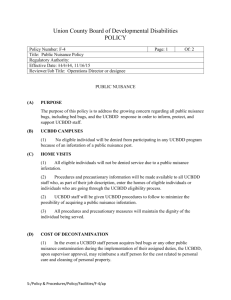
Integrated Cell Processing Work Station
advertisement

Integrated Cell Processing Work Station MHE-PF4025CW2-PA us.panasonic-healthcare.com powered by powered by Discovery starts with a dream. A dream to know who we are. A dream to better our lives... Behind every great discovery, there’s the technology that made it all possible. Panasonic Integrated Cell Processing Work Station (CPWS) Minimizes Cleanroom Expense Self-Contained Space-Saving Easy to Install GMP Compliant for Aseptic Processes User Friendly Energy Efficient, Green Design The Essential Work Station for Cell Therapeutics In designing the industry’s first viable alternative to a conventional Class 10,000 cleanroom for both Class 100 air quality and barrier isolation, Panasonic has introduced the Cell Processing Work Station (CPWS). It brings the potential of cell therapeutics to more facilities, while decreasing acquisition costs, operation costs and protracted time lines. Global Cell Therapy and Tissue Engineering Market ( 2010 & 2015 ) $25,000 Cord Blood and Cell Banking $20,000 Cell/Issue Banking Cancer Cancer General Sur GI,Gyn,Others $15,000 Ophthalmalo Cardiology Neurology General Ophthalmalogy and Organ Replacement/ Preservation Organ $10,000 Dental Dental/Oral Millions of Dollars --------> Skin Urology Osthol $5,000 Orthopedics/Spine Neuro Cardio 2010 2015 Immediate beneficiaries of the CPWS include research focused institutions and scale-up development to treat a broad range of diseases. The focused research can be related to human cells, cellular therapies in clinical trials, and hospitals deploying FDA approved cellular therapeutics. As the science progresses, results are propagated throughout the medical and scientific community. This exponentially expands the need for bench-level research and production. In such situations, Panasonic's approach with free-standing and self contained work station is quite novel considering the conventional layered cleanroom approach of containment and protection. The CPWS brings the capability for on-site cellular therapeutics to more number of facilities rather quickly, at a lower capital and reduced operational cost. All this is within the GMP (Good Manufacturing Practices) performance envelope required of the cell technology itself. 4 Skin Urology Self-Contained System, Small Footprint The Panasonic concept of a self-contained work station is enabled by the company’s demonstrated proficiency for in situ decontamination.This is necessary to separate the processes from one patient to the next, or one protocol to another. By using a highly effective hydrogen peroxide (H2O2) decontamination process, the CPWS can be completely decontaminated without heat and prepared for the next protocol within two hours. This is extremely time efficient as compared to a cleanroom decontamination function that can take up to days or weeks. By increasing throughput within the compliant parameters of GMP criteria, the CPWS is deployed for research, cellular manipulation, cell growth, cell product extraction, and emerging processes that fall within similar guidelines. Current and future applications of the CPWS include organ and tissue regeneration such as skin, cartilage, alveolar bone, cornea, cardiac muscle, nerve, liver, and pancreas regeneration. Immunotherapy applications may extend to dendritic cells, T-cells, and more. Making it Work Improving efficiency in human interaction is the primary goal of the CPWS. All work must be performed without human error, in an aseptic environment with detailed documentation to assure quality and compliance. Gown-up time, expense, and inconvenience is minimized or eliminated altogether. Integrated systems within the CPWS permit cellular extraction, preparation, culturing, and administration with aseptic assurance as well as economic practicality. Because conventional autoclaving is not possible, the Panasonic H2O2 decontamination process diminishes both time and labor associated with this critical step between patients. This positions the CPWS squarely in the equation for cost/ benefit justification associated with investment decision-making. Allowances for integrated centrifuge, microscopy, data acquisition, and incubation functions are important considerations in the CPWS design. A unique docking station permits interface and exchange with an unlimited number of cell culture incubators dedicated to individual patients or cell lines. On the Work Surface Since humans remain the single most common source of contamination, the CPWS provides both physical and work flow benefits to minimize contamination and cross contamination in the work area. Within the four-port glove box, the CPWS delivers more than a conventional Class II, Type A2 biological safety cabinet, typically installed within a cleanroom to achieve the same objective. Here, the manually initiated, automatically deployed H2O2 decontamination process supplements continuous HEPA filtration. As 0.3 micron particles are removed from the fresh air exchange, H2O2 decontamination neutralizes contaminants brought forth by instrumentation or equipment. A number of decontamination sequences are available to protect the aseptic environment. The glove box design offers barrier isolation protection for the operator and the work inside. User comfort and ergonomics are inherent to the CPWS design, including a sloped front for ease of access and glare reduction. The CPWS Advantage The relatively small footprint permits installation into existing or new Class 100,000 lab space with conventional utilities and minimal site preparation. Multi-layered airlocks in multiple treated rooms are avoided. Capital intensive expenses are lowered, while the lead times from decision to operation are shortened. Once in place, operational costs are highly contained and predictable, with decontamination available more frequently and at a fraction of conventional cost. The Panasonic Difference For over forty years, Panasonic has established a reputation as a premier manufacturer of precision biomedical and laboratory equipment. Known throughout the world as a leading brand in consumer electronics and appliances, Panasonic addresses global needs such as energy, food, housing, healthcare, and information technology. As a part of the Panasonic product line worldwide, the CPWS exemplifies our unique vertical component integration approach to product development. This combines ideas and innovations from our global industrial and consumer products network into an integrated product featuring advanced technology, controls, construction, and performance attributes. The CPWS and subcomponent systems have been extensively tested to meet the toughest quality standards for performance, ergonomics, and cost of ownership. The CPWS is designed to minimize its carbon footprint through energy savings and environmental stewardship. 5 Technical Attributes The Panasonic CPWS is a component-based design that permits a long-term or quick turnover of self-contained protocols with efficiency and safety. As an integrated system, all functions associated with good laboratory technique, environmental control, and ergonomic comfort are selected for compatibility and complementary functional performance. The Panasonic CPWS work station is designed to deliver efficient, cost effective, GMP compliant cell therapy, and manufacturing capability without the expense and inconvenience of a class 10,000 cleanroom. The CPWS offers significant advantages over conventional hard wall cleanroom construction. The CPWS is less expensive than a cleanroom. It is quicker to acquire and place into operation. The small footprint increases options for location and orientation. The user-friendly glove box design eliminates gowning, improves operator comfort, and convenience. Operating costs are lower than cleanroom costs Central barrier isolator Pass box interchange Integrated H2O2 decontamination system Optional cell observation system with microscope and monitor The optional incubator and optional centrifuge operate within a Class 100 environment. 6 Work is easily suspended and resumed without the need to de-gown and re-gown, improving user comfort. Fast decontamination increase throughput and deliver quicker return on investment. Record-keeping and process documentation are easier to manage. Optional centrifuge integrated into the work surface Optional CO2 incubator with docking collar Components and operating systems are configured around a central work station with a HEPA filtration and air management system. This is designed to deliver Class 100 air to the work surface within the glove box. 3 1 7 11 4 13 2 5 8 6 9 12 10 1 Hinged front access assembly. Front lifts up when total interior access is required. 2 3 Interchange pass box with manually initiated, automatic sequence H2O2, decontamination system. HEPA supply and exhaust filtration blower motor assembly 4 Interior fluorescent lamps 10 Adjustable leveling feet 5 Glove port 11 Optional cell monitor LCD 6 Electrical compartment 12 7 System controller 8 Centrifuge module accessible for the aseptic work area. 9 Centrifuge controller 13 Incubator cart with locking casters; offset casters nest with frame assembly when docked. Modular CO2 incubator, shown on cart, docked to barrier isolator. 7 Applications Clinical Applications of Cell Therapies DISEASE STATES CELL THERAPIES CANCER HEMATOPOIETIC STEM CELL (HSC) TRANSPLANTATION IMMUNOTHERAPY ORTHOPEDIC NEURODEGENERATIVE DISORDERS/TRAUMA CARDIOVASCULAR DISEASE Autologous and allogeneic HSC; Ex vivo expansion of HSC; ‘Suicide’ T-cells – gene transfer; Stem cell transportation Dendritic cells; NK/T cells; Macrophage-activated killer cells; T-cell expansion; NK cells; Co-stimulatory molecules (gene transfer) Expanded chondrocytes; Mesenchymal stem cells Adult stem cell-derived cells; Embryonic stem cell-derived neural cells Infusion of marrow/blood-derived angio blasts; CD34 stem cells; cardiac cells* ORGAN REPLACEMENT PANCREAS (DIABETES) Pancreatic islet cells; Embryonic stem cell-derived islet cells; Adult stem cell-derived islet cells LIVER (FAILURE, METABOLIC DISORDERS) Bioartificial liver; Isolated hepatocytes; Hepatocyte stem cells KIDNEY (FAILURE) WOUND HEALING INFECTIOUS DISEASES Bioartificial kidney Keratinocytes; Skin stem cells Antigen-loaded dendritic cells; Lymphocyte expansion; Macrophages GENETIC DEFICIENCIES HEMOPHILIA Gene Therapy SCID Gene Therapy CYSTIC FIBROSIS Gene Therapy AUTOIMMUNE DISEASES IMMUNOTHERAPY Dendritic cells; T-cells, Mesenchymal stem cells*; Lymphocyte expansion; Natural Killer cells HSC, hematopoietic stem cells; NK, Natural Killer; SCID, severe combined immunodeficiency * Currently in clinical studies. 8 Applications The Panasonic CPWS enables a broader access to cellular therapeutic research related to both, minimally manipulated and nonminimally manipulated cell products. It lowers the cost of entry, extends the process to the widest range of applications, and minimizes operating expenses, when compared to a conventional cleanroom environment. • Minimally manipulated products are associated with cell washing, enrichment, selection, HSC (PB, BM, CB), cancer therapies, and other under GTP requirements. • Non-minimally manipulated products are associated with expanded, differentiated or transformed cells (DC, MSC, ESC, TC) in cancer centers, biotech labs, stem cell institutes, and contract manufacturing facilities operating under GMP requirements. • GMPs (Good Manufacturing Practices) are mandated by the United States Food and Drug Administration to ensure that drug development and manufacturing is safe, under controlled environment, reproducible, and thoroughly documented. • GMPs typically require expensive hard wall laboratories with laboratory suites that use biological safety cabinets in Class 10,000 cleanrooms within a Class 100,000 room. Cost Benefit Analysis Approximately 35% Less Expensive to Operate The Panasonic CPWS offers significantly lower operating costs when compared to a conventional cleanroom (open system). Comparison methodology and assumptions are available from Panasonic. Soft-cost benefits related to user convenience, user comfort, flexibility, throughput, and other demonstrated advantages of the CPWS can be established through consultative review. Contact Panasonic for details. Comparative Operating Costs1 (Nominal), Panasonic CPWS vs. Conventional Cleanroom, PANASONIC CELL PROCESSING WORK STATION (CPWS) FUNCTION CONVENTIONAL GMP CLEANROOM ANNUAL BUDGET (NOMINAL) FUNCTION ANNUAL BUDGET (NOMINAL) H2 O 2 Cartridge, 25g, Total System Decontamination $9,000 Disinfection, Cleaning $9,000 H2 O 2 Cartridge, 5g, Pass Box Only Decontamination $13,320 Routine Sanitation $12,000 Residual H2 O 2 Detector $2,664 Particle Sensors $3,000 Gloves, 3 gloves, 2× $1,140 Sterilized, Non-Shedding Gowns $18,000 Sleeves, 3 gloves, 1× $3,640 HEPA FILTERS HEPA FILTERS Biosafety Cabinet, Supply Filter $343 $907 Biosafety Cabinet, Exhaust Filter $190 Interchange Pass Box $294 Certifier Labor, BSC Formalin Decontamination Room $833 Cleanroom Air Supply Labor $1,330 Work Station VALIDATION EXPENSES $956 $2,916 Cleanroom Air Exhaust $1,516 Certifier Labor, CR Formalin Decontamination $1,500 $1,440 VALIDATION EXPENSES Decontamination Performance BI Inspection $3,230 Biosafety Cabinet and Report $1,050 Cleanroom and Report $10,000 Centrifuge and Report $1,350 H2 O 2 Mist Uniformity Inspection $720 Centrifuge Module and Report $1,350 CO 2 Incubator Module and Report $1,250 POWER CONSUMPTION $18,057 NOMINAL ANNUAL BUDGET 35% Less Cleanliness and Air Volume Report CO 2 Incubator Module and Report $850 POWER CONSUMPTION $60,000 NOMINAL ANNUAL BUDGET $28,972 $91,600 Comparative operating costs listed herein are nominal and based on actual installations in Japan. Total cost of ownership and operation will vary according to type of installation, method of use, geographic location and other factors. Panasonic offers consultative assistance in developing a cost/ benefit analysis specific to your facility and protocol. Contact Panasonic to arrange a report unique to your prospective application 1 Who Benefits from CPWS MARKET FACILITY PROTOCOL CPWS BENEFIT ACADEMIC Cancer centers Cancer treatment, translational medicine Easy segregation of patient-specific cells ACADEMIC Medical research Regenerative medicine CLINICAL Hospitals PRIVATE INDUSTRY Biotechnology, Pharmaceutical Easier transition from animal models to human clinical trials 9 Feature Benefit Analysis Aseptic Environment Required for Cell Preparation Usually, human derived cells must be guaranteed that they are prepared and cultured in an aseptic environment because they cannot be treated by heat or pressure. Orginal Cells Preparation Extraction Culture CPWS SOLUTION TYPICAL CLEANROOM Glove Box HEPA Filters Change Room More Cost Centrifuge GENERAL PLANNING AND LEAD TIME SPACE/FOOTPRINT ALLOWANCE VALIDATION COSTS OPERATION COST IMPLEMENTATION 10 Class 10,0 00 Class 10,000 Class 100,000 Pass Box * Drawing not to scale PANASONIC CPWS BARRIER ISOLATOR CLEANROOM WITH BIOLOGICAL SAFETY CABINETS Closed system, requires only Class 100,000 air Open system, requires significant investment and maintenance of background environment, Class 10,000 air Minimal; Cost of Class 100,000 room plus CPWS High; Cost of Class 10,000 cleanroom plus Class 100 one or more Class 100 biological safety cabinets Minimal footprint in existing space Dedicated new/retrofit facility with significant requirement for HVAC, filtration, air showers Minimal; Requires Class 100,000 only. High; Requires both Class 100 and Class 100,000. Low High; Repeated decontamination and maintenance costs. Higher consumables cost Weeks Months or Years No second gowning required. Central barrier isolator with ergonomically angled front permits easy access through glove ports. Reduces stress on workforce. Conventional first and second gowning, external air supply, interlock doors/air showers FLEXIBILITY Elimination of hazardous fumes for decontamination permits diverse applications. Elimination of depolymerizing formaldehyde, formalin and other toxic chemicals permits transition between applications seamlessly. Inflexible THROUGHPUT Expanded. Quick changeover extends use, optimizes return on investment. Component integration streamlines workflow while enhancing aseptic processing. Limited due to product changeover criteria DECONTAMINATION Two Hours Up to Two Weeks EQUIPMENT AND INSTRUMENTATION Microscopes and other instrumentation can be dedicated to the work station. Shared instrumentation in an open system exposes processes to cross contamination. ERGONOMICS AND USER COMFORT ISO14644-1 Class 10,000 Class 100,000 Efficient Detachable Incubator Class 100-BSC Class 100,000 35% Class 100 COMPARISON Prepared Cells Administration FDA EU-GMP ANNEX 1 JP WHO-GMP TRS902 ANNEX 6 CLASS 5 Class 100 In Operation 3,520 Grade A In Operation 3,500 Grade A In Operation 3,520 Grade A In Operation 3,500 CLASS 7 Class 10,000 In Operation 35,200 Grade B In Operation 350,000 At rest 3,500 Grade B In Operation 352,000 At rest 3,520 Grade B In Operation 350,000 At rest 3,500 CLASS 8 Class 100,000 In Operation 3,520,000 Grade C In Operation 3,500,000 At rest 350,000 Grade C In Operation 3,520,000 At rest 352,000 Grade C In Operation 3,500,000 At rest 350,000 CLASS 9 — Grade D At rest 3,500,000 Grade D At rest 3,520,000 Grade D At rest 3,500,000 Aseptic Environment Conventional GMP Cleanroom Facility (Open System) with Biological Safety Cabinets Highest aspect level = Aseptic (safety cabinets, etc) Aseptic level low (D) 6 m2 16 m2 PS 18 m2 Aseptic level mid (C) Aseptic level high (B) Aseptic level raised gradually PS PS -152ºC PS PS (A) Air lock General environment (not clean) 18 m2 -32ºC 11 m2 11 m2 10 m2 4 m2 PS PS PS PS PS PS 10 m2 16 m2 7 m2 3 m2 Class A (100) Class B (10,000) Class C IO(100,000) Class D AR(100,000) Panasonic CPWS, Barrier Isolator with Integrated Systems The Panasonic CPWS offers significant throughput potential in a comparatively small footprint. Cleanroom: The biological safety cabinets within the cleanroom are open systems, which require a stricter background environment. Access in and out of the cleanroom must comply with gown-up protocols consistent with GMP facilities. Class 100 air is ultimately delivered to the work product in one or more biological safety cabinet(s) operating independent of the cleanroom. Decontamination is costly and time-consuming; changeover from one protocol to another is difficult and expensive. The CPWS can be installed in manageable Grade D (Class 100,000) environment, providing biological and physical protection between the 6 m2 work and the user. 16 m2 PS 18 m2 Access through the glove ports improves user comfort. The integral H2O2 decontamination system is completed in less than PS two hours, permitting frequent changeover and assuring separation integrity from one cell process to the next. PS -152ºC PS PS 18 m2 -32ºC 11 m2 11 m2 PS 10 m2 PS PS PS PS PS 10 m2 16 m2 7 m2 3 m2 Class A (100) Class B (10,000) Class C IO(100,000) Class D AR(100,000) 11 Series Features Barrier Isolator The barrier isolator forms the central component to the work station and contains the primary operating systems required to establish and maintain aseptic conditions to meet GMP criteria. • The barrier completely isolates the interior work product from the operator. • The barrier isolator creates a closed Class 100 environment, eliminating the need for biological safety cabinets in a cleanroom. • Isolator interior air is 100% Class 100 total exhaust. No recirculated air is used. • The polished stainless steel glove box interior is designed for maximum exposure of all interior surfaces subject to H2O2 decontamination. • Before commissioning and initial use, the isolator front can be opened on a locking hinged frame for installation of instrumentation or other devices larger than the interchange pass box opening. • The HEPA filter and airflow system is mounted on top of the isolator. • The internal airflow system is designed to create a positive pressure to mitigate the possibility of inflow contamination. • Viability of the containment area is not delegated to third-party cleanroom contractors who are hired to decontaminate. Ergonomics and Safety Because second gowning is not required, user comfort and productivity is significantly improved. The workplace routine, including bathroom breaks, is unencumbered by the need to leave and re-enter a cleanroom, bleach, and/or shower. • The inconvenience and expense of cumbersome containments suits with air and vacuum hoses is eliminated. If working with BL3 agents, the buddy system is not required. • The barrier eliminates the potential for room contamination from blood or other aerosols. Workflow is not impacted by routine colds. • Staff confidence in total containment improves morale. • Glove ports permit easier handling of red bag materials when required. • A 6º angled front includes three glove ports to permit access to all interior surfaces. • The interior cabinet includes independent interior fluorescent lamps to supplement ambient light. H2O2 Decontamination System Isolator Interchange • The manually initiated, automatically sequenced H2O2 decontamination system offers a fast, safe, and proven decontamination process. It enhances the performance of the CPWS by allowing more frequent turnover of segregated cell lines. • The interchange pass box allows safe access to the work area for supplies, instruments, devices, sterile media, and labware. • The validated H2O2 system generates an H2O2 vapor that permeates all exposed surfaces from the central interchange pass box nebulizer, which contains a replaceable bottle of enriched hydrogen peroxide. • When deployed, the H2O2 vaporization sequence decontaminates the pass box, work station interior, centrifuge, CO2 incubator exterior, and docking gaskets. • Once the vaporization is complete, the H2O2 program implements a dwell period to ensure that proper exposure times are maintained for a wide range of pathogens. • At the end of the pre-programmed dwell period a resolution process eliminates fumes and toxic residuals. 12 • When materials are brought into the work station they are first positioned inside the interchange for H2O2 decontamination. • Decontamination is manually initiated and automatically sequenced once started. • When the H2O2 decontamination process is complete, the inner door is opened and the transfer is completed. • Door interlocks permit simultaneous opening to protect the barrier isolator. Series Features Decontamination Sequence Total Decontamination A total H2O2 vapor decontamination creates aseptic conditions in the interchange pass box, the glove box and (optional) centrifuge module. Pass Box Only Instruments or materials introduced into the glove box are decontaminated by a pass box cycle only, avoiding the need to decontaminate the entire glove box work area. Work can continue in the glove box while the cycle is in process. Cell Culture Incubator Incubator and Work Area. After aseptic protocols are completed, cultures are transferred to the (optional) CO2 incubator where growth can continue under aseptic conditions after the incubator is detached. Unwanted materials are removed through the pass box interchange. $ Changeover When handling different cells from the previous protocol, the next (optional) cell culture incubator is docked to the work station glove box. The work station, interchange pass box (optional) centrifuge and incubator door are decontaminated prior to opening. The changeover process is completed in less than two hours. Decontaminated Contaminated Possible cell-derived contaminated One CPWS can service multiple CO2 incubators to permit easier cell segregation. One incubator is attached to the work station at the docking collar and subjected to a quick, safe H2O2 decontamination process. When work is completed, the incubator is sealed and returned to the staging area and another incubator is attached and the process is repeated. 13 Series Features CO2 Incubator Incubator Features and Benefits The modular CO2 incubator is an adaptation of the fullperformance Panasonic MCO-5AC(IS). This incubator is designed for precise temperature and CO2 control with elevated relative humidity to minimize cell desiccation. The incubator is a modification of Panasonic’s popular MCO-5AC. The compact 1.4 cu.ft. (49 liter) interior chamber is constructed of Panasonic’s exclusive inCu-saFe® copper-enriched stainless steel, creating a natural germicidal barrier against airborne contamination. • By using multiple, detachable CO2 incubators, the CPWS can manage multiple patient protocols through complete product segregation, thereby assuring aseptic conditions and eliminating any possibility of cross-contamination or mishandling of patientspecific cells. • Segregation permits compliance with published criteria associated with regenerative medicine and in vivo cell therapies. • An integrated microprocessor controller supervises all functions including temperature and CO2 setpoints, control, alarm and monitoring. • The patented Direct Heat and Air Jacket control system assures temperature uniformity and stability essential to the most sensitive cell lines. • The incubator attaches to a docking collar adjacent to the barrier • The rounded-corner, electropolished interior is configured for isolator. use with a variety of standard cell culture vessels. • Once attached, the barrier isolator undergoes a 2-hour H2O2 decontamination process before the incubator door is opened. This process decontaminates the work area, incubator face, centrifuge and pass-thru interchange. • The H2O2 effectively decontaminates the CO2 connection. • All interior components are designed to withstand repeated H2O2 decontamination cycles expected in high turnover environments. Components are removable without tools. Centrifuge • When work is complete, the incubator is sealed, detached and moved to a user-defined staging location on a wheeled cart. The centrifuge is installed beneath the interior work surface and accessible under aseptic conditions without removing cells from the protected environment. • The next incubator can be moved into position to repeat the process for another patient. • The position and orientation of the centrifuge assures thorough decontamination during the H2O2 decontamination cycle. • There is no limit to the number of Panasonic MCO-5AC(IS) incubators that can be used with the work station. • The integrated design eliminates the requirement for additional floor space in a GMP environment. • A variety of fixed and swinging rotors available. • Centrifuge controls are located external to the work area at the front of the centrifuge module. Modular Incubator Model MCO-5AC(IS) docked to the CPWS CPWS Centrifuge 14 Series Features 3 2 1 1. Work station Glove Box The work station creates Grade A Class 100 air quality within the glove box, and permits installation within a Grade D Class 100,000 room, eliminating the need for a typical cleanroom. The selfcontained, closed system protects both the user and the work product without the need for a second gowning. An ergonomically shaped front access is angled 6° for user comfort, glare reduction, and reach throughout the work surface. 2. Pass Box Decontamination 3. Decontamination System Materials are brought into the work chamber from the outside through the pass box interchange. The H2O2 decontamination is manually initiated and automatically sequenced, thoroughly decontaminating the pass thru material and protecting the integrity of the work surface. The interchange contamination process can function alone, independent of the glove box. The CPWS includes an H2O2 decontamination system that generates H2O2 vapor to produce validated results. This system covers the pass box interchange, glove box work area, optional centrifuge, and optional CO2 incubator. The enriched H2O2 solution is contained in a replaceable bottle and inserted as a cartridge inside the decontamination module. Cell Monitoring System (Optional) An integrated cell monitoring system reduces additional footprint required in a GMP environment. Cell monitor components are located within the barrier isolator and designed to withstand repeated H2O2 decontamination sequences. • The interior components include a LCD monitor and a separate collection module. • The collection module includes a high-performance CCD camera (charge coupled device) with objective lens and phase difference filter. • The monitor displays real time images with total magnification of 110×. Images from PC Control/Relay Box PC for Capturing AC Power Camera IF Various Signals IF Lighting VCC,GND Light Dimming Sygnal Cameral IF (power, image signals) • Images can be captured via a noncontact photo sensor, 2.4 × 1.8 mm for monitor display and digital recording. • A standard personal computer, external to the aseptic isolator work area, is used for data acquisition and image management. Power SW Signal Capture SW Signal Observation Module 15 Specifications Component Specifications System Summary Specifications COMPONENT DESCRIPTION COMPONENT ORDER NUMBER BARRIER ISOLATOR Base Unit INTERCHANGE PASS BOX Included BASE WORK STATION, BARRIER ISOLATOR AIRFLOW AND HEPA FILTRATION SYSTEM Included INTERCHANGE Included MHE-PF4025CW2-PA H2O2 SYSTEM Included HEPA FILTRATION SYSTEM Included CENTRIFUGE Included CENTRIFUGE Included CO2 INCUBATOR Optional CELL OBSERVATION MODULE CELL OBSERVATION SYSTEM Optional CO2 INCUBATOR Optional MCO-5ACIS Centrifuge and Module FEATURE EXTERIOR DIMENSIONS (W X F-B X H) MAXIMUM SPEED MAXIMUM CENTRIFUGAL ACCELERATION CONTROLS, ALARMS PRE-SETS TIME SETTABLE RANGE ACCELERATION/DECELERATION SETTINGS MOTOR SHAFT CAPACITY 16 SPECIFICATION 21.25" × 22.9" × 30.3" / 540 x 582 x 771 mm 2100 RPM 10 to 970G centrifugal acceleration settable range Externally mounted, front, with foot switch for cut-off.Door lock status displayed. Up to 4 patterns in memory 10 to 50 seconds (10 second increments); 1 to 99 minutes (1 minute increments); and infinite 3 stage Stainless steel 1000ml (50ml tubes, × 20) Specifications Barrier Isolator DIMENSIONS OVERALL EXTERIOR DIMENSIONS (W X F-B X H) INTERIOR DIMENSIONS (W X F-B X H) INTERIOR WORK SURFACE ELEVATION GLOVE ELEVATION PASS BOX INTERCHANGE (W X F-B X H) NET WEIGHT 115" × 41" × 87.8" / 2920 × 1045 × 2230 mm net of attached optional incubator 70.7" × 28.5" × 34" / 1796 × 726 × 864 mm 31.5" above floor / 800 mm 41.3" above floor / 1050 mm 19.6" × 16.7" × 15.8" / 500 × 425 × 400 mm 2207 lbs / 1000 kg, nominal CONTROLS, ALARMS PRESSURE MONITOR FAN MOTOR Red LCD and audible alarm if pressure falls below alarm setpoint. Audible alarm during motor shutdown CONSTRUCTION EXTERIOR FINISH Painted steel INTERIOR FINISH Polished stainless steel FRONT GLOVE PORT PANEL, ANGLE 6° Polycarbonate with painted steel frame. Hinged for opening to load equipment. AIR SYSTEM AIR QUALITY AIRFLOW SYSTEM AND VELOCITY Grade A Class 100 over work surface; installation in a Grade D, Class 100,000 ambient room Vertical downflow over work surface, 0.2~0.3 m/s ± 20% @6" below supply filter screen (150 mm) FILTRATION SYSTEM Independent HEPA supply and exhaust filters, efficiency 99.99% (0.3 µm PAO), scan tested and passed FILTRATION SYSTEM, PASS BOX Independent HEPA supply and exhaust filters, efficiency 99.99% (0.3 µm PAO), scan tested and passed INTAKE AND EXHAUST Electrically switched system. Room air intake thru HEPA supply filter; return exhaust to room thru HEPA exhaust filter RELATIVE AIR PRESSURE WORK STATION PASS BOX INTERCHANGE Positive pressure after decontamination of work station and pass box; audible notification when ready, 60Pa Positive pressure after decontamination of pass box only; audible notification when ready, 220Pa INTERIOR WORK AREA LIGHTING ELECTRICAL OUTLET Fluorescent lamps (3), 30w each, top of panel, top of right, left Duplex, drip-proof receptacle PASS BOX INTERCHANGE DOOR MATERIAL Front opening to outside; left side opening to interior work area Polished stainless steel DECONTAMINATION SYSTEM FUNCTION H2O2 RESOLUTION AND AERATION DECONTAMINATION CYCLE TIME H2O2 vaporization; simultaneous decontamination of interior barrier isolator work station, pass box interchange, optional centrifuge and optional CO2 incubator Catalytic, external to the work area. Residual H2O2 density at catalytic outlet is at or below 1ppm (by TLV-TWA). Fresh, HEPA filtered air follows resolution Load dependent; typical cycle times range from 1.0 to 2.0 hours from manual initiation of automatic sequence. UTILITIES ELECTRICAL Main system, 4500w, 220V, AC, 60Hz. Electrical outlet 120V, AC, 60Hz. 17 Specifications CO2 Incubator (Modular), Panasonic MCO-5ACIS DIMENSIONS EXTERIOR DIMENSIONS WITHOUT STAND (W X F-B X H) 23.5"W× 22.1" × 24.7" / 597 × 561 × 626 mm) INTERIOR DIMENSIONS (W X F-B X H) 13.8" × 14.9" × 14.8" / 350 × 378 × 375 mm) NET WEIGHT, NOMINAL VOLUME 110 lbs / 50 kg 1.7 cu.ft. / 49 liters nominal OPERATING SYSTEM CO2 SENSOR Thermal INCU-SAFE COPPER ENRICHED STAINLESS STEEL INTERIOR Standard MICROPROCESSOR CONTROLLER/DISPLAY, DOOR MOUNTED Standard DIRECT HEAT, AIR (DHA) JACKET Standard ® DECONTAMINATION H2O2 DECONTAMINATION STANDARD, COPPER ENRICHED STAINLESS STEEL INTERIOR When docked to work station Germicidal protection ENVIRONMENTAL PERFORMANCE TEMPERATURE CONTROL RANGE +7.0°C above ambient to 50°C in a 5°C to 35°C ambient TEMPERATURE CONTROL UNIFORMITY AND DEVIATION ±0.25°C in 25°C ambient, setting 37°C, 5% CO2, no load CO2 CONTROL RANGE AND DEVIATION AIRFLOW INTERIOR HUMIDITY 0% to 20%, ±0.15% in 25°C ambient, setting 37°C, 5% CO2, no load Continuous with inner door closed 95%RH @ 37°C through evaporation via DHA heating system; optional reflective/deflective optical low water sensor CONTROL, MONITORING, ALARM TEMPERATURE AND CO2 CONTROL DISPLAY COMMUNICATIONS Setpoint resolution 0.1°, 0.1% LED, resolution 0.1°, 0.1% Optional 4-20mA connection; Optional PC interface MTR-480 CABINET DESIGN AND CONSTRUCTION SUPERSTRUCTURE, EXTERIOR CABINET AND DOOR INTERIOR AND SHELVES INNER DOOR Galvanized steel exterior, baked-on enamel finish Copper-enriched stainless steel Tempered glass INSULATION Rigid foam polyurethane OUTER DOOR Side oriented for docking with work station ACCESS PORT Single, 1.18" / 30mm with silicone stopper LEVELING FEET Adjustable ENERGY, ELECTRICAL, UTILITIES MAXIMUM POWER CONSUMPTION MAXIMUM HEAT DISCHARGE ELECTRICAL CO2 GAS CONNECTION CO2 GAS INPUT PRESSURE 18 205W 740kJ/h 115V,60Hz 4 to 6 mm barbed fitting Nominal 0.03 MPaG/0.3kg/cm2/5.0lbs/sq.in; from two-stage CO2 regulator Professional Service and Support We provide full product service support to maintain Panasonic standards of product safety, reliability and high performance. The combination of our multi-national network of factory-trained service professionals, detailed documentation of field performance, and high-value on the customer feedback helps us to deliver best-in-class, end-user support for our customers. Convenience of Panasonic Product Service • Panasonic service specialists are trained to: - perform remote and on-site diagnostics - repair and replace worn components - offer preventative maintenance programs as per your needs and budget • Many Panasonic Healthcare products include self-diagnostics that permit authorized service technicians to determine how and when service calls are required. Validation Services Panasonic offers a wide range of high-quality services for all our equipment. These services include on-site validation, customer validation support packages, factory acceptance testing and NIST calibration. Choosing Panasonic as an equipment supplier and validation consultant can greatly reduce the time and cost involved with getting new equipment compliant and ready for use. • We offer training to selected facility biomedical engineers and service staff for authorized in warranty and post-warranty repairs. • Because our products are sold and serviced worldwide, products acquired in one country under grant or facility-sharing programs are easily supported if moved to facilities in the next city or around the world. Unique Services Panasonic Offers: • On-site consultation • Unit specific authorized protocol documents • Customizable testing procedures to meet customer specific requirements • Free archiving of unexecuted testing protocols • Unbiased testing of competitive equipment Pre-delivery Services: On-site Services: • validation support, • installation qualification, • consultation, • operational qualification, • factory acceptance testing, • performance qualification, • calibration • calibration and • temperature mapping • temperature mapping. 19 Panasonic Healthcare Corporation of North America 1300 Michael Drive, Suite A, Wood Dale, IL 60191 Toll Free USA (800) 858-8442, Fax (630) 238-0074 us.panasonic-healthcare.com AW/08/2014 © Panasonic Printed in USA