Lecture Slides
advertisement

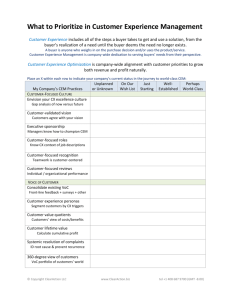
CEM 181H-Lecture 11 The graph shows how the increasing nuclear charge stabilizes (lowers the energy of) the one-electron orbitals. At a given Z, the order of orbital energies defines the loading order for the electrons to first order. 2 hc RH = 4.3597 × 10 −18 J = 27.211eV 1 hartree = 2 Rydbergs = Hartree-Fock/Single-Electron Orbital Energies Describe the Orbital-Filling Sequence to First Order CEM 181H-Lecture 11 2 of 9 1 of 9 You should: 1. understand the origin of the anomalies in electron configurations which cause deviation from the filling orders predicted by 1-electron orbitals. 2. know how to write electron configurations for neutral atoms and ions, including those with anomalous electron configurations. 3. be able to explain and predict the periodic trends for atomic/ionic radii, ionization energies, and electron affinities. Learning Objectives-10/4 valence Ar 3 of 9 CEM 181H-Lecture 11 4 of 9 Electron Density Contracts as the Nuclear Charge Increases CEM 181H-Lecture 11 core The valence electrons are shielded from the full effects of the nuclear positive charge by the core electrons (the especially stable underlying noble-gas configuration). Periodic Trends Arise from Shielding Effects 5 of 9 CEM 181H-Lecture 11 6 of 9 1. Write balanced equations for the processes associated with ionization energies and electron affinities. 2. Explain how atomic radii are determined. In-Class Exercise CEM 181H-Lecture 11 The degenerate p orbitals do not shield each other very much from the increasing nuclear charge, so a significant contraction occurs as they are filled. The Nuclear Charge is Not Well Screened by the p Orbitals CEM 181H-Lecture 11 Periodic Trends in Electron Affinity CEM 181H-Lecture 11 Periodic Trends in Ionization Energy/Potential 8 of 9 7 of 9 CEM 181H-Lecture 11 Periodic Trends in Atomic Radius 9 of 9