university of alabama at birmingham
advertisement

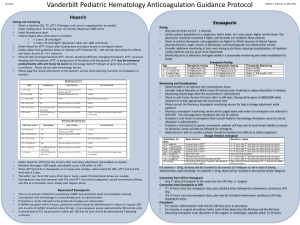
Marquette General Health System Pharmacy and Therapeutics Committee Medication Guideline Drug Classification: 20:12.04.16 Anticoagulants/Heparins Agent: Formulary X Enoxaparin (Lovenox®) Dalteparin (Fragmin®) Tinzaparin (Innohep®) Nonformulary Restricted Nonstock X X X X Pharmacy and Therapeutics Committee-approved Indications for Inpatient Use: Prophylaxis of venous thromboembolism (VTE) including: o Deep vein thrombosis (DVT) in patients who are at risk for thromboembolic complications undergoing abdominal surgery, hip or knee replacement surgery, or medical patients with severely restricted mobility during acute illness. o Ischemic complications of unstable angina and non-Q wave myocardial infarction (MI). Treatment of the following conditions: o DVT in patients with or without pulmonary embolism (PE). o Treatment of acute ST-segment elevation myocardial infarction (STEMI) managed medically or with subsequent percutaneous coronary intervention (PCI). Note: In patients with suspected/presumed heparin-induced thrombocytopenia (HIT) or in patients with a recent history of HIT, enoxaparin should not be utilized. Consider alternative anticoagulants including fondaparinux or the direct thrombin inhibitors (argatroban, bivalirudin). Dosage and Administration: Table 1 depicts specific dosing recommendations based on actual body weight. All patients should be monitored for signs and symptoms of bleeding. Unless otherwise indicated, enoxaparin should be administered by deep subcutaneous (SC) injection. Administration should be alternated between the left and right anterolateral and left and right posterolateral abdominal wall. Bridging recommendations during initiation of warfarin therapy: The following are recommendations from the 2012 CHEST guidelines. discontinue therapeutic enoxaparin once the INR value reaches: 2.5 for mechanical heart valves in mitral and aortic position 1.5 for orthopedic surgery patients 2.0 for all other patient populations Document created: 09/11. Revised: 07/13. Cross Reference: Anticoagulation Monitoring and Safety (100-228). In general, it is recommended to Marquette General Health System Marquette General Hospital Marquette, MI 49855 This is a confidential professional/peer review and quality assessment document of Marquette General Health System of Marquette, MI. It is protected from disclosure pursuant to the provisions of MCL 333.20175, MCL 333.21513, MCL 21515, MCL 331.531, MCL 331.533, MCL 330.1143a, and other state and federal laws. Unauthorized disclosure or duplication is absolutely prohibited. Marquette General Health System Pharmacy and Therapeutics Committee Medication Guideline Table 1: Enoxaparin Dosing Recommendations. Dosing* Indication Prophylaxis VTE Abdominal surgery: 40 mg every 24 hours Knee replacement surgery: 30 mg every 12 hours Hip replacement surgery: 30 mg every 12 hours or 40 mg every 24 hours Medical patients with severely restricted mobility: 40 mg every 24 hours Trauma: 30 mg every 12 hour Pediatric patients < 2 months: initial dose of 0.75 mg/kg every 12 hours Pediatric patients > 2 months: initial dose of 0.5 mg/kg every 12 hours Dosage adjustment for special patient populations: Renal impairment (CrCl <30 mL/minute): 30 mg every 24 hours Moderate to severe obesity (>130% IBW; TBW >150 kg; or BMI >50 kg/m2): 40 mg every 12 hours‡ Pregnancy: 30 mg every 12 hours or 40 mg every 24 hours Usual dose: 1 mg/kg every 12 hours Non-ST-segment elevation acute coronary syndrome Undergoing PCI: If the last dose of enoxaparin was given < 8 h prior to PCI, no additional anticoagulant therapy is needed. If the last dose of enoxaparin was given 8 to 12 hours before PCI, a 0.3 mg/kg bolus of IV enoxaparin at the time of PCI should be administered Dosage adjustment for special patient populations: Renal impairment (CrCl <30 mL/minute): 1 mg/kg every 24 hours Treatment Usual dose: 1mg/kg every 12 hours (with warfarin)† OR 1.5 mg/kg every 24 hours (with warfarin)† Pediatric patients < 2 months: 1.5 mg/kg every 12 hours Pediatric patients > 2 months: 1 mg/kg every 12 hours DVT with/without PE Dosage adjustment for special patient populations: Renal impairment (CrCl <30 mL/minute): 1 mg/kg every 24 hours Alternative dosing in renal impairment: CrCl 30-60 mL/minute: Normal dosing on day 1 followed by 0.8 mg/kg/dose every 12 hours CrCl <30 mL/minute: 1 mg/kg/dose on day 1 followed by 0.65 mg/kg/dose every 12 hours Note: In patients with acute DVT and severe renal failure, heparin is the recommended anticoagulant. Morbid Obesity: (>150 kg or BMI>50 kg/m2): 1mg/kg every 12 hours; adjust dose based on anti-Xa levels. Note: Unfractionated heparin (weight-based IV infusion) is the recommended anticoagulant in patients weighing greater than 150 kg or with a BMI >50 kg/m2. STEMI Pregnancy: 1 mg/kg every 12 hours Patients <75 years of age with preserved renal function (serum creatinine levels 2.5 mg/dL in males and 2 mg/dL in females): Single 30 mg IV bolus dose plus a 1 mg/kg SC dose followed by 1 mg/kg every 12 hours (maximum dose of 100 mg for the first 2 doses only, followed by 1 mg/kg). Renal impairment: Single 30 mg IV bolus plus a 1 mg/kg SC dose followed by 1 mg/kg SC every 24 hours. Patients >75 years of age with preserved renal function: 0.75 mg/kg SC every 12 hours (maximum of 75 mg for the first 2 doses only followed by 0.75 mg/kg) Renal impairment: 1 mg/kg SC every 24 hours *Round doses to the nearest 10 mg for patients weighing 50 kg or more and to the nearest 5 mg for patients weighing less than 50 kg. † Initiate warfarin therapy, if warranted, within 72 hours and continue enoxaparin until INR is within goal range. ‡ Dose increase if the preferred regimen based on medical condition is 30 mg every 12 hours. Monitoring/Outcomes: Baseline laboratory: hematocrit, platelet count, serum creatinine Signs and symptoms of DVT, bleeding/bruising, low blood pressure, tachycardia, neurological impairment Laboratory: complete blood count, stool occult blood test, potassium levels, renal function, anti-Xa levels, liver function tests. Document created: 09/11. Revised: 07/13. Cross Reference: Anticoagulation Monitoring and Safety (100-228). Marquette General Health System Marquette General Hospital Marquette, MI 49855 This is a confidential professional/peer review and quality assessment document of Marquette General Health System of Marquette, MI. It is protected from disclosure pursuant to the provisions of MCL 333.20175, MCL 333.21513, MCL 21515, MCL 331.531, MCL 331.533, MCL 330.1143a, and other state and federal laws. Unauthorized disclosure or duplication is absolutely prohibited. Marquette General Health System Pharmacy and Therapeutics Committee Medication Guideline Anti-Xa Monitoring Routine monitoring of anti-Xa levels with enoxaparin is not required; however, monitoring in specific patient populations receiving enoxaparin for treatment purposes, such as those with renal impairment, obese or pregnant, may be necessary to ensure therapeutic anti-Xa activity. The most adequate anti-Xa range for enoxaparin prophylaxis remains to be defined and is not recommended to be monitored at this time. o A therapeutic peak anti-Xa level with twice daily dosing for treatment purposes is 0.5-1 unit/ml; with once daily dosing anti-Xa activity should be between 1-2 units/ml. o Peak anti-Xa activity should be obtained starting on day two in the special patient populations listed above. o Peak anti-Xa activity should also be considered in patients with hemorrhage or following any dosage adjustment. o If accumulation is suspected, trough anti-Xa levels (drawn prior to next dose) may be obtained. The target trough anti-Xa level is <0.5 units/ml. Table 2: Enoxaparin Dose Adjustments Based on Peak Anti-Xa Levels* Peak Anti- Xa Level Recommended Dosage Adjustment Next Anti-Xa Level <0.35 units/mL Increase dose by 25% Peak level 4 hours after next dose 0.35-0.49 units/mL Increase dose by 10% Peak level 4 hours after next dose 0.5-1 units/mL No change Next day, then 1 week later, then monthly (4 hours after dose) 1.1-1.5 units/mL Decrease dose by 20% Peak level 4 hours after next dose 1.6-2 units/mL Hold dose for 3 hours and decrease dose by 30% Trough level prior to next dose, then peak level 4 hours after next dose Hold all doses until anti-Xa level is 0.5 units/mL, then decrease dose by 40% *Adjustments for twice daily dosing. >2 units/mL Trough level prior to next scheduled dose and every 12 hours thereafter until anti-Xa level <0.5 units/mL Anti-Xa Neutralization: There is no proven method for neutralizing enoxaparin. Protamine sulfate only partially (~60%) neutralizes the antiXa activity of enoxaparin. Unless an immediate effect is needed, discontinuation of enoxaparin is generally sufficient. If an immediate effect is needed, protamine sulfate may be given to attempt reversal of the anticoagulant effect. If enoxaparin was given within 8 hours, protamine sulfate should be administered in a dose of 1 mg per 1 mg enoxaparin (i.e. 80 mg protamine for 80 mg of enoxaparin). If bleeding continues a dose of protamine 0.5 mg per 1 mg of enoxaparin may be administered. Smaller doses of protamine can be given if the time since enoxaparin administration was longer than 8 hours. Protamine should be administered by slow intravenous push at a rate of 5 mg per minute to prevent hypotension. Drug-Drug Interactions: The effects of the following drug-drug interactions with enoxaparin are considered to be potentially life-threatening or capable of causing permanent damage: Heparin Argatroban or Bivalirudin or Dabigatran Fondaparinux Neuraxial anesthesia/spinal puncture Document created: 09/11. Revised: 07/13. Cross Reference: Anticoagulation Monitoring and Safety (100-228). Marquette General Health System Marquette General Hospital Marquette, MI 49855 This is a confidential professional/peer review and quality assessment document of Marquette General Health System of Marquette, MI. It is protected from disclosure pursuant to the provisions of MCL 333.20175, MCL 333.21513, MCL 21515, MCL 331.531, MCL 331.533, MCL 330.1143a, and other state and federal laws. Unauthorized disclosure or duplication is absolutely prohibited. Marquette General Health System Pharmacy and Therapeutics Committee Medication Guideline Interruption of Anticoagulant Therapy For Invasive Procedures: The risk of thromboembolism versus bleeding risk should be evaluated for all patients receiving enoxaparin and undergoing an invasive procedure. Refer to the 2008 Chest guidelines, the perioperative management of antithrombotic therapy, for a complete discussion on risk stratification for thromboembolism and procedures associated with a high bleeding risk. Preoperative management Administer the last dose of therapeutic enoxaparin 24 hours before surgery or procedure, for the last preoperative dose of therapeutic dose enoxaparin approximately half the total daily dose should be administered. Postoperative management Minor surgical or other invasive procedure Therapeutic-dose enoxaparin may be resumed approximately 24 hours after the procedure when there is adequate hemostasis. Major surgery of a high bleeding risk Delay the initiation of therapeutic-dose enoxaparin for 48 to 72 hours post surgery when hemostatsis is secured. Special Handling Procedures: Anticoagulants/heparins are not considered hazardous drugs. Anticoagulants/heparins are considered high-alert drugs. References: Enoxaparin (Lovenox®) [package insert]. Bridgewater, NJ: Sanofi-Aventis;2009. Hirsh J, Baue KA, Donat MB, et al. Prevention of venous thromboembolism. Chest 2008;133;381S-453S. Goodman SG, Menon V, Cannon CP, et al. Acute ST-segment elevation myocardial infarction. Chest 2008;133;708S-775S. Hirsh J, Bauer KA, Donati MB, et al. Parenteral anticoagulants. Chest 2008;133;141S-159S. Harrington RA, Becker RC, Cannon CP, et al. Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes. Chest 2008;133:670S-707S. Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. Chest 2008;133:454S545S. Douketis JD, Berger PB, Dun AS, et al. The perioperative management of antithrombotic therapy. Chest 2008;133:299S339S. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Regional Anesthesia and Pain Medicine 2010;35(1):64-101. Duhl AJ, Paidas MJ, Ural SH, et al. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromboembolism and adverse pregnancy outcomes. Am J Obstet Gynecol 2007;197:457e1-e21. Monagle P, Michelson AD, Bovill E, et al. Antithrombotic therapy in children. Chest 2001;119:344S-370S. Hulot JS, Montalescot G, Lechat P, et al. Dosing strategy in patients with renal failure receiving enoxaparin for the treatment of non-ST-segment elevation acute coronary syndrome. Clin Pharmacol Ther. 2005;77:542-52. Kruse MW, Lee JJ. Retrospective evaluation of a pharmacokinetic program for adjusting enoxaparin in renal impairment. Am Heart J. 2004;148:582-9. Collet JP, Montalescot G, Choussat R, et al. Enoxaparin in unstable angina patients with renal failure. Int J Cardiol. 2001;80:81-2. Document created: 09/11. Revised: 07/13. Cross Reference: Anticoagulation Monitoring and Safety (100-228). Marquette General Health System Marquette General Hospital Marquette, MI 49855 This is a confidential professional/peer review and quality assessment document of Marquette General Health System of Marquette, MI. It is protected from disclosure pursuant to the provisions of MCL 333.20175, MCL 333.21513, MCL 21515, MCL 331.531, MCL 331.533, MCL 330.1143a, and other state and federal laws. Unauthorized disclosure or duplication is absolutely prohibited.