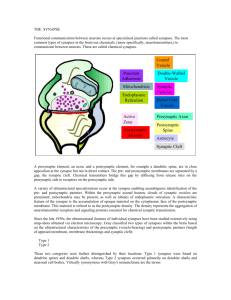
Models of Synaptic Transmission
advertisement

Modelling Synaptic Transmission
Matthias H. Hennig
ANC, Informatics, University of Edinburgh
Contents
1
Overview & Outline
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1
2
Biology of Synapses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2
2.1
Synaptic Morphologies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2
2.2
Types of Synapses
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3
2.2.1
Electrical Synapses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3
2.2.2
Chemical Synapses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4
2.3
Basics of Synaptic Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . .
4
3
Simulation Techniques . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
5
3.1
Microphysiological Simulations
6
3.2
Average Response (Deterministic) Models
. . . . . . . . . . . . . . . . . . . . .
8
3.3
Stochastic Models . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
9
4
Short Term Plasticity
9
4.1
Depression and Facilitation
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
11
4.2
Beyond the Depletion Model . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12
. . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1 Overview & Outline
Synapses are highly specialised structures enabling neurons to exchange signals with other
neurons, or to send signals to non-neural cells such as muscle bres. In contrast to other cellular signalling mechanisms, signal transmission through synapses is very fast. Glutamatergic
synapses for instance can generate a postsynaptic current in less than 0.5ms after the arrival
of the presynaptic action potential.
For simplicity, it is sometimes assumed that synapses are simple excitatory or inhibitory
connections between neurons that can be used to construct neural circuits. For good reasons,
this approach largely ignores the complexity and diversity of synapses. A simple model for
postsynaptic conductance changes is for example the alpha-function:
1
2 Biology of Synapses
Fig. 1:
2
Left: Postsynaptic conductance generated by an alpha function (Eqn. 1) with
(straight line) and
τs = 20ms
τs = 3ms
(dashed line). Right: Possible sites of regulation of the
synaptic ecacy (from Zucker and Regehr, 2002).
gs (t) =
The parameter
τs
t − τt
e s
τs
(1)
species the duration of the response and can be used to distinguish for
instance between fast and slow transmission (e.g.
respectively, see Fig. 1, left).
through AMPA and NMDA receptors,
This model is computationally very ecient, but completely
ignores any aspect dynamical regulation of synaptic transmission.
It is well known that
there is a whole range of dierent processes in synapses regulating and modulating synaptic
transmission (Fig. 1, right), so an alternative view is to consider each single synapse as a
complex computational unit. To better understand how synapses work, computational and
mathematical modelling has emerged as a valuable tool.
The purpose of this review is to
briey summarise the dierent methodologies and some key results from modelling studies of
synaptic transmission.
In what follows, rst a brief overview of the biology of synaptic transmission will be given.
Then, three dierent approaches for simulating synaptic transmission on dierent levels of
complexity will be reviewed.
In the third part, the main focus is on fast use-dependent
synaptic dynamics, also called short-term plasticity.
2 Biology of Synapses
2.1 Synaptic Morphologies
The typical synapse is a contact between neurons with a diameter of about 0.5-2µm. As can
be seen in Figure 2, some chemical synapses show substantial morphological specialisation,
which are thought to reect special functional requirements.
As shown in the top row in
Figure 2, many synapses have a specialised anatomical structure called a synaptic spine. Spines
2 Biology of Synapses
3
Dendritic spines
(from Synapse Web)
Cultured hippocampal
neurons
Human retinal
cone terminal
Fig. 2:
Mouse neuromuscular junction
(Salpeter, 1987)
Axon and spine
(from Synapse Web)
Calyx of Held in rat auditory brainstem
(Saetzler et al, 2002)
Dierent types of chemical synapses.
are little, well, spines on the dendrites of postsynaptic neurons at the site of contact to the
axon of another neuron. Electrophysiological and modelling studies suggest that spines may be
important to make the synapse more ecient by restricting the diusion of neurotransmitter
molecules. A great source of various morphological reconstructions is the web site Synapse
Web (http://synapses.clm.utexas.edu/anatomy/index.stm)
2.2 Types of Synapses
Although synapses come in many forms and shapes, we can distinguish between two main
types: electrical and chemical synapses. Electrical synapses will be mentioned only briey in
the following, before we concentrate on chemical transmission.
2.2.1
Electrical Synapses
This is the simplest form of synapse which consists of intercellular channels allowing ions
and small molecules to pass from one cell to the next.
Clusters of these channels, which
consist of proteins called connexins, form gap-junctions. Intriguingly, these can be found in
almost every mammalian cell. Gap junctions are thought to support synchronisation of larger
populations of neurons (see e.g. Hu and Bloomeld, 2003; Perez Velazquez and Carlen, 2000
for some examples) and long range integration, as for example found in horizontal cells in the
vertebrate retina.
Unlike chemical synapses, gap junctions do not distinguish between pre- and postsynaptic
neuron, there is no dened direction of transmission. Apart from ions, which mediate electrical
activity, gap junctions also allow for the exchange of various small molecules such as cAMP
or IP3 . This may have important, but to date probably still under-appreciated, consequences
for cellular signalling in the brain.
2 Biology of Synapses
2.2.2
4
Chemical Synapses
These are what we would view as a proper synapse:
Presynaptic signals are transmitted
via release of neurotransmitter from the presynaptic neuron, which binds to receptors at the
postsynaptic neuron. Before going into the details, some general remarks.
There are many dierent types of neurotransmitter, and typically a neuron releases only one
type.
However, as always in biology there are exceptions to the rule, for instance retinal
starburst amacrine cells, which release acetylcholine and GABA. At the same time, a neuron
can express many dierent types of receptors, each sensitive to a particular neurotransmitter.
The two main types of neurotransmitter receptors are called ionotropic and metabotropic
receptors. Upon activation, ionotropic receptors directly open a channel, which is more or less
selective for certain ion species. Metabotropic receptors do not have this ion channel - instead
they activate a second messenger cascade, which eventually leads to ion channel opening. As
a rule of thumb, an important dierence is that ionotropic receptors are faster and generate
shorter responses than metabotropic receptors.
The type of transmitter released by a neuron determines the action on the postsynaptic neuron. This can be either excitatory (common: glutamate, acetylcholine) or inhibitory (common:
GABA, glycine). The dierence between excitatory and inhibitory transmission is a consequence of dierences in the reversal potential
ER
of the ionic species involved. The current
generated by a receptor channel can be written as
Isyn = gsyn (V − ER ),
where V is the membrane potential and
(2)
gsyn the synaptic conductance.
The dierence between
the membrane potential, which is usually somewhere between -60mV and -70mV at rest,
and the reversal potential
ER
can either have a positive or negative sign.
If it is negative,
the synapse is depolarising, hence excitatory (e.g. for glutamatergic synapses, mediated by
+ and K+ , which has
Na
ER ≈0mV).
If positive, it is hyperpolarising, hence inhibitory (e.g.
+
responses to GABA are mediated by Cl , which has
ER ≈-70mV).
Shifts in the reversal
potential for certain ions can lead to changes in the sign of a synapse, as for example seen
for GABA, which is excitatory early in development due to a higher
ER (ER ≈
-40mV,
see Ben-Ari, 2002 for a review).
2.3 Basics of Synaptic Transmission
Synaptic transmission takes place at specialised sites in the cell membrane. On the presynaptic side, these sites show a high density of vesicles which contain neurotransmitter molecules.
These are called the active zone (AZ). Postsynaptically, one observes a clustering of neurotransmitter receptors and other molecules in the postsynaptic density (PSD).
Vesicles containing neurotransmitter are typically
≤50nm
in diameter (dierent from those
containing neuropeptides, which are larger) and contain several of thousand molecules each.
Hence typical vesicular transmitter concentrations are a few hundred mM. Because synaptic
transmission involves the triggered release of vesicular neurotransmitter into the synaptic
cleft, the vesicular transmitter content constitutes an unitary or quantal unit for transmission
(in analogy to
~
in quantum mechanics), and each postsynaptic response is the result of a
3 Simulation Techniques
Fig. 3:
5
Simplied schematic diagram of a synapse and the processes taking upon arrival of a
presynaptic action potential.
superposition of many of these unitary events. This is not as simple as it seems however it would make the life of experimentalists much easier if a postsynaptic response could really
be constructed from unitary events, but this is usually not possible because quantal size can
rapidly change due to several factors such as receptor desensitisation.
Electronmicroscopic images show that a small number vesicles are usually attached or docked
to the cell membrane - these vesicles are assumed to be primed or release-ready, while the
remaining are on hold to replace empty docked vesicles. The existence of anatomically distinguishable vesicle populations has led to the concept of vesicle pools : docked vesicles belong to
the releasable pool and those waiting to the reserve pool. There is some electrophysiological evidence for the evidence of other pools, but this matter is still unresolved (and I will not
dwell on this here; for reviews on vesicle pools, see Sudhof, 2004; Rizzoli and Betz, 2005).
What happens when an action potential arrives at the presynaptic terminal is schematically
illustrated in Figure 3.
The action potential causes a depolarisation of the axon terminal,
2+ channels. As a result [Ca2+ ] rapidly
i
which will lead to the opening of voltage gated Ca
increases from about 30nM to 10-30µM in the vicinity of an AZ. This region of highly localised
2+ channels
concentration increase is sometimes called micro- or nanodomain, and one or two Ca
per active zone seem sucient to mediate this.
This in turn triggers the fusion of vesicles
with the cell membrane, and their content is released into the synaptic cleft.
The released
transmitter then diuses passively through the synaptic cleft and nally binds to postsynaptic
receptors, which generate the postsynaptic response.
3 Simulation Techniques
Modelling biological systems such as synapses always requires us to make simplications and
assumptions, and we have to be clear about what we want to achieve. Firstly, we may ask
which components and processes are really relevant and can try to reduce a model's complexity
3 Simulation Techniques
accordingly (reductionism).
6
Secondly, it is important to consider the dimensionality of the
problem at hand, for instance we have to ask which temporal and spatial scales are relevant
(e.g. whether the time course of transmitter release on the scale of
µs is really that important,
or if we can treat this as a point event). Thirdly, we have to be clear how unambiguous the
available experimental data are, that is, how strong are the constraints on the model? It is
worth noting that rejecting a particular model can be as important as providing evidence in
support of an alternative. And nally, we have to keep the underlying physics in mind. For
instance, will we need a stochastic model, or is a deterministic model sucient (note that this
can make a real dierence, see below and Andrews and Arkin, 2006 for examples)?
In models of synaptic transmission, two extremes can be found in the literature: strongly
simplied deterministic ODE models on the one hand, and most detailed biophysical models
on the other (called microphysiological models following Stiles and Bartol, 2001). Somewhere
in-between we also nd more simple stochastic models, or models were complicated spatiotemporal interactions are absorbed into more tractable descriptions, for instance through
systems of PDEs. These dierent model classes are discussed in more detail in the following.
3.1 Microphysiological Simulations
As shown above, chemical synaptic transmission involves release and diusion of neurotransmitter, and binding to receptors.
It has long been speculated that diusion and the ar-
rangement of AZs and PSDs has some inuence on synaptic transmission, but tackling these
questions experimentally is very dicult. Therefore, microphysiological models have been very
useful and successful to answer these questions, which are experimentally very challenging.
Basically, microphysiological models attempt to model reality as accurately as possible on
scales of microseconds and nanometres. These models contain cell membranes, which act as
diusion barriers, agonist molecules which diuse in the intra- or extracellular space, and
eector molecules which generate the postsynaptic response (receptors) or are responsible for
transmitter uptake (transporters). In practice, this is a dicult problem because the diusion
equation has to be solved in complicated three-dimensional geometries, which can be done
analytically only in highly simplied settings (see for example Eccles and Jaeger, 1958 for an
early attempt). On the other hand, these situations can be easily simulated on fast computers
using Monte Carlo simulation techniques.
Very generally, Monte Carlo methods employ random processes to evaluate a quantity or
function. This technique was developed in the 1940's during the Manhattan project and has
been heavily rened ever since. There is a vast amount of literature, but the Wikipedia entry
is a good starting point, Metropolis (1987) summarises the history and Stauer (2005) lists
some interesting examples.
√
1/ N
Generally, Monte Carlo Methods will display convergence with
(N being the number of repetitions), regardless of the number of degrees of freedom,
which is a very useful property if you have sucient computational power.
In synapses and other biological systems, stochasticity arises from diusion and agonist-eector
interactions, both classical random processes. It is conceptionally easy to assign probabilities
to each of these processes, and to simulate the time evolution of the system in very small steps.
Diusion is modelled as Brownian motion of individual molecules, which are reected from
membranes and can bind and unbind to and from eectors. This process can be repeated many
times, and averaging of some desired measured quantity such as the transmitter concentration
3 Simulation Techniques
Fig. 4:
7
Monte Carlo simulations of synaptic transmission at the neuromuscular junction (from
Stiles et al., 2001).
in a specied volume or the opening and closing of receptor channels then yields an approximation of the average behaviour of the synapse.
Since the simulated random process are
equivalent to those in the real system, one also obtains an estimate of the expected variability
of the measured quantity. This variability directly corresponds to the expected trial-to-trial
uctuations in physiological experiments (minus the noise introduced by the recording equipment obviously), and can therefore allow for a detailed comparison between simulated and
experimental data (see Postlethwaite et al., 2007 for an example).
Although in principle not dicult to implement, Monte Carlo simulations of diusion in threedimensional environments tend to get very slow when the number of molecules is increased.
MCell (http://www.mcell.cnl.salk.edu/) is a very useful simulation package which employs
highly optimised Monte Carlo algorithms. The trick is to make excessive use of look-up tables
and to use a clever method to compartmentalise 3D space (Kerr et al., 2008).
MCell was
specially developed for simulations of synaptic transmission, but can also be used in many
equivalent situations (a comprehensive review of available simulation software is given in
Slepchenko et al., 2002).
A number of studies have used Monte Carlo methods to study synaptic transmission in great
detail in dierent systems. One nice example is a series of studies on the neuromuscular junction using MCell, where the complicated geometry was implemented as a three-dimensional
3 Simulation Techniques
Fig. 5:
8
Comparison of simple GABA receptor models with experimental data (data from dentate granule cells; Destexhe et al. (1994a)).
mesh.
This allowed for a detailed investigation of various aspects of vesicular release, dif-
fusion and receptor distribution (see Figure 4; for reviews, see Stiles and Bartol (2001);
Stiles et al. (2001); also interesting is Coggan et al., 2005 - make sure you see the video
at
http://www.mcell.cnl.salk.edu/Publications/ectopic_sciencemag_2005/).
Further
interesting studies that have made use of Monte Carlo simulations include Franks et al. (2003),
Raghavachari and Lisman (2004) and Postlethwaite et al. (2007).
3.2 Average Response (Deterministic) Models
An approach which entirely neglects the stochasticity of biological processes is the description
of the averaged behaviour of the system.
This can be done by using ordinary dierential
equations (ODEs, describing reaction kinetics), if the temporal evolution of some processes
has to be investigated, or partial dierential equations (PDEs, describing reaction-diusion
kinetics) if both temporal and spatial properties are of interest.
In both cases, the dier-
ential equations provide a deterministic description of the average behaviour of substances
and reactions - an accurate description if the number of molecules in the system is very
large and in well-mixed conditions (a famous example is the Hodgkin Huxley model for action potential generation). Amongst others, useful tools to implement these simulations are
NEURON (http://www.neuron.yale.edu/neuron/, Hines and Carnevale (1997)) and GENESIS (http://www.genesis-sim.org/GENESIS/).
Average response models can be used to model various aspects of synaptic transmission. In
the following will look at the gating of neurotransmitter receptors. The most basic model for
a receptor has a closed and an open state, and the corresponding equation is:
do(t)
= αT (t)(1 − o(t)) − βo(t),
dt
(3)
4 Short Term Plasticity
where
o(t) is
9
the relative occupancy of the open state (bounded between [0:1]),
varying transmitter concentration (often modelled as a square pulse),
(expressed in 1/s/mM ) and
β
time-
the rate of opening
the rate of closing (in 1/s ). Because there are only two states,
the closed state occupancy is simply given by
o(t)
α
T (t) the
strongly depends on the shape of
T (t),
c(t) = 1 − o(t).
The precise behaviour of
for which many choices are possible. It can, for
instance, be expressed as a delta function, or evaluated more precisely through Monte Carlo
simulations.
If we take
o(t) (which ranges from 0 to 1) as the fraction of all receptors in the open state, the
I = ḡ·o(t)·(V −Er ), where ḡ is the peak conductance
postsynaptic current can be calculated by
of the whole receptor population (see also Eqn. 2). Figure 5 shows that this simple model
can already reproduce the average time course of a GABA-IPSC quite well (note that
T (t)
was modelled as a square pulse in these examples). Simple extensions of the model, which
add more receptor states, further improve the t. The dierent models shown in Figure 5 are
called Markov models because all transitions between states are time-independent and only
depend on the occupancy of the neighbouring states.
Average response models have been employed to analyse a whole range of properties of
synapses, from the gating of neurotransmitter receptors and other ion channels (see e.g. Destexhe et al., 1994b; Robert and Howe, 2003; Postlethwaite et al., 2007) to models of synaptic
short-term dynamics that will be discussed in more detail below. The assumption that dynamics at synapses can be described by Markov models is not entirely uncontested, but it
provides very useful and plausible constraints for automated tting of experimental data.
3.3 Stochastic Models
Stochastic models are somewhere between microphysiological and average response models
and can be used to simulate the main sources of variability in synaptic transmission while
keeping the model simple and computationally ecient. These models can for instance make
use of the fact that the values calculated by dierential equations such as Equation 3 can also
be interpreted as probabilities, which allows for their pseudo-randomised implementation.
Suppose that a set of ODEs is used to calculate the time- and calcium-dependent transmitter
kr (t) at a synapse (in vesicles/s). Then pr (t) = 1 − exp(∆t/kr (t)) gives the release
[t : t + ∆t]. To model the stochastic character of individual releases,
at every time step a random number from the uniform distribution R ∈ [0 : 1) is generated
and a release event is generated if R < Pr (t).
release rate
probability in the interval
The dierence between activity caused by synaptic transmission simulated with deterministic and stochastic models can be quite profound.
Because the stochastic model introduces
additional uctuations, stimulation which generates weak sub-threshold uctuations in the
deterministic model can lead to spikes if the stochastic nature of vesicle release is taken into
account (Fig. 6).
4 Short Term Plasticity
Short term plasticity (STP) summarises various forms of use-dependent modulation of the
synaptic ecacy on scales of milliseconds to tens of seconds (and even beyond). There are
4 Short Term Plasticity
Fig. 6:
10
Comparison between membrane potential and synaptic current simulated with a deterministic (left) and stochastic (right) model of synaptic transmission (response to
Poisson spike train, taken from de la Rocha and Parga, 2005).
Fig. 7:
Dierent forms of short term plasticity (from Dittman et al., 2000).
4 Short Term Plasticity
Fig. 8:
11
Modelling synaptic depression with a simple depletion model (neocortical pyramidal
cells, compiled from Tsodyks and Markram, 1997).
two main forms of STP, depression and facilitation, that can be observed either alone or in
combination depending on the type of neuron (see Fig. 7 for examples).
4.1 Depression and Facilitation
The simplest model for synaptic depression is the vesicle depletion model. This model assumes
that at each active zone only a limited number of neurotransmitter vesicles are available for
release.
Relling of this release-pool takes time (in the order of seconds), hence it will be
progressively depleted during repetitive stimulation. This type model was formally described
for the rst time (to my knowledge) by Liley and North (1953), although they did not talk
about vesicles at that time. Among many others, important theoretical papers investigating
this model and its consequences include Tsodyks and Markram (1997) (make sure to read the
erratum!) and Abbott et al. (1997).
Formally, the depletion model in its simplest form can be written as
dn(t)
1 − n(t) X
=
−
δ(t − tj ) · p · n(t),
dt
τr
j
| {z }
{z
}
relling |
(4)
release
where n(t) is the occupancy of the release pool (bounded between 0 and 1). The rst term
on the right side makes sure the release pool is relled with a time constant
few seconds).
τr
(typically a
The second term implements the release of vesicles each time a presynaptic
4 Short Term Plasticity
12
action potential arrives (at times tj ,
response magnitude is then
j = 1...N ) with a release probability p.
proportional to p · n(t).
The postsynaptic
It can be calculated that for long stimuli (Poisson spikes) the depletion model produces a mean
response
1997).
∼
1
f , which roughly agrees with experimental data (Fig. 8, Tsodyks and Markram,
The consequence is that with increasing stimulus frequencies the synapse becomes
less eective and reliable to drive the postsynaptic neuron during prolonged high-frequency
stimulation. Furthermore, the amount of depression depends on the release probability, which
in turn determines the response magnitude to the rst stimulus. In particular, for two successive stimuli the model predicts that the amount of depression depend linearly on the release
probability. This is usually not found in experiments, and as a solution it was suggested that
the release probability
p
is not constant, but also changes during stimulation (Betz, 1970).
This leads us to a simple way to add facilitation to this model by increasing the release
probability after each presynaptic action potential (Dayan and Abbott, 2001; Markram et al.,
1998). This can be written as
dp(t)
p0 − p X
=
+
δ(t − tj ) · f · (1 − p(t)),
dt
τf
(5)
j
where
f
p0
is the resting release probability,
τf
the recovery time constant from facilitation and
the amount of facilitation per action potential. In this model, the steady-state facilitation
approaches
hpi = (p0 + f rτf )/(1 + rf τf )
for a Poisson stimulus with rate r.
Hence weak
activity at low rates will cause weak postsynaptic responses, and higher frequencies will lead
to increasingly stronger responses.
Figure 9 shows that this extension of the depletion model can account very well for experimental data where the simple depletion model fails. In particular the relation between stimulus
frequency and steady-state response amplitude is much better captured than by the depletion
model alone. A large survey of cells in the medial prefontal cortex has shown that this model
can, despite large variability in the amount of depression and facilitation, be used to t a
whole range of dierent behaviours extremely well (Wang et al., 2006).
4.2 Beyond the Depletion Model
The depletion model with facilitation is by no means a complete model of synaptic transmission. This is not to say that the model is wrong, but rather that it needs to be extended to
capture the full range of experimentally characterised phenomena.
Here is a list of various
ndings that are not captured by this model (for a good, although now slightly outdated,
review, see Zucker and Regehr, 2002):
•
Receptor desensitisation can contribute to depression and mask facilitation (reviewed in
Jones and Westbrook, 1996; for a model, see Wong et al., 2003).
•
Presynaptic auto-receptors can contribute to depression (Takahashi et al., 1996; Takago
et al., 2005 and more; for a model, see Billups et al., 2005).
•
Calcium channel inactivation can contribute to depression (the rst paper to report this
is, as far as I know, Forsythe et al., 1998).
4 Short Term Plasticity
Fig. 9:
13
Modelling combined synaptic depression and facilitation (neocortical pyramidal cells,
compiled from Markram et al., 1998).
•
The eects of increases in calcium concentration on release probability are not linear,
but increase
•
The recycling of vesicles is accelerated by presynaptic activity (Wang and Kaczmarek,
1998; Sakaba and Neher, 2001; Wu et al., 2005 and many more; for models, see Trommershäuser et al., 2003; Wong et al., 2003; Hennig et al., 2007).
•
Accumulation of intracellular residual calcium can aect facilitation and depression
(for models, see Dittman et al., 2000).
•
Recovery from depression depends on length and frequency of the stimulus (Forsythe et
al., 1998 and many others, for models see Fuhrmann et al., 2004; Hennig et al., 2008).
•
Depression can occur without release of vesicles (reviewed extensively by Zucker and
Regehr, 2002; for a model, see Fuhrmann et al., 2004).
•
Glia cells can contribute to short term plasticity (Haydon, 2001).
As an example, I will illustrate how the reduction of the presynaptic calcium current amplitude
during repetitive stimulation may lead to depression possibly without any apparent vesicle pool
depletion. This eect was recently described at the calyx of Held by Xu and Wu (2005) and
is based on the fact that the relation between vesicle release rate and intracellular calcium
concentration at synapses is highly nonlinear: Presynaptic action potentials lead to a brief
increase of the calcium concentrations to tens of
µM ,
and in this range the release rate scales
4 Short Term Plasticity
Fig. 10:
14
Relation between vesicle release rate and presynaptic calcium concentration (at the
Calyx of Held, from Lou et al., 2005 - this paper also presents a Markov model for
cooperativity during vesicle release).
with the 4th power of the concentration. Hence small changes of the calcium concentration
will lead to signicant changes of the release rate (see Fig.10), and therefore a linear scaling
of the release probability introduced as a model for facilitation (see Eqn.
5) may be too
simplistic.
As it turns out, at the calyx of Held (see Fig. 2), the amplitude of the presynaptic calcium
current recorded during repetitive depolarisation of the axon terminal is, at low stimulus frequencies (2Hz), signicantly reduced even after the rst stimulus. Raising the (normalised)
calcium current amplitude to the power of 3.6 reproduces the depression of the EPSCs recorded
postsynaptically very well (see Fig.11, left part, top). This no longer works at high stimulus frequencies, where a substantial facilitation is visible in the calcium current that is less
pronounced in the EPSCs (see Fig.11, left part, bottom). A model which combines depletion
with a reduction of the calcium current can reproduce the observed behaviour across dierent
stimulus frequencies much better then a simple depletion model (see Fig.11, right part - note
however that the experimental and model curves do not quite overlap; see also Hennig et al.,
2008).
This is just one recent example illustrating how models of synaptic short term plasticity may
need to be amened to include further processes which simultaneously contribute to synaptic dynamics.
It is by no means clear and an important topic of future research what the
computational consequences of synaptic dynamics with multiple, parallel processes are.
4 Short Term Plasticity
Fig. 11:
15
Left part: Presynaptic calcium current and postsynaptic current amplitude during
repetitive stimulation of the calyx of Held. The graphs on the right show the normalised amplitudes of the calcium current, the calcium current raised to the power of
3.6 and the postsynaptic current (recorded during presynaptic depolarisation or bre
stimulation). Right part: Comparison of EPSC amplitudes obtained experimentally
(A), from a depletion model (B) and a depletion model with calcium deactivation (C)
(compiled from Xu and Wu, 2005).
References
Abbott LF, Varela JA, Sen K & Nelson SB (1997).
Synaptic depression and cortical gain
control. Science 275, 220224.
Andrews SS & Arkin AP (2006). Simulating cell biology. Curr Biol 16, 523527.
Ben-Ari Y (2002). Excitatory actions of gaba during development: the nature of the nurture.
Nat Rev Neurosci 3, 728739.
Betz WJ (1970). Depression of transmitter release at the neuromuscular junction of the frog.
J Physiol 206, 629644.
Billups B, Graham B, Wong A & Forsythe I (2005).
Unmasking Group III metabotropic
glutamate autoreceptor function at excitatory synapses. J Physiol 565, 885896.
Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman
MH & Sejnowski TJ (2005). Evidence for ectopic neurotransmission at a neuronal synapse.
Science 309, 446451.
Dayan P & Abbott LF (2001). Theoretical Neuroscience: Computational and Mathematical
Modeling of Neural Systems The MIT Press.
de la Rocha J & Parga N (2005).
Short-term synaptic depression causes a non-monotonic
response to correlated stimuli. J Neurosci 25, 84168431.
4 Short Term Plasticity
16
Destexhe A, Mainen ZF & Sejnowski TJ (1994a). Synthesis of models for excitable membranes,
synaptic transmission and neuromodulation using a common kinetic formalism. J Comput
Neurosci 1, 195231.
Destexhe A, Mainen ZF & Sejnowski TJ (1994b). Synthesis of models for excitable membranes,
synaptic transmission and neuromodulation using a common kinetic formalism. J Comput
Neurosci 1, 195230.
Dittman JS, Kreitzer AC & Regehr WG (2000). Interplay between facilitation, depression,
and residual calcium at three presynaptic terminals. J Neurosci 20, 13741385.
Eccles JC & Jaeger JC (1958).
The relationship between the mode of operation and the
dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond
B Biol Sci 148, 3856.
Forsythe I, Tsujimoto T, Barnes-Davies M, Cuttle M & Takahashi T (1998). Inactivation of
presynaptic calcium current contributes to synaptic depression at a fast central synapse.
Neuron 20, 797807.
Franks KM, Stevens CF & Sejnowski TJ (2003). Independent sources of quantal variability
at single glutamatergic synapses. J Neurosci 23, 31863195.
Fuhrmann G, Cowan A, Segev I, Tsodyks M & Stricker C (2004). Multiple mechanisms govern
the dynamics of depression at neocortical synapses of young rats. J Physiol 557, 415438.
Haydon PG (2001). Glia: listening and talking to the synapse. Nat Rev Neurosci 2, 185193.
Hennig MH, Postlethwaite M, Forsythe ID & Graham BP (2008). Interactions between multiple sources of short-term plasticity during evoked and spontaneous activity at the rat calyx
of held. J Physiol 586, 31293146.
Hennig MH, Postlethwaite M, Forsythe ID & Graham BP (2007).
A biophysical model of
short-term plasticity at the calyx of held. Neurocomput. 70, 16261629.
Hines M & Carnevale N (1997). The NEURON simulation environment. Neural Comput 9,
11791209.
Hu EH & Bloomeld SA (2003). Gap junctional coupling underlies the short-latency spike
synchrony of retinal alpha ganglion cells. J Neurosci 23, 67686777.
Jones MV & Westbrook GL (1996). The impact of receptor desensitization on fast synaptic
transmission. Trends Neurosci 19, 96101.
Kerr RA, Bartol TM, Kaminsky B, Dittrich M, Chang JCJ, Baden SB, Sejnowski TJ & Stiles
JR (2008). Fast monte carlo simulation methods for biological reaction-diusion systems in
solution and on surfaces. SIAM J. Sci. Comput. 30, 31263149.
Liley AW & North KA (1953). An electrical investigation of eects of repetitive stimulation
on mammalian neuromuscular junction. J Neurophysiol 16, 509527.
Lou X, Scheuss V & Schneggenburger R (2005).
Allosteric modulation of the presynaptic
Ca(2+) sensor for vesicle fusion. Nature 435, 497501.
4 Short Term Plasticity
17
Markram H, Wang Y & Tsodyks M (1998). Dierential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci U S A 95, 53235328.
Metropolis N (1987).
The beginning of the monte carlo method.
Los Alamos Science 15,
125130.
Perez Velazquez JL & Carlen PL (2000).
Gap junctions, synchrony and seizures.
Trends
Neurosci 23, 6874.
Postlethwaite M, Hennig MH, Steinert JR, Graham BP & Forsythe ID (2007). Acceleration
of ampa receptor kinetics underlies temperature-dependent changes in synaptic strength at
the rat calyx of held. J Physiol 579, 6984.
Raghavachari S & Lisman J (2004). Properties of quantal transmission at CA1 synapses. J
Neurophysiol 92, 24562467.
Rizzoli SO & Betz WJ (2005). Synaptic vesicle pools. Nat Rev Neurosci 6, 5769.
Robert A & Howe J (2003). How AMPA receptor desensitization depends on receptor occupancy. J Neurosci 23, 847858.
Sakaba T & Neher E (2001). Calmodulin mediates rapid recruitment of fast-releasing synaptic
vesicles at a calyx-type synapse. Neuron 32, 11191131.
Slepchenko BM, Scha JC, Carson JH & Loew LM (2002).
Computational cell biology:
spatiotemporal simulation of cellular events. Annu Rev Biophys Biomol Struct 31, 423441.
Stauer D (2005). Interdisciplinary monte carlo simulations arXiv:physics/0512098.
Stiles JR & Bartol TM (2001). Computational Neuroscience: Realistic Modeling for Experimentalists chapter Monte Carlo methods for simulating realistic synaptic microphysiology
using MCell, pp. 87128. CRC press, Boca Raton.
Stiles J, Bartol T, Salpeter M, Salpeter E & Sejnowski T (2001). Synaptic variability: New
insights from reconstructions and Monte Carlo simulations with MCell In Cowan W, Sudhof
T & Stevens C, editors, Synapses, pp. 581731. Johns Hopkins Press.
Sudhof TC (2004). The synaptic vesicle cycle. Annu Rev Neurosci 27, 509547.
Takago H, Nakamura Y & Takahashi T (2005).
G protein-dependent presynaptic inhibi-
tion mediated by AMPA receptors at the calyx of Held. Proc Natl Acad Sci U S A 102,
73687373.
Takahashi T, Forsythe I, Tsujimoto T, Barnes-Davies M & Onodera K (1996). Presynaptic
calcium current modulation by a metabotropic glutamate receptor. Science 274, 594597.
Trommershäuser J, Schneggenburger R, Zippelius A & Neher E (2003). Heterogeneous presynaptic release probabilities: functional relevance for short-term plasticity.
Biophys J 84,
15631579.
Tsodyks MV & Markram H (1997). The neural code between neocortical pyramidal neurons
depends on neurotransmitter release probability. Proc Natl Acad Sci U S A 94, 719723.
4 Short Term Plasticity
18
Wang L & Kaczmarek L (1998). High-frequency ring helps replenish the readily releasable
pool of synaptic vesicles. Nature 394, 384388.
Wang Y, Markram H, Goodman PH, Berger TK, Ma J & Goldman-Rakic PS (2006). Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci 9, 534542.
Wong A, Graham B, Billups B & Forsythe I (2003).
Distinguishing between presynaptic
and postsynaptic mechanisms of short-term depression during action potential trains.
J
Neurosci 23, 48684877.
Wu W, Xu J, Wu XS & Wu LG (2005). Activity-dependent acceleration of endocytosis at a
central synapse. J Neurosci 25, 1167611683.
Xu J & Wu LG (2005). The decrease in the presynaptic calcium current is a major cause of
short-term depression at a calyx-type synapse. Neuron 46, 633645.
Zucker RS & Regehr WG (2002).
355405.
Short-term synaptic plasticity.
Annu Rev Physiol 64,