Biophysics I. DIFFUSION – OSMOSIS
advertisement

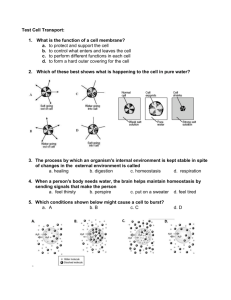
Biophysics I - OSMOSIS 11/09/2012 Biophysics I. - OSMOSIS BROWNIAN MOTION OVERVIEW – DIFFUSION random thermal motion of particles DIFFUSION Biophysics I. DIFFUSION – OSMOSIS due to the non-uniform (inhomogeneous) distribution of particles net transport of particles (Brownian motion) occurs from a region of higher concentration to a region of lower concentration which continues until the distribution of particles is uniform (homogeneous) FICK’S 1st LAW (spatial description) ∆ ∆ DIFFUSION COEFFICIENT: Stokes-Einstein equation 11th September 2012 Dr. Beáta Bugyi University of Pécs, Medical School Department of Biophysics FICK’S 2nd LAW (spatial & temporal description) DIFFUSION THROUGH THE CELL MEMBRANE OSMOSIS – EXPERIMENT 1 Experiment 1: place a dried leaf of salade into water Biophysics I. - OSMOSIS OSMOSIS – EXPERIMENT 2 Biophysics I. - OSMOSIS Experiment 2: place an egg into corn syrup then into water before CORN SYRUP WATER after (3-4 hours) Observation : the egg shrinks before after Observation: the shrinked egg gains its original size, and it continues to get even bigger before after Observation: the leaf of salad becomes bigger and looks fresh again OSMOSIS Biophysics I. - OSMOSIS What is the difference between the „ink” experiment and the „salade/egg” experiment? Biophysics I. - OSMOSIS 1. SOLID (non-permeable) WALL NO TRANSPORT 1 Biophysics I - OSMOSIS 11/09/2012 Biophysics I. - OSMOSIS Biophysics I. - OSMOSIS 2. NO WALL 3. SPECIAL WALL SEMIPERMEABLE – „filter” allows smaller slovent molecules to pass through, but not the larger solute molecules PORE SIZE SELECTIVITY animal skin pellicles, walls of living cells, ceramic plate with holes, cellophane free DIFFUSION both particles (smaller/larger) reach homogeneous distributions Biophysics I. - OSMOSIS 3. SPECIAL WALL Biophysics I. - OSMOSIS type of the wall matter transport yes: non-permeable no no free diffusion yes: SEMIPERMEABLE restricted diffusion: OSMOSIS OSMOSIS unidirectional matter flow, which takes place by means of diffusion semipermeable wall + concentration difference restricted DIFFUSION: OSMOSIS smaller molecules reach a uniform distribution larger molecules remain in the compartment QUANTIFICATION OF OSMOSIS Biophysics I. - OSMOSIS ≫ J OUT J OUT J IN J IN solvent semipermeable membrane concetration difference semipermeable membrane: allows solvent to pass through but not the solute Biophysics I. - OSMOSIS OSMOTIC PRESSURE h J OUT J OUT J IN J IN solvent + solute mixture solvent flow throught the semipermeable membrane the volume of the solvent + solute mixture increases HYDROSTATIC PRESSURE (ph) solvent flow slows down ρ: density h: height g = 10 m/s2 OSMOTIC PRESSURE dynamic equilibrium OSMOTIC EQUILIBRIUM pressure that has to be exerted on the solution connected to pure solvent by a semipermeable membrane to reach dynamic equilibrium, to counteract osmosis pressure that inhibits the net solvent flow 2 Biophysics I - OSMOSIS 11/09/2012 OSMOTIC PRESSURE Biophysics I. - OSMOSIS OSMOTIC PRESSURE Biophysics I. - OSMOSIS VAN’T HOFF’s LAW for dilute solutions and perfect semipermeable membranes using the equation of state of the ideal gas → !"#$% upon OSMOSIS the net particle transport occurs from the lowconcentration regions (of the solute!!!!!) (low osmotic pressure) to the high-concentration regions (high osmotic pressure) V: volume n:: mole fraction T: temperature c: concentration R: universal gas constant it is always the more dense solution which becomes diluted OSMOSIS ~ solvent + solute mixture the osmotic pressure is linearly proportional to the concentration solvent Biophysics I. - OSMOSIS CLASSIFYING SOLUTIONS ON THE BASIS OF OSMOTIC PRESSURE comparision of solutions! HYPERTONIC ISOTONIC HYPOTONIC higher concentration same concentration lower concentration c > cx c = cx c < cx higher osmotic pressure same osmotic pressure lower osmotic pressure p = px p < px p > px SOLUTE - / 0 -#!"#!%! / 0#!"#!%! Biophysics I. - OSMOSIS RED BLOOD CELLS IN DIFFERENT ENVIRONMENT HYPERTONIC (more concentrated: 10% NaCl) ISOTONIC (0.87 % NaCl) pout > pin pout = pin HYPOTONIC (less concentrated: 0.01% NaCl) pout < pin for the cells of the human body, blood: 0.87 % (0.15 M) NaCl physiologic saline solution 3.8 % sodium citrate 5.5 % (0.3 M) glucose x: reference RED BLOOD CELLS IN DIFFERENT ENVIRONMENT net water OUTflux NO net water flux Biophysics I. - OSMOSIS net water INflux Biophysics I. - OSMOSIS PLANT CELLS IN DIFFERENT ENVIRONMENT HYPERTONIC HYPERTONIC ISOTONIC HYPOTONIC ISOTONIC (0.87 % NaCl) net water OUTflux PLASMOLYSIS NO net water flux net water INflux TURGOR PRESSURE HYPOTONIC plasma membrane is pulled away from the cell wall plasma membrane is pushed to the cell wall 3 Biophysics I - OSMOSIS 11/09/2012 Biophysics I. - OSMOSIS OSMOSIS IN THE MEDICAL PRACTICE OSMOSIS IN THE MEDICAL PRACTICE Biophysics I. - OSMOSIS DIALYSIS INJECTION, INFUSION drugs are dissolved in physiological saline solution isotonic environment (compared to the body fluid) water outflow different particles can be sorted by semipermeable membranes pore size of the membrane determines which molecules can pass through the membrane TREATMENT OF OEDEMAS, INFLAMED AREAS abnormal accumulation of fluid beneath the skin or in one or more cavities of the body that produces swelling (fluid accumulation) dextran-solution/bitter salt (MgSO4-solution)-based treatment hypertonic environment is created (compared to the swollen hypertonic areas) induces water outflow from the swollen areas water influx reduced swelling dialysis bag semipermeable membrane concentrated solution TREATMENT OF CONSTIPATION - LAXATIVE SALTS laxative salts are not absorbed by the large intestine hypertonic environment is created in the large intestine results in water influx into the large intestine dilution of colonic content, facilitated excretion hypertonic OSMOSIS IN THE MEDICAL PRACTICE HAEMODIALYSIS treatment of patient with severe kidney disease remove soluble chemicals toxic for the body Schematic diagram of haemodialysis. t=0s Biophysics I. - OSMOSIS t Biophysics I. - OSMOSIS OVERVIEW – the most important things Osmosis Van’t Hoff’s law Osmotic pressure and its significance protein products toxins other waste products 4