Viscosity Notes
advertisement

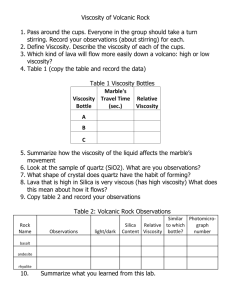
Viscosity Viscosity refers to the thickness (or thinness) of a liquid. It is the term for the resistance that a fluid has to flowing and movement. The stronger the attraction among the particles in a liquid, the greater the resistance of the particles to flowing past one another and the greater the viscosity. Particle theory refers to the amount of particles in a substance and the way they behave. When we talked about the characteristics and behaviour of solids, liquids, and gases, we talked about the particle theory. The particles in a liquid with a high viscosity stick together more than in a thin liquid. A thick liquid will have particles that do not flow easily – they don’t easily pass by each other. Substances have different viscosities because they have different flow rates and thicknesses. Ketchup, maple syrup, molasses, and corn syrup are all examples of liquids with high viscosities. Molasses has a high viscosity – it is very thick. The particles in molasses flow very slowly and don’t pass by each other easily – they stick together. Water has a very low viscosity. Vegetable oil has a relatively low viscosity, as does milk. Viscosity is a concern when you need to know how quickly or how slowly a fluid flows. Viscosity can be very important, take engine oil for example. In the winter, as temperatures decrease, the viscosity of engine oil increases – the oil gets really thick and doesn’t flow easily. This can be harmful to engines if the high viscosity prevents the oil from flowing to all the engine parts. As the engine warms, the viscosity decreases (it gets thinner) and the oil flows easily to all the engine parts. You can measure viscosity by measuring how much time it takes for an object to sink through it. Fill beakers with two different liquids – water and molasses for example. Drop a marble and measure the time it takes for it to hit the bottom. It will take a lot more time for the marble to reach the bottom in the molasses than in the water – molasses has a higher viscosity. We used a hill to measure viscosity in class. Draw two lines 5cm apart on a board placed on an incline. Drop a spoonful of molasses on the incline and measure the amount of time it takes for the molasses to travel the 5cm. This will give you the flow rate of the molasses – flow rate is a measure o the viscosity of the liquid. Density & Viscosity Density and viscosity are different. Density refers to how crowded the particles in a substance are. Viscosity refers to how the particles are attracted to each other. For example, oil is less dense than water. Oil floats on water – the particles in the oil are less crowded than those in water. However, oil is more viscous than water. Oil flows more slowly than water – the particles in the oil are attracted to each other more than the particles in water. Temperature & Viscosity Temperature affects viscosity in the same way it affects density. When a substance is heated, the particles gain more energy and vibrate faster. As more heat is added, this speed of vibration becomes so fast that the force of attraction cannot hold the particles together. The substance becomes less dense - the rigid structure of the solid falls apart, melting occurs, and a liquid is formed. The force of attraction is less and the viscosity decreases. The reverse process occurs when heat is taken away from a gas or a liquid as its temperature decreases.