Lewis Structures: Drawing Molecules & Chemical Bonds
advertisement
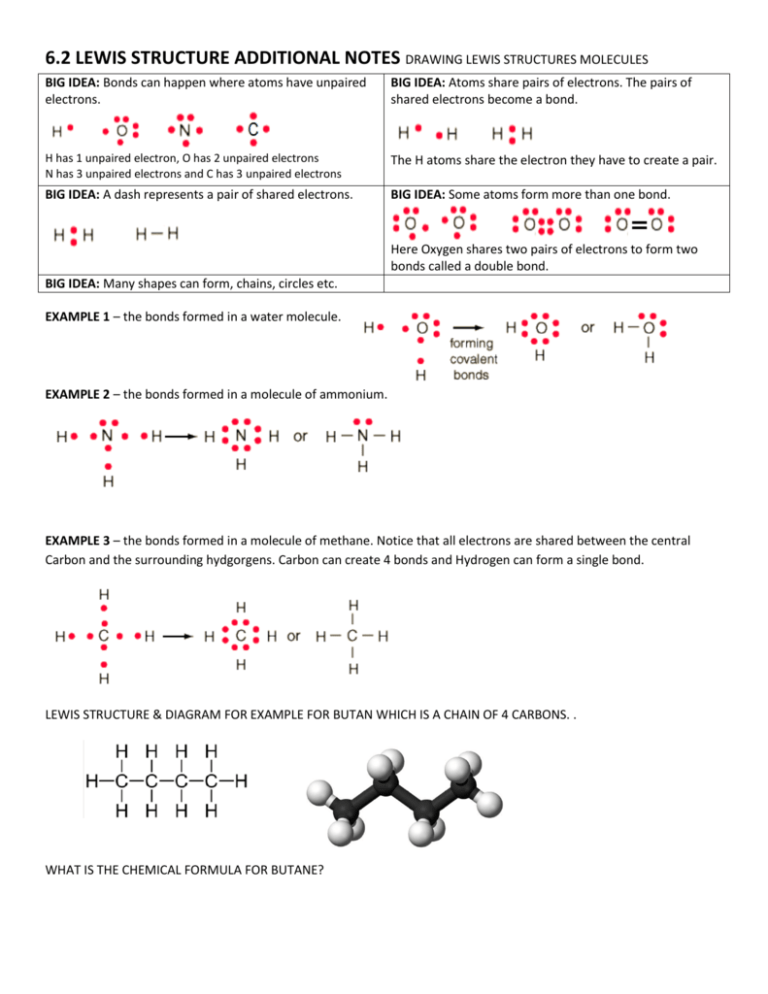
6.2 LEWIS STRUCTURE ADDITIONAL NOTES DRAWING LEWIS STRUCTURES MOLECULES BIG IDEA: Bonds can happen where atoms have unpaired electrons. BIG IDEA: Atoms share pairs of electrons. The pairs of shared electrons become a bond. H has 1 unpaired electron, O has 2 unpaired electrons N has 3 unpaired electrons and C has 3 unpaired electrons The H atoms share the electron they have to create a pair. BIG IDEA: A dash represents a pair of shared electrons. BIG IDEA: Some atoms form more than one bond. Here Oxygen shares two pairs of electrons to form two bonds called a double bond. BIG IDEA: Many shapes can form, chains, circles etc. EXAMPLE 1 – the bonds formed in a water molecule. EXAMPLE 2 – the bonds formed in a molecule of ammonium. EXAMPLE 3 – the bonds formed in a molecule of methane. Notice that all electrons are shared between the central Carbon and the surrounding hydgorgens. Carbon can create 4 bonds and Hydrogen can form a single bond. LEWIS STRUCTURE & DIAGRAM FOR EXAMPLE FOR BUTAN WHICH IS A CHAIN OF 4 CARBONS. . WHAT IS THE CHEMICAL FORMULA FOR BUTANE? Lewis Dot Structures in 5 Steps Follow these step tutorial to draw Lewis Dot Structures for molecules and polyatomic ions. We’re going to be working with 5 different molecules or ions: CO2, H2O, BCl3, PF3, and NH4+. 1) Arrange the atoms appropriately and as symmetrically as possible . The least abundant atom goes in the middle. The middle central atom will typically be the most metallic (least electronegative). Usually, the arrangement that is most symmetrical is correct. Examples: C & Si (4 valence electrons) will be a central atom(s) 2) Count up the total number of valence electrons that should be in the structure at the end . 3) Draw single bonds between the central atom and each surrounding atom . Note that each single bond (dash) represents two electrons which are shared. 4) Place the remaining electrons, in pairs, around the atoms in the structure. I usually start with the outer atoms. Note that H can only participate in a single bond (See water below) These electrons do not participate in a bond so they are drawn as dots. 5) Move pairs around so that each element has an octet (exceptions: H only needs 2 electrons). The final total number of electrons in the structure should equal the number counted in Step 2. BIG IDEA: SHARED ELECTRONS FORM A BOND. BIG IDEA: SHARED ELECTRONS ARE REPRESENTED AS A DASH. http://plone.scottsdalecc.edu/borick/docs/lewis2.pdf/view http://plone.scottsdalecc.edu/borick/docs




