Journal of The Electrochemical Society, 151 共12兲 C850-C854 共2004兲
C850
0013-4651/2004/151共12兲/C850/5/$7.00 © The Electrochemical Society, Inc.
Effects of o-Vanillin as a Brightener on Zinc Electrodeposition
at Iron Electrodes
Soo-Jung Kim,a Hyun-Tae Kim,b and Su-Moon Parka,*,z
a
Department of Chemistry and the Center for Integrated Molecular Systems, Pohang University of Science
and Technology, Pohang 790-784, Gyeongbuk, Korea
POSCO Technical Research Laboratories, Pohang 790-785, Gyeongbuk, Korea
b
Effects of o-vanillin on electrodeposition of zinc on iron electrodes have been studied employing transient electrochemical,
electrochemical quartz crystal microbalance, spectroelectrochemical, and surface analytical techniques. The results indicate that
o-vanillin is adsorbed strongly on the iron surface, raising overpotentials for hydrogen evolution as well as for zinc deposition.
o-Vanillin shows greater effects on the earlier stage of the zinc electrodeposition in the underpotential region than in the bulk
deposition region. The additive appears to adsorb preferentially on the top of the dendritic growth sites, leading to a leveling effect.
The zinc layer obtained in the presence of o-vanillin shows smoother morphology with smaller grain sizes and higher specular
reflectance signals. Reflectance spectroelectrochemistry has been used for the first time to monitor the brightness of the electroplated surface.
© 2004 The Electrochemical Society. 关DOI: 10.1149/1.1814033兴 All rights reserved.
Manuscript submitted February 9, 2004; revised manuscript received May 5, 2004. Available electronically November 5, 2004.
Various metals and alloys including tin, nickel, chromium, and
the zinc-nickel alloy have been electroplated on iron surfaces for
better performances of steel.1-6 Of these, zinc electrodeposition on
iron surfaces is a particularly important industrial process for protection of iron surfaces from corrosion.7,8 Organic additives are often added to the zinc electroplating baths to make the electroplated
surface more durable, uniform, and compact for better performances
in terms of corrosion protection. The concentrations of additives
range between 100 M and 10 mM and yet prevent whiskers or
dendrites from growing during the electroplating process. The additives can provide leveling and/or brightening effects during electroplating of metals, affecting the qualities of the surfaces of electroplated metals such as their roughness and brightness. Recently, the
effects of the additives have been studied extensively employing
conventional electrochemical experiments along with surface characterization techniques including scanning electron microscopy
共SEM兲, scanning probe microscopy 共SPM兲, and other surface analytical techniques.
We have been studying effects of organic additives such as polyethylene glycol and benzoic acid on zinc electrodeposition at iron
electrodes employing transient electrochemical, electrochemical
quartz crystal microbalance 共EQCM兲, impedance, and SPM
techniques.9,10 Similar studies have been carried out on the effects of
ethoxylated naphthalene sulfonic acid 共ENSA兲 on the electrodeposition of tin on the iron surface.11 We now expand this study to
o-vanillin, which has been reported to suppress corrosion of
aluminum.12 Effects of additives including o-vanillin on zinc electrodeposition have been studied in various aqueous electrolyte
solutions.13-17 In this study, we examine the effects of o-vanillin as a
brightener of the electrodeposited zinc surface on irons employing
transient electrochemical, EQCM, and reflectance spectroelectrochemical experiments.
An electrochemical cell with a three-electrode configuration was
used for electrochemical experiments. An iron rod 共Mateck,
99.99%兲 with a diameter of 2.0 mm, a platinum foil, and an Ag/
AgCl 共in saturated KCl兲 electrode were used as working, counter,
and reference electrodes, respectively. The iron electrode was polished successively with 1.0, 0.3, and 0.05 m alumina slurries 共Fis-
Experimental
Zinc chloride (ZnCl2 : Kanto Chemicals, 99.0 ⫹ %), potassium
chloride 共KCl: Samchun Chemicals, 99.99%兲, and o-vanillin 共Acros
Organics, 99.0%兲 were used as received. The electroplating solution
contained 1.38 M ZnCl2 , 4.30 M KCl with or without 165 M
o-vanillin with its pH adjusted to 4.5 using a small amount of HCl.
The solution was bubbled with argon for 3 h and maintained at 60°C
during electrodeposition experiments. The solution has the same
composition as the electroplating bath used in industries when it
contains all the additives.5
* Electrochemical Society Active Member.
z
E-mail: smpark@postech.edu
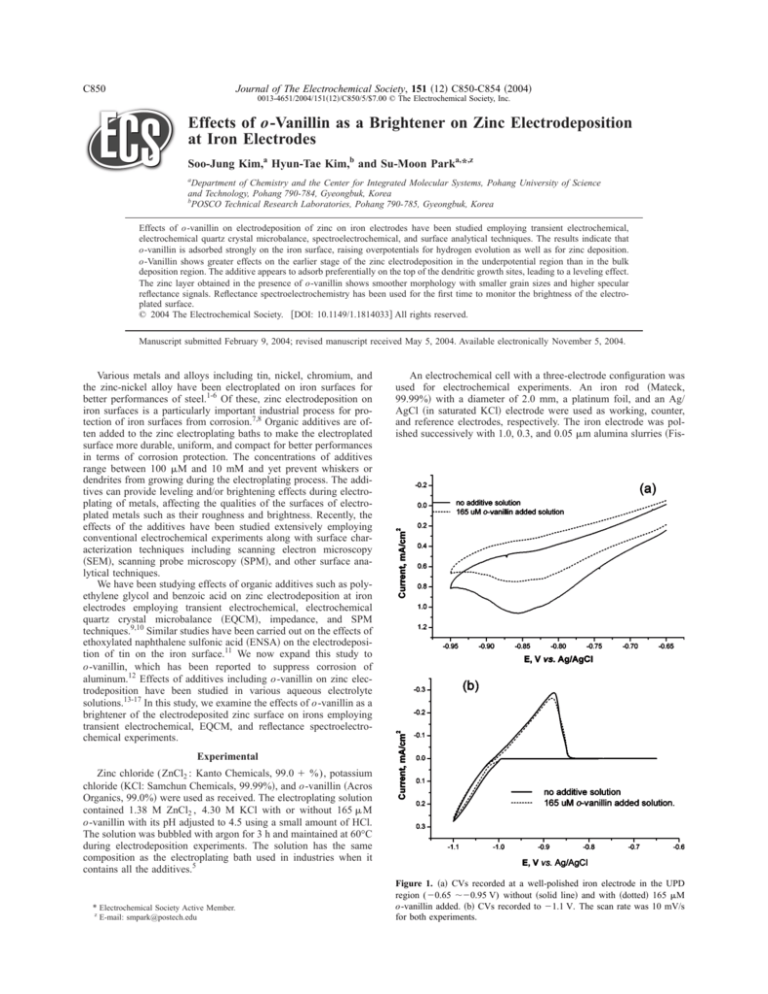
Figure 1. 共a兲 CVs recorded at a well-polished iron electrode in the UPD
region (⫺0.65 ⬃⫺0.95 V) without 共solid line兲 and with 共dotted兲 165 M
o-vanillin added. 共b兲 CVs recorded to ⫺1.1 V. The scan rate was 10 mV/s
for both experiments.
Journal of The Electrochemical Society, 151 共12兲 C850-C854 共2004兲
C851
Figure 2. 共a兲 Chronoamperometric behavior for zinc deposition at ⫺1.0 V for 30 s in a solution with and without o-vanillin, 共b兲 linear sweep voltammogram
共LSV兲 for dissolution of zinc deposited at ⫺1.0 V, 共c兲 chronoamperometric behavior zinc deposition at ⫺1.2 V in a solution with and without o-vanillin present,
共d兲 LSV for dissolution of zinc deposited at ⫺1.2 V. The sweep rate was 10 mV/s for LSV experiments.
cher兲 and then cleaned ultrasonically with doubly distilled, deionized water.
Electrochemical experiments were carried out with an EG&G
Princeton Applied Research model 273A potentiostat/galvanostat.
EQCM measurements were made with a Seiko EG&G model 917
quartz crystal analyzer along with the potentiostat/galvanostat. For
quartz crystal analysis 共QCA兲 and EQCM analyses on the iron electrode, the iron thin film 共area 0.20 cm2 ) was deposited onto a 9 MHz
AT-cut gold-plated quartz crystal by sweeping the potential between
⫺0.40 and ⫺1.40 V at 10 mV/s in an aqueous solution containing
0.10 M Fe2 SO4 , 0.40 M H3 BO3 , and 0.50 M Na2 SO4 . 9 Gold plated
quartz electrodes were cleaned with doubly distilled, deionized water followed by immersing in a 1:3 共v/v兲 H2 O2 ⫹ H2 SO4 solution
共piranha solution兲 before these experiments. The QCA electrode had
a sensitivity of 6.7 ng/Hz/cm2 . Reflectance spectra were taken with
an Oriel InstaSpec IV spectrometer with a charge-coupled device
共CCD兲 array detector, which was configured in a near normal incidence reflectance mode using a bifurcated quartz optical fiber. The
light source was a 60 W xenon lamp. The wavelength of the spectrograph was calibrated using a mercury lamp. The details of the
setup have been described elsewhere.18-20
Results and Discussion
Figure 1 shows cyclic voltammograms 共CVs兲 recorded for zinc
reduction with and without o-vanillin in two different potential
ranges between: 共a兲 ⫺0.65 and ⫺0.95 V and 共b兲 ⫺0.65 and
⫺1.10 V, respectively. The CVs in the underpotential deposition
共UPD兲 region shown in Fig. 1a display that the addition of
o-vanillin decreases the proton reduction current by adsorbing onto
the active sites of the electrode. Also, a small amount of zinc underpotentially deposited at these potentials raises the overpotential for
hydrogen evolution, resulting in lower currents.9 This is supported
by the fact that the peak current was shown to be adsorption controlled by the scan rate dependency 共not shown兲 when the additive
was present as was the case for other additives,9,11 as well as by the
other experimental results described below. This effect is extended
to the bulk deposition region as can be seen in Fig. 1b. The overpotential increases and the current is seen to be suppressed even for the
anodic stripping of the deposited zinc. This indicates that the zinc
dissolution is made more difficult by the presence of the additive
due to better interlayer adherence of the deposited zinc.
Potentiostatically prepared films display different stripping behaviors depending on whether or not there is an additive as shown in
Fig. 2, which shows the current recorded during potentiostatic deposition of zinc at ⫺1.0 and ⫺1.2 V 关共a兲 and 共c兲兴 and their subsequent
stripping by sweeping the potential in a reverse direction from
⫺1.10 to ⫺0.65 V 关共b兲 and 共d兲兴. At ⫺1.0 V, where the bulk deposition starts to take off, significant suppression of both the zinc
deposition and stripping currents is noticed 共Fig. 2a and b兲. However, these effects are significantly reduced at a higher overpotential,
⫺1.2 V 共Fig. 2c and d兲, and intermediate behaviors were observed at
potentials in between 共not shown兲. At ⫺1.0 V, the zinc deposition
current is significantly reduced when o-vanillin is added and the
stripping behavior of the deposited zinc in the presence of the additive is quite different from when it is absent. Apparently, different
fractions of two different zinc crystalline forms, which dissolve at
different potentials, one at about ⫺1.03 and the other at about
⫺0.97 V, are formed in the presence and absence of the additive as
Journal of The Electrochemical Society, 151 共12兲 C850-C854 共2004兲
C852
Table I. Current efficiencies of zinc deposition obtained at different deposition potentials.
a
E 共V兲
Q total
共mC兲
Q Zn 共mC兲
Q HERa
共mC兲
Columbic
efficiency 共%兲
Without
o-vanillin
⫺1.00
⫺1.05
⫺1.10
⫺1.20
7.62
119.9
199.8
237.4
2.92
114.9
194.4
231.2
4.70
5.00
5.40
6.20
38.3
95.8
97.3
97.4
With
o-vanillin
⫺1.00
⫺1.05
⫺1.10
⫺1.20
5.64
114.9
183.1
237.3
2.24
111.7
179.6
232.9
3.40
3.20
3.50
4.40
39.7
97.2
98.1
98.1
Q HER is the charge spent for the hydrogen evolution reaction 共HER兲
calculated from the total charge Q total and the charge for zinc deposition Q Zn , which was calculated from the amount of zinc deposited.
can be seen from Fig. 2b. These results indicate that the adsorption
of o-vanillin affects more on the earlier stage of the deposition than
that at higher overpotentials, and the earlier effects seem to be carried over to the overpotential region as was the case for other
additives.9-11 The corrosion of the zinc layer deposited at ⫺1.0 V
with the additive present is definitely inhibited in comparison to that
obtained without the additive, as can be seen from the shifted potential and the reduced current for the dissolution 共Fig. 2b兲.
That o-vanillin molecules compete effectively with protons for
adsorption sites is also suggested by the current efficiencies for zinc
deposition as shown in Table I. The enhancement in current efficiencies is about the same whether the potential is more or less negative,
and thus the roles of the additive playing in earlier stages are carried
over to the large overpotential region. As the overpotential increases,
the effect becomes slightly less important due to a large overpotential for hydrogen evolution reaction.
Figure 3 shows the mass increase at the iron coated EQCM electrode upon injection of o-vanillin into the 4.3 M KCl solution
at pH 4.5 to make its final concentration of 165 M at an
applied potential of ⫺0.90 V to maintain the integrity of the iron
surface. As can be seen, a decrease of the frequency by 251 ng/cm2
was observed in about 150 s with its final mass remaining
about constant. The amount of o-vanillin calculated using the sensitivity of the QCA electrode is 50.1 g, corresponding to about
2 ⫻ 1014 molecules/cm2 . Assuming the molecular area of 0.39 nm2
for the following structure, the monolayer adsorption would require
Figure 3. Frequency change at the iron-coated EQCM electrode upon injection of 165 M o-vanillin to the electrolyte solution. The potential was
maintained at ⫺0.90 V during this experiment.
Figure 4. Frequency changes recorded during a potentiodynamic scan at 10
mV/s from ⫺0.70 to ⫺0.95 V in the absence 共䊉兲 and presence 共䊊兲 of 165
M o-vanillin.
2.56 ⫻ 1014 and, thus, this corresponds to slightly less than a monolayer of adsorption because the iron covered QCA electrode surface
would be relatively rough and thus should be larger than its geometric area
The dimension of the molecule was calculated from the ChemDraw program. From this observation, we conclude that o-vanillin
covers the iron surface with less than a single layer, blocking the
surface from protons. We believe these molecules are preferentially
adsorbed on the peak of the dendritic growth sites, preventing fur-
Figure 5. The same experiment as in Fig. 4 but the scanned potential range
was between ⫺0.70 and ⫺1.10 V.
Journal of The Electrochemical Society, 151 共12兲 C850-C854 共2004兲
C853
Figure 6. SEMs of the zinc layers deposited at ⫺1.0 V in the absence of o-vanillin at different magnifications 关共a兲 and 共b兲兴: 共a兲 5000 X and 共b兲 15,000 X, and
in the presence of o-vanillin: 共c兲 5000 X and 共d兲 15,000 X. Both deposits used for SEM imaging were obtained by electrodeposition at ⫺1.0 V for 500 s.
ther growth of their peaks, leading to a smoother surface as was the
case for benzoic acid.9 When the dendritic sites are not blocked, the
deposition current shows a slight increase as seen Fig. 2a, whereas a
slow decrease in cathodic current is observed in the presence of
o-vanillin because the growth sites are masked.
Although we have already seen how the dissolution of the zinc
layer deposited with and without o-vanillin present is affected in
Fig. 2, it is now examined again by employing the EQCM experiments, and the result obtained for the zinc deposited with and without o-vanillin by a potentiodynamic scan between ⫺0.70 and
⫺0.95 V is shown in Fig. 4. The total amount of zinc deposited in
the presence of o-vanillin is about 10% less than that obtained without o-vanillin 共3.08 vs. 3.35 g/cm2 ), but the amount of zinc left
over after its anodic stripping by scanning back to ⫺0.70 V is significantly larger for the case where o-vanillin was present than without it 共1.45 vs. 0.87 g/cm2 ). The greater amount of Zn left over
after its anodic stripping indicates that proton reduction has been
diminished during Zn electrodeposition in the presence of
o-vanillin. This must have led to the denser layers with improved
interlayer adherence due to the lack of hydrogen adsorbed or hydrogen gas bubbles trapped during the deposition.
This trend is also seen when the vertex potential is more negative
at ⫺1.10 V as shown in Fig. 5. Here the frequency decreases due to
the zinc deposition in the absence and presence of o-vanillin are
0.105 and 0.099 mg/cm2 , respectively. The differences in frequen-
cies when the anodic scanning is over are 1.52 and 0.099 g/cm2 ,
respectively, in the presence and absence of o-vanillin. While the
relative effect is not as large as for the case when the cathodic vertex
potential is less negative, the initial roles played by the additive
appears to remain effective.
That the additive affects the interlayer adhesion of the electrodeposits leading to deposition of zinc layers of better qualities can also
be seen by examining their surfaces. Figure 6 presents SEM images
of electrodeposits prepared on iron surfaces in the absence 关共a兲 and
共b兲兴 and presence 关共c兲 and 共d兲兴 of o-vanillin. It can be seen clearly
from these images that the grains are much larger when o-vanillin is
absent during the electrolytic deposition. The presence of o-vanillin
makes grain sizes on the zinc layer much smaller and more homogeneous.
As a final check of the qualities of electrodeposits thus obtained,
we used reflectance spectrum in the uv-vis region and the results are
shown in Fig. 7. Shown in Fig. 7 are reflectance signals plotted as a
function of wavelengths of incident light. In these experiments, the
monochromatic light was brought to the surface of the electroplated
zinc with one branch of the bifurcated optical fiber and the light
reflected off the surface was detected with another branch, at the end
of which a charge coupled device detector was attached.13 The
brightness of the surface as measured in terms of the reflected light
intensity from the surface obtained in the presence of o-vanillin over
that of the electrodeposit without o-vanillin ranges from about as
C854
Journal of The Electrochemical Society, 151 共12兲 C850-C854 共2004兲
Figure 7. Reflectance spectra obtained from zinc layers deposited in the
absence 共solid line兲 and the presence 共dotted line兲 of 165 M o-vanillin.
small as 1.3 times at 550 nm to more than twice of those at 430 and
800 nm. In other words, how bright the surface is depends on the
wavelengths. The difference in brightness results from the relative
flatness of the surface obtained with and without the additive in
regard to the wavelength of the incident light. Our result clearly
shows that the grains making up the surface are much smaller, making the surface macroscopically flatter and more homogeneous,
when o-vanillin is used as an additive during the electroplating of
zinc.
Conclusions
From our results, we conclude the following about the roles of
o-vanillin as an additive for zinc electrodeposition
1. The presence of o-vanillin imposes a larger overpotential on
both the zinc deposition and the hydrogen evolution reactions compared to the case when no additive is used. This effect is greater
during an early stage of electrodeposition affecting the interlayer
adhesion. This was also shown by the determination of current efficiencies for zinc deposition.
2. The QCA experiments showed that o-vanillin is quickly adsorbed onto the iron surface, raising the overpotentials for electrochemical processes as mentioned above. Both linear sweep voltammetric and EQCM experiments confirm that improved corrosion
properties are obtained for the electrodeposited zinc layer during
anodic dissolution experiments when the zinc layer was obtained in
the presence of o-vanillin as an additive.
3. The qualities of the electrodeposited zinc layer are improved
significantly as evidenced by the surface analytical measurements
such as SEM and reflectance signal recording.
In conclusion, o-vanillin significantly affects not only the earlier
stage of electrodeposition in the UPD region but also the process in
the overpotential region to a smaller extent. o-Vanillin appears to act
as a leveling agent by being adsorbed on the peak of the microscopic
dendrites formed during crystal growth in the same way as benzoic
acid does.9,10 Perhaps use of other additives such as polyethylene
glycol, which adsorbs strongly on the surface and controls the transport of zinc ions, along with o-vanillin may further improve the
quality of electrodeposited zinc layers.10,11 We believe the
o-vanillin/polyethylene glycol mixed additive should provide better
performances in zinc plating compared to the polyethylene glycolbenzoic acid mixed additive, which is currently used in industrial
zinc plating baths. This is because o-vanillin provides better performance than benzoic acid10,11 does as was seen in this study.
The stable sub-monolayer adsorption of o-vanillin is an evidence
on a molecular level for suppressing the hydrogen as well as zinc
reduction current at the electrode surface, which, to our knowledge,
has not been reported in the literature. Further, this type of conclusion was drawn indirectly in most of previous studies from the voltammetric curves and/or other transient electrochemical experiments
without presenting direct evidence of adsorption with an assumption
that the additives are being adsorbed.
Finally, we should point out that the reflectance spectroscopy,
which was used in this study for the first time for evaluation of the
electrodeposited surface, offers an excellent tool for a quantitative
assessment of the effects of surface brightener during or after electroplating. The experiment is straightforward enough for in situ examinations of electrodeposited surface but provides the data difficult
or impossible to obtain otherwise. The reflectance spectroelectrochemical technique would be very useful in quantitatively monitoring the color of the reflected light from the electroplated surface so
that organic additives may be developed for a specific purpose. Also,
it may be developed to an in situ sensor for monitoring the surface
of electrodeposited layers.
Acknowledgment
This research was supported by the Korea Science and Engineering Foundation with a grant through the Center for Integrated Molecular Systems at POSTECH and by the BK21 program from the
Korea Research Foundation for graduate stipends for S.J.K.
Pohang University of Science and Technology assisted in meeting the
publication costs of this article.
References
1. R. Renuka and S. Ramamurthy, J. Power Sources, 76, 197 共1998兲.
2. J. Kelly and C. Tian, J. Electrochem. Soc., 146, 2540 共1999兲.
3. M. Monev, L. Mirkova, I. Krastev, Hr. Tsvetkova, St. Rashkov, and W. Richtering,
J. Appl. Electrochem., 28, 1107 共1998兲.
4. F. Ganne, C. Cachet, G. Maurin, and R. Wiart, J. Appl. Electrochem., 30, 665
共2000兲.
5. J.-R. Park and H.-T. Kim, Plat. Surf. Finish., 86, 108 共1999兲.
6. T. Sonada and H. Nawafune, Plat. Surf. Finish., 79, 79 共1992兲.
7. M. Schlesinger and M. Paunovic, Modern Electroplating, 4th Ed., p. 423, Wiley,
New York 共2000兲.
8. H. Geduld, Zinc Plating, ASM International, p. 13, Teddington, England 共1988兲.
9. J.-Y. Lee, J.-W. Kim, M.-K. Lee, H.-J. Shin, H. T. Kim, and S.-M. Park, J. Electrochem. Soc., 151, C25 共2004兲.
10. J.-W. Kim, J.-Y. Lee, and S.-M. Park, Langmuir, 20, 459 共2004兲.
11. J.-Y. Lee, J.-W. Kim, B.-Y. Chang, H. T. Kim, and S.-M. Park, J. Electrochem.
Soc., 151, C333 共2004兲.
12. A. Y. El-Etre, Corros. Sci., 43, 1031 共2001兲.
13. F. Huang, S. Pan, and Y. Liang, Plat. Surf. Finish., 79, 64 共1992兲.
14. G. N. K. Ramesh Bapu, G. Devaraj, and J. Ayyapparaj, J. Solid State Electrochem.,
3, 48 共1998兲.
15. M. Chandran, R. L. Sarma, and R. M. Krishnan, Bull. Electrochem., 15, 242
共1999兲.
16. R. Sekar, C. Kala, and R. M. Krishnan, Trans. Inst. Metal Finish., 80, 173 共2002兲.
17. A. J. Rethinam, G. N. K. Ramesh Bapu, and V. S. Muralidharan, Trans. Inst. Metal
Finish., 81, 136 共2003兲.
18. C.-H. Pyun and S.-M. Park, Anal. Chem., 58, 251 共1986兲.
19. C. Zhang and S.-M. Park, Anal. Chem., 60, 1639 共1988兲.
20. C. Zhang and S.-M. Park, Bull. Korean Chem. Soc., 10, 302 共1989兲.