Activation Energies Worksheet (Solutions)
advertisement

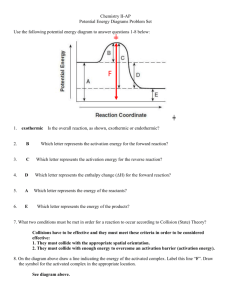
CHEMISTRY 12 – ACTIVATION ENERGIES WORKSHEET Use the following diagram to answer question 1 1) The above diagram shows reactant molecules approaching one another. a) What is happening to the kinetic energy and the potential energy? (2 marks) KE increasing and PE decreasing b) Draw the structure of the activated complex? (1 mark) 0 Cl \ / N ' ' 0 a 2) Describe the relationship between activation energy and the rate of a chemical reaction. (2 marks) If the activation energy is high, the rate of a chemical reaction is slow and if the activation energy is low, the rate of a chemical reaction is fast 3) If ΔH = - 40 kJ and Ea(f) = 20 kJ, what is the value of Ea(r)? (2 mark) Eats 20 Each = Each = Each 60 = DH + 40 - KJ 4) Consider the following reaction: formation H2 (g) + I2 (g) → 2 HI (g) ΔH = +28 kJ decomposition The activation energy for the formation of HI is 167 kJ. What is the activation energy for the decomposition of HI? Is the decomposition reaction endothermic or exothermic? (2 marks) Each 167 Each Each = + Each = = 139 KJ SH +28 since is the formation endothermic , the decomposition is exothermic 5) A reaction has Ea(f) = 40 kJ and Ea(r) = 10 kJ. Is the reaction exothermic or endothermic? Explain. (2 marks) Each 40 DH Eacrs = 10 = = endothermic + +30 , DH + SH KJ since AH positive is 6) Sketch a PE diagram for the reacting system where: (3 marks) ΔH = - 30 kJ / mol Ea = 50 kJ / mol the DH = -30 KJ 7) Consider the following potential energy diagram for a reversible reaction: (3 marks) a) Calculate the activation energy for the forward reaction 15 kJ b) Calculate ΔH for the forward reaction - 10 kJ c) Calculate the activation energy for the reverse reaction 25 kJ 8) Consider the following reaction: H2 (g) + I2 (g) → 2 HI (g) What is true of the activated complex, in terms of KE and stability, relative to the reactants? (1 mark) The activated complex has lower KE and has less stability 9) Describe what happens to the KE and PE as an activated complex forms products? (1 mark) KE increases and PE decreases 10) Consider the following reaction: CO + NO2 → CO2 + NO ΔH = -234 kJ The activation energy of the forward reaction is 134 kJ. Is the reverse reaction endothermic or exothermic? What is the activation energy for the reverse reaction? (2 marks) Each 134 Eacr ) Eacr ) = Each = 368 = DH + -234 endothermic KJ 11) In a certain reaction ΔH = -136 kJ and Ea = 96 kJ. Is the reverse reaction endothermic or exothermic? What is the activation energy for the reverse reaction? (2 marks) EACH 96 Each = Each = Eacr ) = DH + - 232 KJ 136 endothermic 12) Define “activated complex”. (2 marks) A chemical species that is short-lived and unstable due to its high PE 13) Consider the following potential energy diagram for a reversible reaction: (5 marks) a) Calculate the activation energy for the reverse reaction 50 kJ b) Calculate ΔH for the forward reaction + 50 kJ c) Calculate the activation energy for the forward reaction 100 kJ d) Calculate the energy of the activated complex 200 kJ e) Is the reaction endothermic or exothermic in the reverse direction? Exothermic