MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
PETROLEUM SCIENCE AND TECHNOLOGY
Vol. 21, Nos. 1 & 2, pp. 275–282, 2003
Green Catalytic Oxidation of Cyclohexanone
to Adipic Acid
Shi-gang Zhang, Heng Jiang,* Hong Gong,
and Zhao-lin Sun
Department of Materials Science, Fushun Petroleum
Institute, Fushun, P.R. China
ABSTRACT
Synthesis of adipic acid by catalytic oxidation of cyclohexanone with
30% hydrogen peroxide in the presence of sodium tungstate catalyst
can be well performed under reflux temperature. The adipic acid
isolated yield reaches to 82.1%, and the purity of the product is also
very high. The catalytic oxidation reaction takes place without any
organic solvent and phase-transfer agent. The effects over different
acidic ligands and the amount of the ligand on the catalytic oxidation
were investigated. The catalysts exhibit high activity for the catalytic
oxidation reaction of the mixture of cyclohexanone and cyclohexanol
to adipic acid by 30% hydrogen peroxide under reflux temperature.
*Correspondence: Heng Jiang, Department of Materials Science, Fushun
Petroleum Institute, Fushun 113001, P.R. China; E-mail: hjiang78@hotmail.
com.
275
DOI: 10.1081/LFT-120016948
Copyright & 2003 by Marcel Dekker, Inc.
1091-6466 (Print); 1532-2459 (Online)
www.dekker.com
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
276
Zhang et al.
Key Words: Adipic acid; Green catalytic oxidation; Cyclohexanone; Hydrogen peroxide.
INTRODUCTION
Adipic acid is an important chemical mainly used for manufacture of
nylon-6,6, whose production is up to 2.2 million metric tons per year
(Xuan, 1999). Currently the industrial production of adipic acid uses
nitric acid oxidation of cyclohexanol or a tow-step oxidation of cyclohexane process by nitric acid. The nitrous oxide is the inevitable stoichiometric waste produced by this process. Despite the efforts made to decrease
the emission of this waste by recovering or recycling of the nitrous oxide,
about 400,000 metric tons are still emitted into the environment each year,
which corresponds to 5–8% of the worldwide anthropogenic emission of
N2O (Noyori et al., 1998). As it is well known that the nitrous oxides cause
the ozone depletion as well as acid rain and smog which are harmful
for our earth. Together with the development of the environmental
legalization and the environmentally conscious of the common people,
to find a new clean way to produce adipic acid has become very necessary.
Many attempts have been made to substitute the classical process, such as
the direct oxidation of cyclohexane to adipic acid by molecular oxygen
(Schulz and Onopchenko, 1981) or by air (Park and Goroff, 1993),
manufacture of adipic acid by hydrocarboxylation of pentenic acid
(Denis et al., 1995; Bruner et al., 1998), and synthesis of adipic acid
from biomass-derived carbon sources (Frost and Draths, 1996) or from
D-glucose (Draths and Frost, 1994). But these processes would not be the
ideal one because of the low yield or the terminal products are too complex
for the isolation of the wanted product.
Aqueous hydrogen peroxide as an ideal clean oxidant also achieves
great interest. Noroyi et al., reported a practical method of oxidation
cyclohexene with 30% hydrogen peroxide in presence of small amounts
of Na2WO4 and [CH3(n-C8H17)3N]HSO4 as a phase transfer catalyst.
A novel clean peroxy tungstate-organic complex catalyst was also used
to catalyze oxidation of cyclohexene by 30% hydrogen peroxide to
produce adipic acid at a high yield (Ma et al., 2001). Long chain carbon
alkyl ammonium sulfate was used to substitute the expensive phrase
transfer catalyst to produce adipic acid and obtained a yield of 81.7%
(Gong et al., 2000). The industrial production of cyclohexene is mainly
by partially hydrogenation of benzene or by dehydration of cyclohexanol.
As the process needs critical conditions, the price of cyclohexene is very
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
Cyclohexanone Oxidation
277
high which cause much effect on the production cost of the adipic acid.
However the industrial process of oxidation cyclohexane to cyclohexanol
and cyclohexanone has become quite efficient. In this paper, we chose
Na2WO42H2O as catalyst to oxidize cyclohexanone to adipic acid by
30% hydrogen peroxide in the presence of different acidic ligands. The
effects of the kinds of the ligands, the amounts of the ligand and the reaction time on the catalytic reaction are studied. Further researches show
that the catalytic system also gives a well catalytic ability for the oxidation
of mixture of cyclohexanone and cyclohexanol. Based on these works,
we discuss the catalytic mechanism of this kind of catalysis oxidation.
EXPERIMENTAL
The reagents used in the reaction are analytical purity reagents.
0.825 g Na2WO4H2O (2.5 mmol), desired amounts of acidic ligand
and 44.5 mL 30% hydrogen peroxide (440 mmol) were placed in an
100 mL flask, stirred for 10–15 min to get a clear solution at room
temperature. Then 10.5 mL cyclohexanone (100 mmol) was added into
it without stopping stirring. Continue stirring the reaction mixture at a
reflux temperature for 8 h. After reaction was completed the reaction
mixture was cooled in the refrigerator for 12 h. Then white crystalline
product was obtained after filtration, washed with ice water and dried
in the air. The product was weighted and the isolated yield of the adipic
acid was calculated which was based on the cyclohexanone that was put
in the reaction flask. A melting point tube was used to measure the
melting point of the product (the thermometer is not justified). The
melting point of the product is mainly between 149 and 151 C (the
reported result is 152 C).
RESULTS AND DISCUSSION
The effect of different acidic ligands on the reaction is showed in
Table 1. It can be seen that there is no adipic acid crystalline obtained
when no acidic ligand is added into the reaction mixture. The isolated
yield increases noticeably when acidic ligand is present. It can be
explained that when the ligand is added into catalytic system, it can
coordinate with the water soluble sodium tungstate to form a water
soluble complex, which is easily to contact with the oil phase, thus the
reaction speed is accelerated. The highest yield showed in the Table 1
—
9.45
9.44
10.0
—
4.09
5.10
4.17
1.23
3.22
2.90
2.85
4.21
—
—
25 C pKa
0
74.8
72.1
71.1
23.7
62.4
74.3
67.0
58.6
59.7
15.9
70.1
20.4
55.2
44.8
Isolated yield (%)
Anthranilic acid
Phthalic acid
Benzoic acid
Hydroxylamine hydrochloride
Diaminehydrochloride
Hydrazine sulfate
Phosphoric acid
Phosphorous acid
Metaphosphoric acid
NaH2PO42H2O
NaHSO4
Sulfosalicylic acid
Salicylic acid
Sulfamic acid
p-Toluenesulfonic acid
Acidic ligand
2.05
2.95
4.21
—
—
—
2.12
2.15
—
—
—
—
2.98
—
—
25 C pKa
The effect of different acidic ligand on the catalytic reaction.
66.3
77.6
1.8
66.7
48.3
61.7
75.0
73.4
3.4
0
73.7
71.2
75.4
66.3
18.1
Isolated yield (%)
278
Reaction conditions: Na2WO42H2O, 2.5 mmol; catalyst/acid ligand ¼ 1/1 (mol ratio); 30% H2O2, 44.5 mL; cyclohexanone,
100 mmol; reaction time, 8 h; reflux temperature.
None
Pyrocatechol
Resorcinol
Hydroquinone
o-Phenylenediamine
2,4-Dinitrophenol
8-Quinolinol
L(þ)Ascorbic acid
Oxalic acid
Tartaric acid
Bromoacetic acid
Malonic acid
Succinic acid
Nicotinic acid
i-Nicotinic acid
Acidic ligand
Table 1.
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
Zhang et al.
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
Cyclohexanone Oxidation
279
is 77.6% when phthalic acid is used as the acidic ligand. It also can be
seen from Table 1 that for the same kind of ligands, the isolated yield of
adipic acid increases with the increasing acidity of the acidic ligand. The
acid condition is favored for the oxidation when hydrogen peroxide
is used as oxidant, but this is not the only factor. Although the acidity
of 8-quinolinol, pyrolatechol, resorcinol and hydroquinone is low, their
isolated yield of adipic acid is much higher than that of 2,4-dinitrophenol,
and bromoacetic acid. There may be something to do with the coordinate
effect of the ligand when it coordinates with sodium tungstate. The pKa
value of pyrocatechol, resorcinol and hydroquinone is in the range of 9–
10, however, the isolated yield of adipic acid is very high. Since these
phenolic compounds are radical inhibitors, we can infer that the oxidation reaction is not a free radical mechanism but coordination catalysis
mechanism.
The effect of different amounts of ligands on the reaction is showed
in Table 2. Phosophoric acid, sulfosalicylic acid, hydroquinone,
Table 2.
Acidic ligand
Phosphoric acid
Sulfosalicylic acid
Hydroquinone
Pyrocatechol
The effect of acidic ligand amount.
Ligand amount (mmol)
Product isolated
yield (%)
1.014
1.523
2.567
5.22
10.22
0.675
1.25
2.5
5.0
10.0
2.5
5.0
10.0
2.5
5.0
10.0
9.6
29.1
75.0
65.7
60.4
27.4
82.1
71.2
71.4
59.6
71.1
76.3
71.1
74.8
78.6
71.7
Reaction condition: Na2WO42H2O, 2.5 mmol; cyclohexanone,
100 mmol; 30% H2O2, 44.5 mL; reaction time, 8 h; reflux
temperature.
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
280
Zhang et al.
pyrocatechol are used with different amounts to study these effects.
Table 2 shows that the isolated yield of adipic is low if the amount of
acidic ligand is too little and too much. Using sulfosalicylic acid as ligand,
the isolated yield of adipic acid reaches the highest when the mole ratio of
the catalyst and the ligand is 2/1. When the amount of ligand is too little,
sodium tungstate cannot form coordinate complex with ligand
thoroughly, which will affect the catalyst to contact with the oil
phase. When the ligand is too much, the ligand will form with the
Na2WO42H2O to multiple chelate, thus the peroxide bonds in the catalyst structure may be destroyed. This also can be partly proved by the
phenomena of using tartaric acid as ligand. Though acid ability of
tartaric acid is high, the isolated yield is very low as Table 1 showed
because the tartaric acid can coordinate with the catalyst to form multiple
chelate. Furthermore, too much ligand dissolved in the reaction mixture
will increase the solubility of the product that will decrease the isolated
yield, too.
Effect of the reaction time on the reaction is showed in Table 3. From
Table 3 we can see that the isolated yield of adipic acid increase at first
when the reaction time prolonged and reaches the highest isolated yield
at 10 and 6.5 h, respectively. Long reaction time leads to the decreasing
Table 3.
The effect of reaction time on the catalytic oxidation.
Acidic ligand
Reaction time (h)
Product isolated
yield (%)
Hydroquinone
4
6
8
10
12
14
2
3
4.5
5
6.5
8
70.1
73.5
71.1
76.9
76.4
70.1
9.30
18.8
47.0
59.5
63.7
39.5
L(þ)ascorbic
acid
Reaction condition: Na2WO42H2O, 2.5 mmol; catalyst/acid
ligand ¼ 1/1 (mole ratio); 30% H2O2, 44.5 mL; cyclohexanone,
100 mmol; reflux temperature.
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
Cyclohexanone Oxidation
281
yield of adipic acid slightly. The further oxidation of the adipic acid to
soluble products such as succinic acid and -valeroactone may be the
probable reason.
Based on the above researches, we investigate the catalytic behavior
of catalysis system for the mixture of cyclohexanone and cyclohexanol.
The results are summarized in Table 4. The results indicate that the
catalysis system exhibit the highest catalytic activity for the oxidation
of mixture of cyclohexanol and cyclohexanone with the mole ratio of
cyclohexanol and cyclohexanone being 1/2. The isolated yield reaches
to 79.1% when sulfosalicylic acid was used as the ligand, but it is still
lower than 82.1% isolated yield of adipic acid when pure cyclohexanone
is used as substrate. It also shows that the oxidation of cyclohexanol is
not easier than that of cyclohexanone.
According to the research of Noyori et al., (1998) the oxidation
reaction of cyclohexene to obtain adipic acid catalyzed by Na2WO42H2O
Table 4.
Catalytic oxidation of the mixture of cyclohexanone and cyclohexanol.
Ligand
Sulfosalicylic acid
Hydroquinone
Pyrocatechol
Ligand
amount
(mmol)
1
2.5
1.25
2.5
2.5
62.3
68.6
37.5
45.9
Cyclohexanol/cyclohexanone (mol ratio)
4/1
68.6
71.4
44.8
46.2
2/1
72.0
74.8
52.7
50.2
1/1
73.3
76.6
58.3
53.9
1/2
74.3
79.4
61.9
60.3
1/4
73.2
78.2
63.2
52.2
0
71.2
82.1
71.1
74.8
Reaction conditions: Na2WO42H2O, 2.5 mmol; 30%H2O2, 44.5 mL; cyclohexanone þ cyclohexanol ¼ 100 mmol; reaction time, 8 h; reflux temperature.
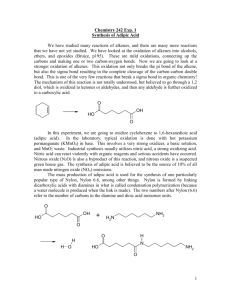
Scheme 1.
The proposed reaction mechanism.
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016
©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc.
282
Zhang et al.
should follow multiple steps involving three kinds of oxidation
reactions (olefin epoxidation, two alcohol oxidation, and Baeyer–
Villiger oxidation) and hydrolyses. The oxidation of cyclopentanone to
obtain -valeroactone by hydrogen peroxide reported by Fischer and
Hölderich, (1999) also shows that glutaric acid is one of side product.
According to these facts and our research results, the proposed reaction
mechanism is depicted in Sch. 1.
REFERENCES
Bruner, H. S., Lane, S. L., Murphree, B. E. (1998). Manufacture of adipic
acid. US Patent 5,710,325.
Denis, P., Grosslin, J., Metz, F. (1995). Preparation of adipic acid by
hydrocarboxylation of pentenic acids. US Patent 5,420,346.
Draths, K. M., Frost, J. W. (1994). Environmentally compatible synthesis of adipic acid from D-glucose. J. Am. Chem. Soc. 116:399.
Fischer J., Hölderich, W. F. (1999). Baeyer–Villiger-oxidation of cyclopentanone with aqueous hydrogen peroxide by acid heterogeneous
catalysis. Appl. Catal. A: General 180:435.
Frost, J. W., Draths, K. M. (1996). Synthesis of adipic acid from biomass-derived carbon sources. US Patent 5,487,987.
Gong, H., Jiang, H., Lu, Z. B. (2000). A new green route to the adipic
acid. Chem. J. Chinese Universities 21(7):1121.
Ma, Z. F., Deng, Y. Q., Wang, K., Cheng, J. (2001). Clean catalytic
oxidation to adipic acid. Chemistry Bulletin (Chinese) 2:116.
Park, C. M., Goroff, N. S. (1993). One step air oxidation of cyclohexane
to produce adipic acid. US Patent 5,221,800.
Sato, K., Aoki, M., Noyori, R. (1998). A green route to adipic acid:
direct oxidation of cyclohexanes with 30 percent hydrogen peroxide. Science 281:1646.
Schulz, J. G. D., Onopchenko, A. (1981). A process for converting cyclohexane to adipic acid. US Patent 4,263,453.
Xuan, E. F. (1999). The production and market analysis of adipic acid.
Chemical Production and Technology (Chinese) 6(4):59.
Received December 31, 2001
Accepted February 23, 2002