Mr. Westfield's Class AP Chemistry Summer Assignment
advertisement

AP Chemistry Summer Assignment
Welcome to college chemistry!
Congratulations on making the decision to take one of the most challenging college-level
courses. Many would-be engineers and doctors change their careers because of the difficulty of
taking this course while in college. By taking college chemistry in high school, you could earn
college credit for the course (so you don’t have it take it in college). At a minimum, you will
prepare yourself very well for chemistry in college so you CAN get that elusive “A” in college
chemistry and become whatever you want to be!
I love teaching students college level chemistry! I am an ex-chemical engineer and have been
teaching at Cooper High School since the doors were opened way back in 2008. I work very
hard to help you meet your goals. I promise to provide you with appropriate, focused materials
and help you build skills to do your best on the AP chemistry exam next May. I am here to help
you.
The folks that run the AP program (College Board), say you will need to study 5-10 hours per
week outside of class to be successful. Be assured that I work very hard to provide materials that
focus you on what’s the most important. This should minimize that time requirement and allow
us to “do more with less”. But, no doubt about it, you will need to study outside of class.
To be honest, I dislike summer assignments. However, I feel that it is unfair for me to expect you
to be able to name chemical compounds on Day Two without providing you with practice. Much
(or hopefully all) of the naming you have already learned in honors chemistry. But you should
refresh yourself for the naming quiz on Day Two. All of this information can be found at
www.mrwestfieldsclass.yolasite.com. (Login: westfield, password: 5jags5). Be sure to lower
case letters for Login. There are also naming practice videos on the website if you want more
help
Assignment:
Things to buy:
Lab Notebook: Lab Notebook 100 Carbonless Pages Spiral Bound; by Barbakam
(Author) (about $15 on Amazon)
Classroom Materials: 3” 3-ring binder, paper, scientific calculator, writing
utensils.
Note: I will be analyzing new AP study guides over the summer as new ones are
being developed. Over the course of the school year, I will ask you to purchase
one that I believe is the best.
1
Things to Study and Practice:
Study and complete the practice naming work below. There will be a quiz on the second
day of school. The quiz will count as your first quiz grade.
After the quiz, you will show me that you completed AT LEAST the three practice
quizzes and self-assed them. This will be your first homework grade.
Again, welcome to our class. Please contact me at daryl.westfield@boone.kyschools.us if you
have any questions. During the school year, I will check this e-mail address regularly. During the
summer, I plan on not checking e-mail often.
Mr. Westfield
2
Nomenclature (Naming) Practice
Instructions: Use the material provided below to review naming.
Notes:
1. This is for you to practice. There’s nothing to turn in to me. After the quiz, you will show
me that you completed and self-assessed at LEAST the three Practices Quizzes at the end
and self-assessed your work.
2. There will be a quiz on the second day of school. You will have a periodic table only as a
reference. Use only the periodic table provided for practice.
3. This is for a REVIEW and will not tell you how to name. If you need help to understand
any of this, you can go to my website or use other materials to help. You can also send
me an email. If you have specific questions.
3
4
Common Element/Symbol List
NOTE: Spelling counts. Capitalization counts on symbols. In a symbol, the first letter is
ALWAYS a capital letter. If the symbol has two letters, the second is ALWAYS a lower case
letter.
Hydrogen
Helium
Lithium
Beryllium
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Neon
Sodium
Magnesium
Aluminum
Silicon
Phosphorous
Sulfur
Chlorine
Argon
Potassium
Calcium
Scandium
Titanium
Vanadium
Chromium
Manganese
Iron
Cobalt
Nickel
Copper
Zinc
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg (NOT Manganese!)
Al
Si
P
S (Sulphur is British spelling)
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn (not Magnesium!)
Fe
Co (not CO)
Ni
Cu
Zn
Gallium
Germanium
Arsenic
Selenium
Bromine
Krypton
Rubidium
Strontium
Yttrium
Zirconium
Palladium
Silver
Cadmium
Tin
Antimony
Tellurium
Iodine
Xenon
Cesium
Barium
Tungsten
Platinum
Gold
Mercury
Thallium
Lead
Bismuth
Radon
Radium
Uranium
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Pd
Ag
Cd
Sn
Sb
Te
I
Xe
Cs
Ba
W
Pt
Au
Hg
Tl
Pb
Bi
Rn
Ra
U
5
Polyatomic Ion List for AP Chemistry
Some Rules:
-ite is one less oxygen than the –ate {ex: nitrate (NO3-) to nitrite (NO2-)}
Hypo- is one less oxygen than the –ite {ex: chlorite (ClO2-) to hypochlorite (ClO-)}
Per- is one more oxygen than the –ate {ex: chlorate (ClO3-) to perchlorate (ClO4-)}
Hydrogen can be added to -2 or -3 ions to make a “new ion” {ex: H2PO4 – is dihydrogen
phosphate (note the – charge went up 1 for each H+ added)}
Strategy: Learn the bold ions below first. They are the most important. Then, learn the others.
Use the Rules above to help. Make flashcards and practice a few minutes daily until you get
these!
+1
ammonium, NH4+
-1
acetate, C2H3O2-, or CH3COO chlorate, ClO3cyanide, CNhydrogen carbonate, HCO3- (also called bicarbonate)
hydroxide, OHhypochlorite, ClOnitrate, NO3nitrite, NO2permanganate, MnO4perchlorate, ClO4-2
carbonate, CO3 -2
chromate, CrO4 -2
dichromate, Cr2O7 -2
peroxide, O2 -2
sulfate, SO4 -2
sulfite, SO3 -2
-3
phosphate, PO4 -3
phosphite, PO3 -3
6
Periodic Table and Ionic Charges
You need to know:
1. Group 1A ions always have a +1 charge (i.e. Li+)
2. Group 2A ions have a +2 charge (i.e. Ca2+
3. Group 3A ions typical have a +3 charge (i.e. Al3+)
4. Group 4A ions vary
5. Group 5A ions typically have a -3 charge (i.e. N3-)
6. Group 6A ions typically have a -2 charge (i.e. O2-)
7. Group 7A ions typically have a -1 charge (i.e. Cl-)
8. Noble gases only form ions in extreme cases
9. Transitions elements typically have variable charge, however you need to know these
fixed transition element ion charges:
a. Silver ion Ag+
b. Zinc ion Zn2+
c. Cadmium ion Cd2+
Common Diatomic and Polyatomic Elements
These elements, when uncombined with other element, exist as diatomic or polyatomic elements:
Hydrogen H2
Chlorine
Cl2
Nitrogen
N2
Oxygen
O2
Fluorine
F2
Bromine
Br2
Iodine
I2
phosphorous P4
Common Names of Common Chemicals
1. H2O
water
2. CH4
methane
3. NH3
ammonia
7
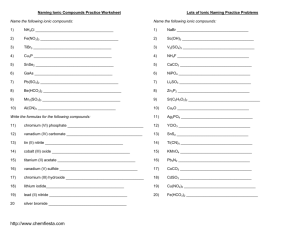
Ionic Naming Practice 1
Name the following ionic compounds:
1)
NH4Cl
_____________________________________
2)
Fe(NO3)3
_____________________________________
3)
TiBr3
_____________________________________
4)
Cu3P
_____________________________________
5)
SnSe2
_____________________________________
6)
GaAs
_____________________________________
7)
Pb(SO4)2
_____________________________________
8)
Be(HCO3)2
_____________________________________
9)
Mn2(SO3)3
_____________________________________
10)
Al(CN)3
_____________________________________
Write the formulas for the following compounds:
11)
chromium (VI) phosphate
_____________________________________
12)
vanadium (IV) carbonate
_____________________________________
13)
tin (II) nitrite
_____________________________________
14)
cobalt (III) oxide
_____________________________________
15)
titanium (II) acetate
_____________________________________
16)
vanadium (V) sulfide
_____________________________________
17)
chromium (III) hydroxide
_____________________________________
18)
lithium iodide
_____________________________________
19)
lead (II) nitride
_____________________________________
20
silver bromide
_____________________________________
8
Answers: Ionic Naming Practice 1
1)
ammonium chloride
2)
iron (III) nitrate
3)
titanium (III) bromide
4)
copper (I) phosphide
5)
tin (IV) selenide
6)
gallium arsenide
7)
lead (IV) sulfate
8)
beryllium bicarbonate
9)
manganese (III) sulfite
10)
aluminum cyanide
11)
Cr(PO4)2
12)
V(CO3)2
13)
Sn(NO2)2
14)
Co2O3
15)
Ti(CH3COO)2
16)
V2S5
17)
Cr(OH)3
18)
LiI
19)
Pb3N2
20)
AgBr
9
Ionic Naming Practice 2
Name the following ionic compounds:
1)
NaBr
__________________________________
2)
Sc(OH)3
__________________________________
3)
V2(SO4)3
__________________________________
4)
NH4F
__________________________________
5)
CaCO3
__________________________________
6)
NiPO4
__________________________________
7)
Li2SO3
__________________________________
8)
Zn3P2
__________________________________
9)
Sr(CH3COO)2
__________________________________
10)
Cu2O
__________________________________
11)
Ag3PO4
__________________________________
12)
YClO3
__________________________________
13)
SnS2
__________________________________
14)
Ti(CN)4
__________________________________
15)
KMnO4
__________________________________
16)
Pb3N2
__________________________________
17)
CoCO3
__________________________________
18)
CdSO3
__________________________________
19)
Cu(NO2)2
__________________________________
20)
Fe(HCO3)2
__________________________________
10
Write the formulas for the following ionic compounds:
21)
lithium acetate
__________________________________
22)
iron (II) phosphate
__________________________________
23)
titanium (II) selenide
__________________________________
24)
calcium bromide
__________________________________
25)
gallium chloride
__________________________________
26)
sodium hydride
__________________________________
27)
beryllium hydroxide
__________________________________
28)
zinc carbonate
__________________________________
29)
manganese (VII) arsenide
__________________________________
30)
copper (II) chlorate
__________________________________
31)
cobalt (III) chromate
__________________________________
32)
ammonium oxide
__________________________________
33)
potassium hydroxide
__________________________________
34)
lead (IV) sulfate
__________________________________
35)
silver cyanide
__________________________________
36)
vanadium (V) nitride
__________________________________
37)
strontium acetate
__________________________________
38)
molybdenum sulfate
__________________________________
39)
platinum (II) sulfide
__________________________________
40)
ammonium sulfate
__________________________________
11
Answers: Ionic Naming Practice 2
Name the following ionic compounds:
1)
NaBr
sodium bromide
2)
Sc(OH)3
scandium (III) hydroxide
3)
V2(SO4)3
vanadium (III) sulfate
4)
NH4F
ammonium fluoride
5)
CaCO3
calcium carbonate
6)
NiPO4
nickel (III) phosphate
7)
Li2SO3
lithium sulfite
8)
Zn3P2
zinc phosphide
9)
Sr(CH3COO)2 strontium acetate
10)
Cu2O
copper (I) oxide
11)
Ag3PO4
silver phosphate
12)
YClO3
yttrium(I) chlorate
13)
SnS2
tin (IV) sulfide
14)
Ti(CN)4
titanium (IV) cyanide
15)
KMnO4
potassium permanganate
16)
Pb3N2
lead (II) nitride
17)
CoCO3
cobalt (II) carbonate
18)
CdSO3
cadmium sulfite
19)
Cu(NO2)2
copper (II) nitrite
20)
Fe(HCO3)2
iron (II) bicarbonate
12
Write the formulas for the following ionic compounds:
21)
lithium acetate
LiCH3COO
22)
iron (II) phosphate
Fe3(PO4)2
23)
titanium (II) selenide
TiSe
24)
calcium bromide
CaBr2
25)
gallium chloride
GaCl3
26)
sodium hydride
NaH
27)
beryllium hydroxide
Be(OH)2
28)
zinc carbonate
ZnCO3
29)
manganese (VII) arsenide
Mn3As7
30)
copper (II) chlorate
Cu(ClO3)2
31)
cobalt (III) chromate
Co2(CrO4)3
32)
ammonium oxide
(NH4)2O
33)
potassium hydroxide
KOH
34)
lead (IV) sulfate
Pb(SO4)2
35)
silver cyanide
AgCN
36)
vanadium (V) nitride
V3N5
37)
strontium acetate
Sr(CH3COO)2
38)
molybdenum sulfate
Mo(SO4)3
39)
platinum (II) sulfide
PtS
40)
ammonium sulfate
(NH4)2SO4
13
Ionic Naming Practice 3
Write the formulas of the following ionic compounds
1)
iron (II) arsenide
_____________________________________
2)
lead (II) sulfate
_____________________________________
3)
lead (IV) hydroxide
_____________________________________
4)
copper (II) acetate
_____________________________________
5)
beryllium chloride
_____________________________________
6)
ammonium chromate
_____________________________________
7)
silver oxide
_____________________________________
8)
potassium sulfide
_____________________________________
Write the names of the following ionic compounds
9)
KI
_____________________________________
10)
Mn2(SO3)7
_____________________________________
11)
SnBr4
_____________________________________
12)
Mg3P2
_____________________________________
13)
NaF
_____________________________________
14)
Sr(MnO4)2
_____________________________________
15)
Cr(PO4)2
_____________________________________
16)
Al2Se3
_____________________________________
14
Answers: Ionic Naming Practice 3
Write the formulas of the following ionic compounds:
1)
iron (II) arsenide
Fe3As2
2)
lead (II) sulfate
PbSO4
3)
lead (IV) hydroxide
Pb(OH)4
4)
copper (II) acetate
Cu(CH3COO)2
5)
beryllium chloride
BeCl2
6)
ammonium chromate
(NH4)2CrO4
7)
silver oxide
Ag2O
8)
potassium sulfide
K2S
Write the names of the following ionic compounds:
9)
KI
potassium iodide
10)
Mn2(SO3)7
manganese (VII) sulfite
11)
SnBr4
tin (IV) bromide
12)
Mg3P2
magnesium phosphide
13)
NaF
sodium fluoride
14)
Sr(MnO4)2
strontium permanganate
15)
Cr(PO4)2
chromium (VI) phosphate
16)
Al2Se3
aluminum selenide
15
Naming Acids and Bases
Memorize the acids and bases in the table below first. If needed, see summer assignment web
page for more help on how to name acids and bases.
Formula
Acids H2SO4
HCl
HBr
HI
HClO3
HClO4
HNO3
HF
CH3COOH
H2CO3
Bases LiOH
NaOH
KOH
RbOH
CsOH
Ca(OH)2
Sr(OH)2
Ba(OH)2
NH3
Name
sulfuric acid
hydrochloric acid
hydrobromic acid
hydroiodic acid
chloric acid
perchloric acid
nitric acid
hydrofluoric acid
acetic acid
carbonic acid
lithium hydroxide
sodium hydroxide
potassium hydroxide
rubidium hydroxide
cesium hydroxide
calcium hydroxide
strontium hydroxide
barium hydroxide
ammonia
Name the following acids and bases:
1)
NaOH
_______________________________________
2)
H2SO4
_______________________________________
3)
H2SO3
_______________________________________
4)
H3PO4
_______________________________________
5)
NH3
_______________________________________
6)
HCN
_______________________________________
7)
Ca(OH)2
_______________________________________
8)
Fe(OH)3
_______________________________________
9)
H3P
_______________________________________
16
10)
CH3COOH
______________________________________
11)
KOH
___________________________________
12)
HNO3
________________________________________
13)
HNO2
________________________________________
14)
HClO3
_____________________________________
15)
HClO4
________________________________________
16)
H2CO3
_______________________________________
Write the formulas of the following acids and bases:
17)
hydrofluoric acid
_______________________________________
18)
hydroselenic acid
_______________________________________
19)
carbonic acid
_______________________________________
20)
lithium hydroxide
_______________________________________
21)
nitrous acid
_______________________________________
22)
cobalt (II) hydroxide
_______________________________________
23)
sulfuric acid
_______________________________________
24)
beryllium hydroxide
_______________________________________
25)
hydrobromic acid
_______________________________________
26)
nitric acid
_________________________________________
27)
ammonia
____________________________________
28)
acetic acid
______________________________________
17
Answers: Naming Acids and Bases
Name the following acids and bases:
1)
2)
3)
4)
5)
6)
7)
8)
9)
10)
11)
12)
13)
14)
15)
16)
NaOH
H2SO4
H2SO3
H3PO4
NH3
HCN
Ca(OH)2
Fe(OH)3
H3P
CH3COOH
KOH
HNO3
HNO2
HClO3
HClO4
H2CO3
sodium hydroxide
sulfuric acid
sulfurous acid
phosphoric acid
ammonia
hydrocyanic acid
calcium hydroxide
iron (III) hydroxide
hydrophosphoric acid
acetic acid
potassium hydroxide
nitric acid
nitrous acid
chloric acid
perchloric acid
carbonic acid
Write the formulas of the following acids and bases:
17)
18)
19)
20)
21)
22)
23)
24)
25)
26)
27)
28)
hydrofluoric acid
hydroselenic acid
carbonic acid
lithium hydroxide
nitrous acid
cobalt (II) hydroxide
sulfuric acid
beryllium hydroxide
hydrobromic acid
nitric acid
ammonia
acetic acid
HF
H2Se
H2CO3
LiOH
HNO2
Co(OH)2
H2SO4
Be(OH)2
HBr
HNO3
NH3
CH3COOH
18
Covalent Naming Practice
Write the names of the following covalent compounds:
1)
SO3
____________________________________
2)
N2S
____________________________________
3)
PH3
____________________________________
4)
BF3
____________________________________
5)
P2Br4
____________________________________
6)
CO
____________________________________
7)
SiO2
____________________________________
8)
SF6
____________________________________
9)
NH3
____________________________________
10)
NO2
____________________________________
Write the formulas of the following covalent compounds:
11)
nitrogen trichloride
__________________
12)
boron carbide
__________________
13)
dinitrogen trioxide
__________________
14)
phosphorus pentafluoride
__________________
15)
methane
__________________
16)
sulfur dibromide
__________________
17)
diboron tetrahydride
__________________
18)
oxygen difluoride
__________________
19)
carbon disulfide
__________________
20)
nitrogen monoxide
__________________
19
Answers: Covalent Naming Practice
Write the names of the following covalent compounds:
1)
SO3
sulfur trioxide
2)
N2S
dinitrogen sulfide
3)
PH3
phosphorus trihydride
4)
BF3
boron trifluoride
5)
P2Br4
diphosphorus tetrabromide
6)
CO
carbon monoxide
7)
SiO2
silicon dioxide
8)
SF6
sulfur hexafluoride
9)
NH3
ammonia
10)
NO2
nitrogen dioxide
Write the formulas of the following covalent compounds:
11)
nitrogen trichloride
NCl3
12)
boron carbide
BC
13)
dinitrogen trioxide
N2O3
14)
phosphorus pentafluoride
PF5
15)
methane
CH4
16)
sulfur dibromide
SBr2
17)
diboron tetrahydride
B2H4
18)
oxygen difluoride
OF2
19)
carbon disulfide
CS2
20)
nitrogen monoxide
NO
20
Practice Quiz 1
Name each compound.
1)
Na2CO3
_________________________________________
2)
NH4OH
_________________________________________
3)
NH3
_________________________________________
4)
FeSO4
_________________________________________
5)
SiO2
_________________________________________
6)
Ga(NO3)3
_________________________________________
7)
H2SO4
_________________________________________
8)
B2H4
_________________________________________
9)
CO
_________________________________________
10)
HClO4
_________________________________________
Write the formulas of the following chemical compounds:
11)
dinitrogen trioxide
_________________________________________
12)
nitrogen
_________________________________________
13)
methane
_________________________________________
14)
lithium acetate
_________________________________________
15)
phosphorus trifluoride _________________________________________
16)
vanadium (V) oxide _________________________________________
17)
aluminum hydroxide _________________________________________
18)
zinc sulfide
_________________________________________
19)
carbonic acid
_________________________________________
20)
silver phosphate
_________________________________________
21
Answers: Practice Quiz 1
Name each compound.
1)
Na2CO3
sodium carbonate
2)
NH4OH
ammonium hydroxide
3)
NH3
ammonia
4)
FeSO4
iron (II) sulfate
5)
SiO2
silicon dioxide
6)
Ga(NO3)3
gallium nitrate
7)
H2SO4
sulfuric acid
8)
B2H4
diboron tetrahydride
9)
CO
carbon monoxide
10)
HClO4
perchloric acid
Write the formulas of the following chemical compounds:
11)
dinitrogen trioxide
N2O3
12)
nitrogen
N2
13)
methane
CH4
14)
lithium acetate
LiCH3COO
15)
phosphorus trifluoride
PF3
16)
vanadium (V) oxide
V2O5
17)
aluminum hydroxide
Al(OH)3
18)
zinc sulfide
ZnS
19)
carbonic acid
H2CO3
20)
silver phosphate
Ag3PO4
22
Practice Quiz 2
Name each compound.
1)
HI
______________________________________
2)
CaSO4
______________________________________
3)
C2Br6
______________________________________
4)
Cr(CO3)3
______________________________________
5)
Ag3P
______________________________________
6)
IO2
______________________________________
7)
HCl
______________________________________
8)
PbS
______________________________________
9)
CH4
______________________________________
10)
N2O3
______________________________________
Write the formulas of the following chemical compounds:
11)
tetraphosphorus triselenide
____________________________________
12)
potassium acetate
______________________________________
13)
iron (II) phosphide
______________________________________
14)
disilicon hexabromide
______________________________________
15)
titanium (IV) nitrate
______________________________________
16)
diselenium diiodide
______________________________________
17)
copper (I) phosphate
______________________________________
18)
ammonium oxide
______________________________________
19)
nitric acid
______________________________________
20)
phosphorus
______________________________________
23
Answers: Practice Quiz 2
Name each compound.
1)
HI
hydroiodic acid
2)
CaSO4
calcium sulfate
3)
C2Br6
dicarbon hexabromide
4)
Cr(CO3)3
chromium (VI) carbonate
5)
Ag3P
silver phosphide
6)
IO2
iodine dioxide
7)
HCl
hydrochloric acid
8)
PbS
lead (II) sulfide
9)
CH4
methane
10)
N2O3
dinitrogen trioxide
Write the formulas of the following chemical compounds:
11)
tetraphosphorus triselenide
P4Se3
12)
potassium acetate
KCH3COO
13)
iron (II) phosphide
Fe3P2
14)
disilicon hexabromide
Si2Br6
15)
titanium (IV) nitrate
Ti(NO3)4
16)
diselenium diiodide
Se2I2
17)
copper (I) phosphate
Cu3PO4
18)
ammonium oxide
(NH4)2O
19)
nitric acid
HNO3
20)
phosphorus
P4
24
Practice Quiz 3
Name each compound.
1)
NaBr
______________________________________________
2)
Ca(C2H3O2)2
______________________________________________
3)
P2O5
______________________________________________
4)
HBr
______________________________________________
5)
FePO4
______________________________________________
6)
K3N
______________________________________________
7)
SO2
_____________________________________________
8)
CuOH
______________________________________________
9)
Zn(NO2)2
______________________________________________
10)
V2S3
______________________________________________
Write the formulas for the following chemical compounds:
11)
silicon dioxide
_________________________
12)
nickel (III) sulfide
_________________________
13)
manganese (II) phosphate
_________________________
14)
silver acetate
_________________________
15)
diboron tetrabromide
_________________________
16)
magnesium sulfate heptahydrate
_________________________
17)
potassium carbonate
_________________________
18)
ammonium oxide
_________________________
19)
acetic acid
_________________________
20)
ammonia
_________________________
25
Answers: Practice Quiz 3
Name the following chemical compounds:
1)
NaBr
sodium bromide
2)
Ca(C2H3O2)2
calcium acetate
3)
P2O5
diphosphorus pentoxide
4)
HBr
hydrobromic acid
5)
FePO4
iron(III) phosphate
6)
K3N
potassium nitride
7)
SO2
sulfur dioxide
8)
CuOH
copper(I) hydroxide
9)
Zn(NO2)2
zinc nitrite
10)
V2S3
vanadium(III) sulfide
Write the formulas for the following chemical compounds:
11)
silicon dioxide
SiO2
12)
nickel (III) sulfide
Ni2S3
13)
manganese (II) phosphate
Mn3(PO4)2
14)
silver acetate
AgC2H3O2
15)
diboron tetrabromide
B2Br4
16)
magnesium sulfate heptahydrate
MgSO4.7H2O
17)
potassium carbonate
K2CO3
18)
ammonium oxide
(NH4)2O
19)
acetic acid
CH3COOH
20)
ammonia
NH3
26