) found some axon terminals with synaptic vesicles having mean diameters of 45nm and other with mean diameters of 40nm. They deduced that terminals with the larger vesicles are excitatory and the others inhibitory in function. Over the years it has been shown that this deduction is correct. Asymmetric and symmetric synapses As in tissue fixed primarily in osmic acid, both the large and the smaller synaptic vesicles appear spherical in the freeze- fractures studies that were abundant in the 1970s (e.g. Akert et al., 1972; Sandri et al., 1977). However, the images of synapses changed when glutaraldehyde was introduced as a primary fixative (Sabatini et al., 1963). Prior to the advent of glutaraldehyde, preservation of central nervous system tissue was generally poor, but this changed in the late 1970s, when central nervous tissue began to be fixed by perfusion with mixtures of glutaraldehyde and formaldehyde, followed by osmication before embedding. This procedure greatly improved preservation, and while the vesicles within some terminals (Gray type I) retained their spherical shapes in glutaraldehyde fixed tissue, in other terminals some of the vesicles appear elongate (Gray type II). And studying cerebral cortex after glutaraldehyde fixation, Colonnier (1968) concluded that the two types of synapses described by Gray (1959) really represent the extremes of a continuum of morphology. Colonnier suggested that the two extremes should be referred to as asymmetric and symmetric synapses, on the basis of the prominence of the density on the cytoplasmic faces of the postsynaptic components. Asymmetric synapses are those with spherical synaptic vesicles and a prominent postsynaptic density, and in general such synapses are excitatory in function. Symmetric synapses lack a prominent postsynaptic density, and have pleomorphic vesicles, that is some vesicles have round profiles and others are elongate (Figures 3 and 4). Figure 3. Synapses in the cerebellum. In the upper half of the figure are two axon terminals (At1 and At2) from granule cells and they are making asymmetric synapses with the spines (sp1 and sp2) of Purkinje cells. In the spines are cisternae of smooth endoplasmic reticulum (SR). The synapses are surrounded by processes of astrocytes (As). The rest of the field is occupied by axons (Ax) of granule cells. Rhesus monkey. x60,000. In the lower half of the figure is a terminal (At) from a basket cell, and it is forming a symmetric synapse (arrow) with the perikaryon of a Purkinje cell (N). Note the neurofilaments (nf) and mitochondria (mit) in the axonal cytoplasm. Rhesus monkey. x35,000. In general asymmetric synapses are excitatory in function, and use transmitters such as glutamate and aspartate, although in some locations such as the spinal cord, substantia nigra, striatum and globus pallidus, superior colliculus and inferior olive, some asymmetric synapses are GABAergic (see lower Fig. 4, and Peters et al. 1991). Figure 4. Symmetric and asymmetric synapses. In the upper picture are two different axon terminals (At1 and At2) forming symmetric synaptic junctions (arrows) with the cell body (N) of pyramidal cell in cerebral cortex. The different packing densities and shapes of the synaptic vesicles in the two terminals suggest that they are from different types of presynaptic neurons. Note the punctum adhaerens (triangle) at one of the junctions. Cerebral cortex of rat. x80,000. In the lower picture is the axon terminal (At) of a basket cell in cerebellum. It is recognized by the presence of neurofilaments (nf) and pleomorphic vesicles (sv) in its cytoplasm. These inhibitory terminals are unusual in that they form asymmetric synapses with dendritic spines of Purkinje cells (sp1 and sp2). Rhesus monkey. x50,000. https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 5/12 12/15/12 Ev ernote Web In general, however, symmetric synapses are inhibitory in function. Attempts have been made to fit synapses in all parts of the nervous system into these two basic categories, and while there has been some success, a number of variations have been encountered, even in cerebral cortex in which Peters and Harriman (1990) distinguished at least three types of axon terminals forming symmetric synapses. Nevertheless, this number pales in the light of studies on spinal cord, in which Bodian (1972; 1975), Conradi (1969) and McLaughlin (1972) have described as many as six types of axon terminals that can be distinguished from each other on the sizes and shapes of their synaptic vesicles. An example of the kinds of variation that can occur is shown in Figure 5, which is from the cochlear nucleus. Figure 5. A variety of synapses. Occupying the center of the field is a protrusion from the cell body of a neuron in ventral cochlear nucleus. It is surrounded by a number of axon terminals. Two of the terminals (At1 and At2) contain small, spherical vesicles, but two other terminals (At3 and At4) contain larger spherical vesicles. The other axon terminals (At5 to At9) contain some elongate vesicles. The synaptic junction formed by axon terminal At5 is noteworthy since it has a regular array of presynaptic densities of the presynaptic grid (arrows). Adult rat. x44.000. Finally, it must be mentioned that in addition to clear vesicles, other vesicles in some terminals contain a dense granule. Such granular vesicles are associated with the presence of catecholamines (Figure 6, lower half). Figure 6. Axoaxonic synapse and dense core vesicles. The upper picture shows an axo-axonal synapse between a pyramidal cell axon initial segment (Ax) and the axon terminal of a chandelier cell (At), which has pleomorphic synaptic vesicles. The axon initial segment is characterized by the presence of fascicles of microtubules (m) in its cytoplasm and the dense undercoating (D) of the axolemma. The synaptic junction is symmetric and inhibitory. Rat cerebral cortex. x90,000. The lower picture shows two axon terminals (At1 and At2) synapsing with a dendrite in the dentate nucleus. One of the terminals (At2) contains agranular, or clear vesicles, while the other one (At1) contains some granular vesicles with dark cores. Monkey. x90,000. Disposition of synapses In terms of their frequency, in all parts of the central nervous system, asymmetric synapses outnumber GABAergic symmeteric ones by a factor of about 4:1. And when the disposition of synapses on a postsynaptic neuron is examined, it is generally found that inhibitory synapses are preferentially located on proximal dendrites, the cell body and axon initial segment of the postsynaptic neuron; locations where inhibition can be most effectively applied. Asymmetric synapses tend to be preferentially located on distal dendrites and dendritic spines, but it is not uncommon to find symmetric and asymmetric synapses existing side-by-side. For example in cerebral cortex this occurs on the cell bodies of inhibitory neurons and on some dendritic spines. Synaptic junctions Presynaptic components. At asymmetric synapses there is usually only one synaptic junction. At the smallest synapses, this junction may have the form of a complete disc, but at larger synapses the junction may have on or two perforations (see Peters and Kaiserman-Abramof, 1969). Consequently, in thin sections taken at right angles to the plane of large junction, it often appears that two or three discontinuous densities are present. The reason for the perforations is not known. However, the number of perforations has been shown to increase during development and to be enhanced by complex behavioral environments (see Greenough and Chang, 1988). It might be mentioned that at the neuromuscular junction of striated muscle (Figure 2), there are numerous junctional zones or vesicle release sites, which consist of many strips arrange at right angles to the length of the axon terminal (e.g., Dreyer et al., 1973; Heuser and Reese, 1973) since this type https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 6/12 12/15/12 Ev ernote Web of synapse needs to fire in a predicable fashion. Multiple release sites also occur at excitatory synapses between bulbs and Held and the neurons of the ventral cochlear nucleus (Gulley et al., 1978). At symmetric synapses there may be multiple small synaptic junctional zones, or vesicle release sites, where vesicles accumulate near the pre-synaptic membrane. In addition there may be a number of puncta adhaerentia (Peters et al., 1990). The puncta are adhesion sites, and not active zones, since vesicles do not accumulate next to them (see Figure 4, upper picture; Figure 7, lower picture). Gray (1963) found that when tissue is treated with phosphotungstic acid before it is embedded, some dense particles appear within the presynaptic density. These particles form an hexagonal grid, which is referred to as the presynaptic grid. (see Figure 5; e.g., Akert et al., 1972). Such grids can be found at both asymmetric and symmetric synapses, and it seems that the depressions in the grid are places where vesicle attachments sites are located (see Triller and Korn, 1985; Sur et al. 1995). In other terminals the role of the presynaptic grid appears to be assumed by a presynaptic ribbon. Such ribbons occur in rods and cones of the retina, in the lateral lines of fishes and in hair cells of the cochlear, and their role appears to be to guide synaptic vesicles towards the presynaptic membrane (see Peters et al., 1991). Postsynaptic components. Postsynaptic densities can be isolated and a number of studies have examined their composition (e.g., Carlin et al., 1983; Hunt et al., 1996). At some synapses there is a postsynaptic apparatus. Thus, as first shown by Taxi (1961) in sympathetic ganglia, in addition to the post synaptic density on the plasma membrane there may also be subsynaptic material that consists of two dense bars, or a row of dense particles beneath the postsynaptic membrane density. Another postsynaptic element that is often encountered in the cerebral and cerebellar cortices is the spine apparatus, which consists either simple smooth endoplasmic cisternae, as in the cerebellum, or of parallel smooth endoplasmic reticulum cisternae separated by sheets of dense material, as in the cerebral cortex. Presynaptic dendrites In the early electron microscopic studies of the central nervous system it was assumed that only axons could be presynaptic, and so synapses could be described as being axo-dendritic, axospinous, axo-somatic, or axo-axonic, but then in 1966 Rall and his colleagues (Rall, 1966) produced evidence that in the olfactory bulb there are dendro-dendritic synapses between mitral cell and granule cell dendrites. To cloud the field even further, it was found that not only are both components of these synapses dendrites, but they are also unusual in being reciprocal synapses: at the same interface both the mitral cell and the granule cell can be pre- and postsynaptic (see Figure 7). Figure 7. Dendro-dendritic and dendro-somatic synapses. The upper picture shows the dendrite (Den) of a mitral cell in olfactory bulb, synapsing with the gemmule (gem) of a granule cell dendrite. At the interface between the two there are reciprocal synaptic junctions with opposite polarities. Where the mitral cell dendrite is presynaptic (left side arrow) vesicles accumulate against its plasma membrane and the synapse is asymmetric, and excitatory. When the gemmule is presynaptic (right side arrow) the synapse is symmetric, and inhibitory. Olfactory bulb of rhesus monkey. x80,000. The lower picture shows gemmules (gem) of granule cells synapsing with the cell body (N) of a mitral cell. As in the upper picture, at the synapses where the mitral cell is presynaptic (arrows) the junctions are asymmetric. The other junction between the mitral cell and the gemmule on the right is a punctum adhaerens (triangle). Olfactory bulb of rhesus monkey x 60,000. Where the mitral cell is presynaptic the junction is asymmetric (upper Figure 7, Den to gem) and the vesicles are round, so that the synapse is excitatory. In contrast, at the granule cell–to-mitral 7/12 12/15/12 Ev ernote Web cell synapse, the junction is symmetric and the vesicles are pleomorphic, so that this synapse is inhibitory (Upper Figure 7, gem to Den). It is assumed that at these synapses the mitral cell-togranule cell synapses are activated first, and that the excitation is then inhibited by the reciprocal synapses. Other examples of dendro-dendritic synapses are to be found in the synaptic glomeruli of the thalamus. These are aggregations of synapsing processes that are often surrounded by an astrocytic sheath. The most widely studied of these glomeruli are those in the lateral geniculate nucleus (e.g. Famiglietti and Peters, 1972; Wilson et al. 1984; Hamos et al., 1985). The main elements in these glomeruli (see Figure 8) are the terminals from the optic tract (Ax1). Figure 8. A glomerulus. Lateral geniculate nucleus. This large synaptic glomerulus has a prominent central axon terminal (Ax1), which is an optic nerve terminal. It is forming asymmetric synapses (arrows) both with the dendrite (Den1) of a geniculocortical relay neuron and with a vesicle-filled dendritic terminal from an interneuron (Den2). The vesicle filled dendritic terminals (Den2) also synapse (arrowhead), at inhibitory synapses, with the dendrites (Den1) of the geniculocortical relay neuron, so that a synaptic triad is formed. A fourth type of profile (Ax2) with somewhat flatter vesicles is also present in the glomerulus. Cat. x40,000. These are excitatory and form asymmetric synapses with two kinds of elements. One is a dendrite (Den1) belonging to the principal cell in the LGN, and the other is a vesicle-containing dendrite (Den2) of a local circuit GABAergic inhibitory neuron. These three components form triads in which the optic tract terminals excite both dendritic components, after which the vesicle-containing dendrites (Den2) one synapse delay later inhibit the dendrites (Den1) of the principal cells (e.g. see Fitzpatrick et al., 1984). Thus, as in the olfactory bulb there is excitation followed after one synaptic delay by inhibition. Other places where synaptic glomeruli are found are in the granular layer of the cerebellum (see Palay and Chan-Palay, 1974), the olfactory bulb (see Shepherd and Greer, 1990), and in the dorsal horn of the spinal cord. And in addition to dendro-dendritic synapses, examples of dendro-somatic, and somato-somatic synapses have been found, as well as dendro-axonic and somato-axonic synapses, although these latter are not very common (see Peters et al., 1991). Electrotonic synapses Not long after the separation had been shown between synapsing elements in the nervous system, intracellular recording showed that between some neurons, there are connections at which there is no synaptic delay. These connections are electrotonic synapses. The first one to be examined morphologically was the electrotonic synapse at the motor junction of the crayfish (Robertson, 1953), and this was also the first synapse of this kind to be examined electrophysiologically (Furshpan and Potter, 1969). Since then a number of these synapses have been examined in the nervous systems of fishes by Bennett and Pappas and their co-workers (see Bennett, 1977). Other electronic synapses occur in the retina, in which nearly all type of neurons participate in electrotonic networks (see Vaney, 2002), as well as in locations such as the lateral vestibular nucleus, the inferior olive, the cerebellum, and the cerebral cortex. In all cases it has been shown that these electrotonic synapses have the features of gap junctions. Thus, in thin sections taken at right angles to a junction, the outer faces of the apposed plasma membranes are separated by a distance of only 2nm (Fig. 9 lower half), and in freeze-fractured preparations arrays of hexagonal particles are evident in the plasma membranes at the sites of the junctions (Figure 9; upper half). Figure 9. Electrotonic synapses. At the upper left is a freeze fractured gap junction, or electrotonic synapse, between two elements in the retina. The particles (arrows) are connexins and they are on the P face of one membrane. Corresponding pits, or depressions, are apparent on the E face of the other membrane forming the junction. Inner plexiform layers of rhesus monkey retina. x150,000. https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 8/12 12/15/12 Ev ernote Web At the upper right is a ribbon-like gap junction between a rod and a cone. The particles of the junction (arrow) are in a row. Outer plexiform layer of rhesus monkey. x120,000. In the lower picture is a gap junction or electrotonic junction between a presynaptic (pre) mossy fiber (At) and a postsynaptic (post) granule cell dendrite (Den). The gap between the two plasma membranes is about 3nm. At each end of the junction are puncta adhaerentia (arrows). Cerebellum of viper. X 230,000. These particles are connexins. Connexins are perforated by a hydrophilic channel, and when the plasma membranes become apposed, the connexins in each of them line up to provide channels through which ions can pass from one cell to the other. The main features of electrotonic synapses are that they are faster than chemical ones, and they are bidirectional, so that it is sometimes difficult to establish which neuron is the functional presynaptic one. In the 1970s it was thought that electrotonic junctions in the cerebral cortex were rare, and indeed such synapses do not commonly occur between excitatory neurons. But in the past 10 years or so, it has been established that electrotonic junctions are common between fast spiking, inhibitory cortical neurons (see Galarretta and Hestrin, 2001, Connors and Long, 2004). The electrotonic junctions in cerebral cortex are mainly dendro-dendritic and they tend to be most common between neurons of the same type, for example inhibitory neurons that are parvalbumin positive, and these same neurons can also be interconnected by chemical synapses. Finally it should be mentioned that there have been descriptions of mixed synapses, at which there is either morphological or physiological evidence for both chemical and electrotonic transmission at the same synaptic interface. But such synapses are not common, and for more information consult Peters et al. 1991. Summary It is evident that there are many morphological varieties of synapses, and almost any part of a neuron can be presynaptic, or postsynaptic, to another. With the advent of antibodies, and techniques for recording from neurons and then intracellular filling them, it has been possible to analyze the physiology and morphology of some neurons in detail and to determine where their axon terminals form their synapses. However, detailed information about how many synapses individual neurons receive, and what are the sources of those synapses is still largely missing. Only when that information becomes available will we be able to know how the central nervous system really functions. Some of the information about local neuronal circuits may eventually become available through making extensive three-dimensional reconstructions from serial thin sections, but determining the precise connections of neurons with long axonal projections presents much more of a challenge. Alan Peters Waterhouse Professor Department of Anatomy and Neurobiology Boston University School of Medicine Boston, MA, USA Figures All the figures are reproduced from Peters, Palay and Webster, The Fine Structure of the Nervous System. Neurons and their Supporting Cells, 3rd edition. 1991. Oxford University Press. New York. References Akert, K., Pfenninger,K., Sandri, C and Moor, H. 1972. Freeze-etching and the cytochemistry of vesicles and membrane complexes in synapses of the central nervous system. In: Structure and Function of Synapses (eds. Pappas, G, D. and Purpura, D.P.), pp. 67–86. New York, Raven Press. Bennett, M.V.L. 1977. Electrical transmission: a functional analysis and comparison to chemical https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 9/12 12/15/12 Ev ernote Web transmission. In: Handbook of Physiology. Section 1, The Nervous System, vol.1 Cellular Biology of Neurons (ed. Kandel. E.R.), pp. 357–416. Bethesda, MD, American Physiological Society. Bodian, D. 1972. Synaptic diversity and characterization by electron microscopy. In: Structure and Function of Synapses (eds. Pappas, G.D. and Purpura, D.P.). pp 45–65. New York, Raven Press. Bodian, D. 1975. Origin of specific synaptic types in the motoneuron neuropil of the monkey. J. Comp. Neurol. 159: 225–244. Carlin, R.C., Bartelt, D.C., and Siekevitz, P. 1983. Identification of fodrin as a major calmodulinbinding protein in postsynaptic density preparations. J. Cell Biol., 96: 449–454. Colonnier, M. 1968. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. Brain Res,. 9: 268–287. Connors, B.W. and Long, M.A. 2004. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 27: 393 – 418. Conradi, S. 1969. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbrosacral spinal cord of the adult cat. Acta Physiol. Scand, (suppl.) 332: 5–48. Dryer, F. K., Peper, K. Akert, K., Sandri, C. and Moor, H. 1973. Ultrastructure of the active zone in the frog neuromuscular junction. Brain Res. 62: 373–380. Famiglietti, E.V. and Peters, A. 1972. The synaptic glomerulus and the intrinsic neurons in the dorsal lateral geniculate nucleus of the cat. J. Comp. Neurol. 144: 285–334. Fitzpatrick, D., Penny, A. R., and Schmechel. I.M. 1984. Glutamic acid decarboxylaseimmunoreactive neurons and terminals in the lateral geniculate nucleus of the cat. J. Neurosci. 4:1809–1829. Foster, M. and Sherrington, 1897. A Text Book of Physiology. Vol 3. The Central Nervous System. 7th edn. London, Macmillan. Fulton, J.F. 1938. The Physiology of the Nervous System. London, Oxford Medical Publications. Furshpan, E.J. and Potter, D.D. 1959. Transmission at the giant motor synapses of the crayfish. J. Physiol. 145: 289–325. Galarreta, M. and Hestrin, S. 2001. Electrical synapses between GABA-releasing interneurons. Nature Rev. Neurosci. 2: 425–433. Gray, E.G. 1959. Axosomatic and axodendritic synapses of the cerebral cortex. J. Anat. Lond. 934: 420–433. Gray, E.G. 1963 Electron microscopy of presynaptic organelles of the spinal cord. J. Anat. Lond. 97: 101–106. Greenough ,W.T. and Chang, F.-L. 1988 Plasticity of synapse structure and pattern in the cerebral cortex. In: Development and Maturation of the Cerebral Cortex. Cerebral Cortex vol. 7 (eds. Peters, A. and Jones, E.G.). pp 391–440. New York, Plenum Press. Gulley, R.L., Landis, D. M.D. and Reese, T.S. 1978. Internal organization of membranes at endbulbs of Held in the anteroventral cochlear nucleus. J. Comp. Neurol. 180: 707–742. Hamos, J.E., Van Horn, S.C., Raczkowski, SD., Uhlrich, J. and Sherman, S.M. 1985. Synaptic connectivity of a local circuit neuron in lateral geniculate nucleus of the cat. Nature, 317: 618– 621. Heuser, J.E. and Reese, T.S. 1973. Evidence for recycling of synaptic membrane during transmitter release at the frog neuro-muscular junction. J. Cell. Biol. 57: 315–344. https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 10/12 12/15/12 Ev ernote Web Hunt, C.A., Schenker, L.G. and Kennedy, M. 1996. PSD-95 is associated with the presynaptic membrane at forebrain synapses. J. Neurosci. 16: 1380–1392. Lenn, N.J. and Reese, T.S. 1966. The fine structure of the nerve endings in the nucleus of the trapezoid body and the ventral cochlear nucleus. Am. J. Anat. 118: 375–389. McLaughlin, B.J. 1972. The fine structure of neurons and synapses in the motor nuclei of the cat spinal cord. J. Comp. Neurol. 144: 429–460. Palade, G.E. and Palay, S.L. 1954. Electron microscope observations of interneuronal and neuromuscular synapses. Anat. Rec. 118: 335–336. Palay, S.L. 1956. Synapses in the central nervous system. J. Biophys, Biochem. Cytol. 2 (suppl) 193–202. Palay, S.L. and Chan-Palay V. 1974. Cerebellar Cortex: Cytology and Organization. New York and Heidelberg, Springer Verlag. Peters, A. and Harriman, K.M. 1990. Different kinds of axon terminals forming symmetric synapses with the cell bodies and initial axon segments of layer II/III pyramidal cells. I. Morphometric analysis. J. Neurocytol. 19: 154–174. Peters, A. and Kaiserman-Abramof. I. 1969. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z. Zellforch. 100: 487–506. Peters, A., Palay, S.L. and Webster, DeF. H. 1991. The Fine Structure of the Nervous System, Neurons and Their Supporting Cells. New York, Oxford University Press. Peters, A., Sethares, C. and Harriman, K.M. 1990. Different kinds of axon terminals forming symmetric synapses with the cell bodies and initial axon segments of layer II/III pyramidal cells. II. Synaptic junctions. J. Neurocytol. 19: 584–600. Rall, W., Shepherd, G.M., Reese, T.S. and Brightman, M.W. 1966. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exptl. Neurol. 14: 44–56. Robertson, J.D. 1953. Ultrastructure of two invertebrate synapses. Proc. Soc. Exptl. Biol. Med. 82: 219–223. Sabatini, D., Bensch, K. and Barnett, R.J. 1963. Cytochemistry and electron microscopy: the preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell. Biol. 17: 19 – 58. Sandri, C., Van Buren, J.M. and Akert, K. 1977. Membrane Morphology of the Vertebrate Nervous System. A Study in Freeze-etch Technique. Brain Research vol 46. Amsterdam. Elsevier. Shepherd, G.M. and Greer, C.A. 1990 Olfactory bulb. In: The Synaptic Organization of the Brain. 3rd edn. (ed. Shepherd, G.M.), pp 133–169. New York, Oxford University Press. Sherrington, C.S. 1906. The Integrative Action of the Nervous System. New Haven, Yale University Press. Sur, C., Triller, A. and Korn, H. 1995. Morphology of the release sites of inhibitory synapses on the soma and dendrites of an identified neuron. J. Comp. Neurol. 351: 247–260. Taxi. J. 1961. Etude del’ultrastructure des zones synaptique dans les gangliones sympathetiques de la grenouille. Compte R. Acad. Sci. 252: 174–176. Triller, A. and Korn, H. 1985. Activity-dependent deformation of presynaptic grids at central synapses. J. Neurocytol. 14: 177–192. https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 11/12 12/15/12 Ev ernote Web Vaney, D.I. 2002. Retinal neurons: cell types and coupled networks. Prog. Brain. Res. 136: 239– 254. Wilson, J.R. Friedlander, M.J. and Sherman, S.M. 1984. Fine structural morphology of identified Xand Y- cells in the cat’s lateral geniculate nucleus. Proc. Roy. Soc., Ser. B. 221: 411–436. https://www.ev ernote.com/edit/b5b0a889-9e26-4cc0-a678-ded644f 98de7#st=p&n=b5b0a889-9e26-4… 12/12