Electrophilic Aromatic Substitution
advertisement

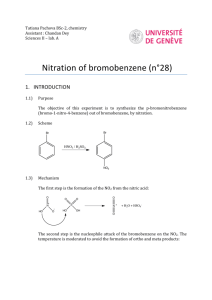
Electrophilic Aromatic Substitution (EAS) Nitration - an example of an electrophilic aromatic substitution General scheme HNO3 nitric acid benzene very stable undergoes EAS NO2 H2SO4 catalyst nitrobenzene Caution: Nitric acid-turns skin yellow, corrosive Step 1: Generation of the Electrophile (NO2+) O H O S O H O + H O H O N Mechanism H O N + O O H protonated nitric acid O O N O O S O H O O N O O O H protonated nitric acid H O H nitronium ion actual electrophile Step 2: Reaction between the Electrophile and Benzene H O N O H NO2 H H N-C bond forms Step 3: Proton abstraction H H NO2 H H O NO2 H + H H O H Scheme: dependent upon unknown liquid Br Br HNO3 nitric acid Br NO2 + H2SO4 catalyst NO2 1-bromo-4-nitrobenzene (major) 1-bromo-2-nitrobenzene (minor) Bromine is said to be an ortho-para directing group O O O O HNO3 nitric acid plus trace amounts of ortho- and para- H2SO4 catalyst NO2 ~ 90 % methyl-3-nitrobenzoate A carbonyl is said to be a meta directing group Procedure: TA assigns unknown letter (A or B) 1) In an ice bath, place a beaker containing 4 mL of conc. HNO3 (wear gloves) 2) With swirling, add 6 mL of H2SO4 dropwise (Caution: exothermic) 3) Dropwise (w/ mixing) add 1 mL of unknown over ca. 2 min 4) Allow to sit at room temperature for 20 min with occasional swirling 5) Pour slowly into 30 mL of ice cold water (Caution: exothermic) 6) Collect precipitate on a filter 7) Recrystallize from ethanol ~ 5-10 mL 8) Interpret 1H NMR spectrum for unknown 1 H NMR Spectrum a) 1-Bromo-4-nitrobenzene Here, two sets of equivalent protons give rise to two sets of signals. Br HA – a doublet of doublets HA HA HB – a doublet of doublets HB HB NO2 b) Methyl-3-benzoate Here four distinct protons will give rise to four sets of signals O O HA – a doublet with different degrees of splitting. HA HD HB – a triplet with strong splitting HC – doublet with different degrees of splitting HB NO 2 HD – a singlet with some splitting HC 9) Identify the substitution pattern 10) Identify the unknown liquid