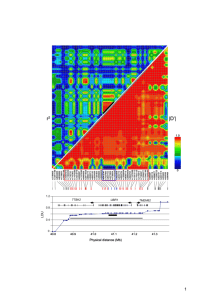
Genome-Wide Genetic Interaction Analysis of Glaucoma Using
advertisement

GENOME-WIDE GENETIC INTERACTION ANALYSIS OF GLAUCOMA USING EXPERT KNOWLEDGE DERIVED FROM HUMAN PHENOTYPE NETWORKS TING HU† , CHRISTIAN DARABOS† , MARIA E. CRICCO, EMILY KONG, JASON H. MOORE Institute for the Quantitative Biomedical Sciences, Geisel School of Medicine, Dartmouth College Hanover, NH 03755, U.S.A. E-mail: jason.h.moore@dartmouth.edu The large volume of GWAS data poses great computational challenges for analyzing genetic interactions associated with common human diseases. We propose a computational framework for characterizing epistatic interactions among large sets of genetic attributes in GWAS data. We build the human phenotype network (HPN) and focus around a disease of interest. In this study, we use the GLAUGEN glaucoma GWAS dataset and apply the HPN as a biological knowledge-based filter to prioritize genetic variants. Then, we use the statistical epistasis network (SEN) to identify a significant connected network of pairwise epistatic interactions among the prioritized SNPs. These clearly highlight the complex genetic basis of glaucoma. Furthermore, we identify key SNPs by quantifying structural network characteristics. Through functional annotation of these key SNPs using Biofilter, a software accessing multiple publicly available human genetic data sources, we find supporting biomedical evidences linking glaucoma to an array of genetic diseases, proving our concept. We conclude by suggesting hypotheses for a better understanding of the disease. Keywords: GWAS; Epistasis; Gene-gene interaction; Human phenotype network; Statistical epistasis network; Biofilter; Eye diseases; Glaucoma; SNPs; Pathways. 1. Introduction With the rapid development of genotyping technologies and exponential increase in computational power, we are able to leverage the wealth of genome-wide association studies (GWAS) data to test millions of genetic variations (single nucleotide polymorphisms, SNPs) for their associations with common human diseases.1,2 However, common disorders are usually the result of the non-linear combined effect of many variations. The study of these complex, epistatic interactions among multiple genetic attributes is crucial in explaining portions of the genotype-to-phenotype associations.3,4 Detecting and analyzing complex genetic interactions in GWAS data pose great statistical and computational challenges. Epistatic effects potentially involve any number of SNPs, from a couple to hundreds or even thousands. In turn, the uncertainty of size of the interacting SNP set would require interaction studies to comprehensively explore all possible combinations of SNPs. A typical GWAS dataset encompasses hundreds of thousands of variations, making the enumeration of all pairwise SNP interactions computationally infeasible, and the computational cost increases exponentially with the number of features at hand. This inevitably becomes a computational bottleneck of processing and analyzing GWAS data. Therefore, genetic interaction studies meant to process modern GWAS data require the development of advanced informatics methodologies. These novel algorithms must be able to † Co-first authors – TH and CD have contributed equally to this work. simultaneously analyze large sets of interactions and identify genetic attributes potentially involved in higher-order interactions. Network modeling has emerged as a suitable framework for such purposes,5–7 thanks to its ability to represent a large number of entities, as vertices, and their relationships, as edges. In this study, we propose an informatics framework of detecting and analyzing genetic interactions for GWAS data. We use the example of the GLAUGEN study, a GWAS on glaucoma consisting of over a million attributes. We pre-screen the dataset using a knowledge-based filter by building human phenotype network (HPN) to prioritize SNPs and reduce the search space according to their known relationship to the disease/phenotype in question. This reduced SNP list is then used to quantitatively evaluate all pairwise epistatic interactions and to build statistical epistasis network (SEN) to characterize their global interaction structure. The key SNPs identified by the SEN are functionally annotated and evaluated for their interaction with other disorders. This new framework has the potential to elucidate large parts of the complex genetic architecture of common human diseases, such as glaucoma, and identify key SNPs for further biological validations. 2. Dataset and Methods 2.1. Glaucoma and the GLAUGEN Study Glaucoma, a neurodegenerative disease, is the primary cause of irreversible blindness, affecting over 60 million people worldwide. The most common kind of glaucoma, in all populations, is primary open-angle glaucoma (POAG). Currently the only modifiable risk factor of POAG is intraocular pressure (IOP), but even lowering that will only slow the process, not stop it.8 However, a substantial amount of POAG has been shown to have a genetic basis. Familial aggregation of POAG has been long recognized and studied to find multiple loci linked to them, causing the discovery of glaucoma-causing genes myocilin (MYOC), optineurin (OPTN) and WDa -repeat domain 36.9 About 5% of POAG is presently attributed to a single-gene or Mendelian forms of glaucoma. More cases of POAG are caused by the combined epistatic effects of many genetics risk factors.10 The Glaucoma Gene Environment (GLAUGEN) seeks to illuminate the origin of the disease, to discover genetic loci associated with POAG, and to identify gene-gene and geneenvironment associations.8 GLAUGEN is a GWAS, case-control study, with about 2,000 unrelated cases and over 2,300 controls. To be involved in the study, both the cases and the controls had to be at least 40 years of age and European derived or Hispanic Caucasian. Subjects were genotyped with 1,048,965 SNPs examined.8 2.2. Human Phenotype Network (HPN) The human disease network,11 or the more general human phenotype networks (HPNs),12,13 are mathematical graph models where nodes represent human genetic disorders and edges link those nodes with shared biology.14 The underlying connections of the HPN contribute to a tryptophan-aspartic acid 300" Obesity Renal sinus fat Bone Density 1000$ Follicule stimulating hormone Heart rate variability traits Neuranatomic and neurocognitive phenotypes Epirubicin-induced leukopenia Anthropometry Neuroanatomy Hormone measurements Adiponectin Azoospermia Anthropometric traits Oocytes Ceramides Syndrome Adverse response to lamotrigine and phenytoin Osteoporosis Creutzfeldt-Jakob fibrin fragment D [Supplementary Concept] Serum selenium levels Prion diseases Male fertility Thyroid volume Goiter Endothelial function traits Oligospermia Coronary spasm Coronary Vasospasm Anticonvulsants Ovarian reserve Iron deficiency Thyroid cancer Leukopenia Left Atrial Function Recombination rate (males) radiation-related) Thyroid cancer (Papillary Blood Platelets Ileal carcinoids Sphingolipids Glucosylceramides Partial Thromboplastin Time Anemia Fuchs's corneal dystrophy Caudate nucleus volume Echocardiographic traits Tumor biomarkers DNA Caudate Nucleus Forced Vital Capacity Carcinoid Tumor Brachial Artery Genetic Recombination Left Ventricular Function Thyroid Neoplasms Platelet function and related traits Fuchs Endothelial Dystrophy Circulating vasoactive peptide levels Primary Ovarian Insufficiency Kidney Calculi Kidney stones QT interval Heart Atria Papillomaviridae Folic Acid Vitamin B 6 N-glycan levels Vitamin B 12 Circulating cell-free DNA Blood Coagulation Factor Inhibitors Insulin-Like Growth Factor Binding Protein 3 5-HTT brain serotonin transporter levels Acetaminophen Cortical structure Gastric cancer Sphingomyelins Dupuytren's disease Cleft Palate Citalopram Amygdala Hepatitis B vaccine response Social Desirability Sleep duration Polysaccharides Dupuytren Contracture Serotonin Plasma Membrane Transport Proteins Intracranial volume Dengue Hemorrhagic Fever Uterine fibroids Nasopharyngeal Neoplasms Blood Vessels Ovarian cancer Antidepressive Agents Morbidity-free survival Visual Cortex Endometriosis Hepatitis B Vaccines Dengue shock syndrome Small Cell Lung Carcinoma Chronic Hepatitis B Insulin-like growth factors Cleft Lip Response to antineoplastic agents Response to acetaminophen (hepatotoxicity) Functional MRI Nasopharyngeal carcinoma Fibromyoma Fructose-Bisphosphate Aldolase Folate pathway vitamin levels Chronic hepatitis B infection Event-related brain oscillations Ovarian Neoplasms Transferrin Receptors Myoglobin Lymphoma Jaw Abnormalities Response to cerivastatin Marijuana Abuse Religion and Psychology Insulin-Like Growth Factor Binding Protein 5 Germinoma Complement C4b-Binding Protein Leukocyte Count Inflammation White matter integrity Insulin-Like Growth Factor Binding Protein 4 Response to TNF antagonist treatment Pubertal anthropometrics Personality dimensions Walking Floxacillin alpha-Macroglobulins Benzodiazepines Extraversion (Psychology) Stomach Venous thromboembolism Neoplasms Leptin Kawasaki disease Aortic-valve calcification Rhabdomyolysis Tobacco UseInterleukin-10 Disorder Depressive Disorder Interleukin-18 Waves Cardiac structure and function Interleukin-2 Receptor alpha Subunit Movement Disorders Acute lung injury Insulin-Like Growth Factor I Iron-Regulatory Proteins Survival Brain Systemic lupus erythematosus and Systemic sclerosis T-Lymphocytes Immunoglobulin A Smoking Testicular cancer Sensory disturbances after bilateral sagittal split ramus osteotomy IgE levels Behavior Eosinophils Thyroxine Drug-Induced Liver Injury Mucocutaneous Lymph Node Syndrome Idiopathic CD8-Positive T-Lymphocytes Myopia Exploratory Behavior Rheumatoid arthritis Hypertrophic Pyloric Stenosis Interleukin-12 Vitamin D pulmonary fibrosis Response to tamoxifen in breast cancer Risperidone Corneal Topography Testicular Neoplasms Blood trace element (Zn levels) Cortical thickness Word reading Brain Mapping Immunoglobulin E Cardiac Arrhythmias Addiction Blood Sedimentation Smoking behavior Vascular Endothelial Growth Factor A Hodgkin Disease Tamoxifen Personality Glioma Biliary Liver Cirrhosis Lymphocytic Leukemia Resting heart rate Alpha-Globulins Interleukin-8 Keratoconus Hodgkin's lymphoma Volumetric brain MRI Primary biliary cirrhosis Non-substance related behavioral disinhibition Emphysema-related traits Erythrocyte Indices Sleep Carotene Vascular endothelial growth factor levels Allergic sensitization Inflammatory biomarkers Membranous Glomerulonephritis Moyamoya disease beta Astigmatism Interleukin 1 Receptor Antagonist Protein Serum uric acid levels Lung cancer Bleomycin sensitivity Self-reported allergy Brain Urinary albumin excretion Lung Neoplasms Transferrin Infantile hypertrophic pyloric stenosis PR interval Red blood cell count mean corpuscular hemoglobin concentration Parietal Lobe Celiac disease lipoprotein A-I [Supplementary Concept] bipolar disorder and depression (combined) Entorhinal Cortex Schizophrenia Emphysema Systemic Scleroderma Chemokine CCL2 Brugada syndrome Substance-Related Disorders Response to iloperidone treatment (PANSS-T score) Sclerosis Protein quantitative trait loci Glycoproteins Menarche Heart Function Tests Antipsychotic-induced QTc interval prolongation Aorta Allergic Rhinitis Exfoliation Syndrome Venous Thrombosis Frontal Lobe Butyrylcholinesterase Ferritins Homocysteine Angiotensin-Converting Enzyme Inhibitors Acquired Immunodeficiency Syndrome Vascular Diseases Isoxazoles Angiotensin-converting enzyme activity Behavioural disinhibition (generation interaction) Monocyte count Weight Face Prostate cancerCerebrum Hyperactive-impulsive symptoms Temporal Lobe Nephropathy Parathyroid Hormone vWF and FVIII levels Vitamin K 1 Taste Antipsychotic Agents Menopause Pancreatic cancer Stevens-Johnson Syndrome Alanine Transaminase Reading and spelling Lymphocytes Duodenal Ulcer Aging traits Upper aerodigestive tract cancers Sudden Death Gout Asperger Syndrome Cervical cancer Celiac disease and Rheumatoid arthritis Glaucoma Interleukin-6 Receptors carbohydrate-deficient transferrin [Supplementary Concept] Vascular constriction Open-Angle Glaucoma Muscular Diseases Urinary Bladder Neoplasms Clozapine Foot Tumor Necrosis Factor-alpha Acenocoumarol Dietary macronutrient intake Tooth Eruption Head and Neck Neoplasms Vitamin K Perphenazine Corneal curvature Autoimmune Diseases Chemokine CCL4 Non-Small-Cell Lung Carcinoma Atrioventricular conduction Hepatocellular carcinoma Graves Disease Warfarin Aortic root size External Ear Esophageal Neoplasms Aging Mental Disorders Basal Cell Carcinoma End-stage Permanent tooth development IgA levels Chronic lymphocytic leukemia coagulation Brain structure Sepsis Menarche and menopause (age at onset) Cystatin C Essential Tremor Select biomarker traits cancer Memory human [Supplementary Concept] F8 protein Odontogenesis Apolipoprotein A-I Esophageal Transforming Growth Factor beta1 Psychological Sexual Dysfunctions Erectile Dysfunction Common traits (Other) Eye IGA Glomerulonephritis Allopurinol von Willebrand Factor Chronic Kidney Failure Vitamin E Central Nervous System Sneezing Life Expectancy Illicit drug use Myeloid Leukemia Hematological and biochemical traits Hippocampus Myasthenia gravis Optic Disk Melanoma Chronic kidney disease Sj�gren's syndrome gamma-Glutamyltransferase Neuropsychological Tests Inattentive symptoms Facial morphology Interleukin-6 Cornea Kidney Diseases Blood Cells Metabolome Pain Non-albumin volume Progranulin levels Carotid intima media thickness Cardiovascular disease risk factors cancer protein levels Maximal Midexpiratory Flow Rate Angiography Bladder Coronary restenosis HippocampalDysplastic Response to Vitamin E supplementation Levels Nevus Syndrome Suntan Two-hour glucose challenge Apolipoprotein Autism Urinary metabolites Albuminuria ErythropoietinBlood Dehydroepiandrosterone Tanning Proteins Treatment response for severe sepsis Hair Aspartatealpha-Tocopherol aminotransferase L-Lactate Dehydrogenase β2-Glycoprotein I (β2-GPI) plasma levels Myeloproliferative Disorders Interleukin-1beta Melanosis Hair Color Serum dimethylarginine levels (symmetric) Lipid Metabolism Response to platinum-based chemotherapy in non-small-cell lung cancer Optic Nerve total Cholesterol AIDS Atherosclerosis Anti-cyclic Citrullinated Peptide Antibody Blood pressure measurement (cold pressor test) Haptoglobins Longevity Pulmonary function Sunburns Chronic myeloid leukemia Precursor Cell Lymphoblastic Leukemia-Lymphoma CD40 Ligand Alcohol Drinking Sex Hormone-Binding Globulin Glycemic traits Abdominal Aortic Braces Aneurysm Lymphoid Leukemia Eye color Blue vs. green eyes Colorectal Neoplasms Monocyte early outgrowth colony forming units Response to platinum-based agents Paget's disease Biomedical quantitative traits 1-Alkyl-2-acetylglycerophosphocholine Esterase gemcitabine [Supplementary Concept] Liver Cirrhosis Comprehensive strength and appendicular lean mass Cyclic Peptides Dialysis-related mortality Beta-2 microglubulin Tolerance Test plasma levels Protein C Brain imaging Neurotic Disorders Iris color Circadian Rhythm Carotid Stenosis Glucose Cognitive test performance Endometrial cancer Blue vs. brown eyes Atrial Natriuretic Factor Mortality Metabolic Syndrome X Metabolic traits Handedness in dyslexia Functional Laterality Hair morphology Nevus count Apolipoproteins C Behcet's disease Colorectal cancer Gestational Diabetes Eye color traits Neurobehavioral Manifestations Digit length ratio Autistic Disorder Cardiovascular Diseases Blood Viscosity Blood Glucose Chemokines Mortality among heart failure patients Thoracic Aortic Aneurysm Palmitic acid (16:0) plasma levels Multiple myeloma pigmentation Endometrial Neoplasms Colony-Forming Units Assay Colonic Neoplasms Stearic acid (18:0) plasma levels Blood Flow Velocity Pain Skin Alkaline Phosphatase Measurement Amyloid beta-Peptides Cognitive decline Nevi and Melanomas Response to treatment for acute lymphoblastic leukemia Tissue Plasminogen Activator Glomerulosclerosis Paget's disease Vital Capacity Palmitoleic acid (16:1n-7) plasma levels Speech Perception Peroxidase Cystatins Luteinizing Hormone Follicle Stimulating Hormone Glucose Transporter Type 2 Phospholipids Psychomotor Performance LDL Lipoproteins of Fallot Alopecia Carotid Arteries oxaliplatin [Supplementary Concept] Response to gemcitabine in pancreatic cancer Telomere length Tetralogy Brain Natriuretic Peptide Serum alkaline phosphatase levels Cardiomegaly Oleic acid (18:1n-9) plasma levels Deformans Information processing speed Chemerin Osteitis levels Intercellular Adhesion Molecule-1 Intra-Abdominal Fat Working memory Aromatase Inhibitors Natriuretic peptide levels Proinsulin levels Mean forced vital capacity from 2 exams Apolipoproteins E Quantitative traits Anxiety Occipital Lobe Intestinal Diseases Iris characteristics Carotid Artery Diseases Hypothyroidism Adverse response to aromatase inhibitors Iris Phytosterols Hyperkalemic Periodic Paralysis HIV-1 Retinal vascular caliber Drug Toxicity Heart Diseases Common Carotid Artery Focal Segmental Glomerulosclerosis Soluble E-selectin levels Major CVD Thyroid hormone levels Thyrotoxic hypokalemic periodic paralysis Primary sclerosing cholangitis Soluble levels of adhesion molecules Androgen levels Lupus Vulgaris Telomere Short-Term Memory Pulse Adverse response to carbamapezine Internal Carotid Artery Migraine with aura Abdominal Fat P-Selectin Hand Strength gamma-Glutamylcyclotransferase Subclinical atherosclerosis traits (other) Pure-Tone Audiometry Dehydroepiandrosterone Sulfate Crohn's disease and celiac disease Carbamazepine Sclerosing Cholangitis Subcutaneous Fat Attention Deficit and Disruptive Behavior Disorders Digestive system disease (Barrett's esophagus and esophageal adenocarcinoma combined) Vitamin A Migraine without aura HIV-1 susceptibility Gallbladder Diseases Retinal Vein Retinol levels Hoarding Gallbladder cancer Thyrotropin Soluble ICAM-1 Hearing Loss T-tau Migraine Barrett's esophagus Urinalysis Depression E-Selectin Abdominal Aorta Esophageal adenocarcinoma HIV-1 viral setpoint Migraine - clinic-based Vascular Calcification Coffee Cardiac hypertrophy Ulcerative colitis Caffeine consumption Intuition tau Proteins Hearing impairment Ankle Brachial Index Migraine Disorders Esophagitis Coffee consumption Reasoning Meningococcal disease Gallbladder Neoplasms P-tau181p Addictive Behavior Radiation response Meningococcal Infections Response to radiation Caffeine Cytomegalovirus Vaccines Cytomegalovirus antibody response frequency" 100$ 200" Insulin 10$ Forced Expiratory Volume 1$ 100" 0" 0.1$ 1$ 1" 5" 10$ 9" 13" degree" Asthma 100$ Electrocardiography 17" Pancreatic Neoplasms Prostatic Neoplasms Neutrophils Respiratory Function Tests Attention Deficit Disorder with Hyperactivity Coronary Disease Macular Degeneration Type 2 diabetes Gambling Crohn Disease Metabolism Inflammatory bowel disease Breast Neoplasms Breast size Fig. 1. The SNP-based human phenotype network, filtered (edge weight cutoff = 0.002). Vertices are colored by “modules”13 to increase readability. The vertex sizes are proportional to the number of associated SNPs. The degree distribution is given on both linear and logarithmic scales. the understanding of the basis of disorders, which in turn leads to a better understanding of human diseases. In the present work, we rely on the SNP-based HPN, where connected diseases share common SNPs. We use two sources for the phenotype-to-SNPs mapping: the National Human Genome Research Institute GWAS catalog,15 a manually curated list of all the National Institute of Heath (NIH) funded GWAS studies, and the NIH’s database of Genotypes and Phenotypes (dbGaP, http://www.ncbi.nlm.nih.gov/gap). To build the HPN, all traits listed in the GWAS catalog and dbGaP are annotated with their risk-associated SNPs. All the traits become nodes in our network. Then, traits that have one or more SNPs in common are linked in the HPN by an edge, the weight of which is proportional to the size of the SNPs’ overlap. The GWAS catalog and dbGaP report 1,252 traits combined, annotated with 37,681 SNPs in 16,411 loci. The resulting bipartite network is projected in the space of phenotype vertices to obtain the HPN shown in Fig. 1. The HPN contains 985 vertices and over 26,000 edges, and encompasses all phenotypes listed in the GWAS catalog and dbGaP, provided that they are connected to at least one other trait. The resulting network is extremely dense, with an average degree greater than 500. 2.3. Statistical Epistasis Network (SEN) Statistical epistasis network (SEN) is designed to study a global aggregation of pairwise epistatic interactions among a large number of factors.16,17 First, pairwise epistatic interaction is quantified using the information-theoretic measure called information gain18 for all possible pairs of genetic attributes. Specifically, the genotypes of two SNPs A and B , and the discrete phenotypic class C are regarded as variables. Mutual information I(A; C) or I(B; C) measures the shared information between (A or B ) and C calculated as I(A; C) = H(A)+H(C)−H(A, C) or I(B; C) = H(B) + H(C) − H(B, C), where entropy H measures the uncertainty of a random variable or joint multiple random variables. Therefore, mutual information I(A; C) or I(B; C) describes the reduction of the uncertainty of the phenotypic class C given the knowledge of the genotype of A or B , known as the main effect of A or B on C . Similarly, the joint mutual information I(A, B; C) measures the total effect of combining A and B on explaining the phenotype C . Subtracting the individual mutual information I(A; C) and I(B; C) from I(A, B; C) captures the gained information on C by considering A and B together rather than individually. This information gain IG(A; B; C) is a practical measure for the epistatic interaction effect of SNPs A and B on phenotype C , and has been used as an efficient non-parametric and model-free statistical quantification of pairwise epistasis in genetic association studies.19–23 Second, all pairwise SNP-SNP interactions are quantified and ranked. We build statistical epistasis networks where vertices are SNPs and edges are weighted pairwise interactions. We only include pairs of SNPs if their interaction strengths are stronger than a preset threshold. We analyze the network topological properties at each cutoff value of the threshold, such as the size of the network (the number of its vertices and the number of its edges), the connectivity of the network (the size of its largest connected component), and its vertex degree distribution. Then, a threshold of the pairwise interaction strength is determined systematically by finding the cutoff where the topological properties of the real-data network differentiate the most from the null distribution of permutation testing. Such a SEN provides a significant global structure of clustered strong pairwise epistatic interactions associated with a particular phenotype. It serves as a map for further network properties investigation and key SNPs prioritization as discussed in the following section. 2.4. Network Property Analysis of SEN The assortativity of a network measures the propensities of vertices with similar characteristics to connect to one another.24,25 In the context of SENs, we are interested in looking into the main effect assortativity, i.e., whether there exists a correlation of main effects between pairs of interacting SNPs. Such main effect assortativity is calculated as the Pearson correlation coefficient r of the main effects at either ends of an edge in a SEN, P P i 2 M −1 i ji ki − [M −1 i ji +k 2 ] r= P 2 i2 P ji +ki 2 , −1 M −1 i ji +k − [M i 2 ] 2 (1) where M is the total number of edges, and ji and ki are the main effects, calculated as the mutual information of a SNP and the phenotypic class of the vertices (SNPs) at the ends of the i-th edge, with i = 1, 2, ..., M . The coefficient r lies between -1 and 1, with r = 1 indicating perfectly assortative, r = 0 for non-assortative, and r = −1 for complete disassortative networks. At the vertex level, centrality measures the importance of individual vertices in a network. The most commonly used centrality measure is the node’s degree CD (v), which is the total Phenotype Exfoliation Syndrome Coronary Artery Disease Cardiovascular Diseases Corneal curvature Optic Disk Open-Angle Glaucoma Glioma Eye Glaucoma Coronary restenosis #SNPs 1 639 70 13 17 6 12 13 18 56 Degree 1 2 2 4 4 5 2 4 9 3 (a) (b) Fig. 2. Glaucoma network neighborhood. (a) Phenotypes, including the number of SNPs associated with each phenotype and the “strength” of the interaction (degree) with the rest of the sub-network. (b) a depicted representation of the Glaucoma-centered sub-network, in which the node sizes are proportional to the number of associated SNPs. number of edges connected to vertex v . The degree of a SNP in the statistical epistasis network shows the number of other SNPs with which it is interacting. Betweenness centrality is a more sophisticated metric that quantifies the number of times a vertex is part of the shortest path P between any pair of vertices,26 represented as CB (v) = s6=v6=t∈V σstσst(v) , where σst is the total number of shortest paths from vertex s to vertex t and σst (v) is the number of those paths that pass through vertex v . Closeness centrality, defined as CC (v) = P 1 dvs , where dvs is the s6=v distance between vertices v and s.27,28 This metric describes how easily a given vertex can reach all other vertices in a network. In the context of SENs, centrality measures are used to identify key SNPs that play an essential role in the global interaction structure. 3. Results 3.1. Pre-screening of Glaucoma Data Using HPN To reduce the computational cost (in time and resources) of analyzing a GWAS dataset, the data must be filtered, selected and/or prioritized prior to processing. Algorithmic filtering methods fall into two main families: knowledge-driven and data-driven. Knowledge-driven filters relies on the actual known biology behind the data, whereas data-driven filtering solely relies on the statistical pre-processing of the data, regardless of their type. ReliefF, SURF, and TuRF29,30 are examples of data-driven statistical methods of preprocessing GWAS data. For a complete review of the knowledge-based vs. data-driven filtering, refer to Sun et al.31 The method we propose to use here starts with the full HPN described in Section 2.2. We identify the glaucoma vertex and establish a list of the disorders and phenotypes in its immediate neighborhood. The 10 members of the “glaucoma neighborhood” are presented in table in Fig. 2. Using the sub-HPN, we compile a list of 948 SNPs that have been associated with any of the phenotypes in the sub-network. We subsequently annotate each of the risk-associated SNPs with its linkage disequilibrium32 SNPs, indirectly associated with an increased risk. The full list of 4,388 features containing both direct and indirect risk-associated SNPs is used to filter the full one million SNPs of the GLAUGEN GWAS data. The subset made of the overlap between our priority-list and the GLAUGEN dataset, composed of 2,380 SNPs, is used to build the SNP interaction network presented in the following section. 3.2. Statistical Epistasis Network of Glaucoma Using the list of 2,380 SNPs, we evaluate all the pairwise epistatic interactions (>2.8 million) using the information gain measure. The strongest interacting pair is SNPs rs13123790 and rs6572380 with a strength of 1.163% association of the disease class. We set the pairwise interaction strength threshold decreasing from the strongest observed value with a step of 0.0001. At each cutoff, a SEN is built by including pairs of SNPs as edges and two end vertices if their interaction strength is above the cutoff. Then for each network we evaluate its topological properties including the number of non-isolated vertices, the number of edges, the sum of weighted edges, and the size of its largest connected component. A 100-fold permutation test assesses the significance of these observed network properties. We permute the data 100 times by randomly shuffling the disease class column, and for each permuted dataset all the pairwise interaction strengths are calculated and networks are built using the same cutoffs as the read-data networks. We observe a network at cutoff 0.693% that has a largest connected component including significantly more vertices (p = 0.05) than those permuted-data networks at the same cutoff. This largest connected component has 713 vertices and 789 edges (Fig. 3). The main effect assortativity of this network is -0.053 with a significance of p = 0.04 using a 100-fold edge swapping permutation test where we randomly pick 10 × |E| (|E| is the total number of edges) pairs of edges and swap their end vertices. This significant negative assortativity indicates that the main effects of interacting SNPs are negatively correlated, i.e., high main-effect vertices tend to interact more with low main-effect vertices. The degree, betweenness, and closeness centralities of all the vertices in the network are evaluated, and their distributions are shown in Fig. 4. Most of vertices have degrees equal to or less than 3. However there are a minority vertices with a degree higher than 5, meaning that they interact with larger number of other SNPs. In the distribution of betweenness centrality, the majority of vertices have low betweenness. However, some vertices have a much higher betweenness than the rest. The closeness centrality follows a normal distribution indicating that most vertices have similar access to all other vertices. We sort the vertices by descending centrality. At the top, we find the key SNPs that are potentially involved the genetic processes responsible for glaucoma. We further investigate the clinical and biomedical implications of these SNPs through Biofilter33 functional annotations. Biofilter provides a single interface to multiple publicly available online human genetic datasources (https://ritchielab.psu. edu/software/biofilter-download). 4. Discussion In this study, we proposed a genetic interaction analysis framework of using human phenotype network (HPN) as knowledge-based data filter and subsequently statistical epistasis network (SEN) for quantitative epistatic interaction detection and visualization. Our method was successfully applied to GLAUGEN GWAS data and we were able to identify a connected network of interacting SNPs that included significantly more SNPs than expected randomly. Such a rs7481311 rs1057510 rs16852600 rs1540923 rs9498099 rs918911 rs766992 rs7792939 rs2080401 rs12359685 rs10807323 rs13102884 rs10493900 rs2047870 rs7208097 rs1559777 rs8190893 rs10738886 rs2048327 rs2223662 rs10828213 rs9540221 rs1859428 rs13117608 rs10816943 rs6895327 rs1861612 rs1167242 rs1151008 rs9863717 rs2123808 rs12418204 rs10518221 rs2701248 rs4800148 rs1325273 rs6566726 rs11943513 rs7604827 rs11196686 rs1434709 rs9285397 rs1048228 rs701276 rs7090871 rs4421636 rs1754073 rs9521112 rs4979264 rs13240504 rs1216472 rs1480577 rs2554380 rs4845405 rs9399799 rs16940375 rs17261321 rs2439312 rs10485733 rs1473533 rs1145153 rs6795970 rs6565681 rs7648727 rs9521509 rs10495936 rs6869688 rs7044529 rs815815 rs2869832 rs4507859 rs2273301 rs6780510 rs6779469 rs4346352 rs16861329 rs16860271 rs885389 rs1538138 rs6774494 rs4952125 rs1932397 rs11749320 rs7239883 rs1357523 rs11921014 rs400337 rs12420080 rs2833836 rs6013871 rs1870661 rs2168159 rs12597778 rs7129429 rs795955 rs38831 rs9294851 rs2405781 rs1484948 rs17041869 rs17128272 rs3771395 rs6129032 rs12576239 rs10801913 rs4792192 rs7498403 rs1239947 rs10506627 rs560713 rs2410501 rs17286397 rs1950702 rs1950500 rs553346 rs7502462 rs1558139 rs727957 rs6504218 rs2627775 rs2750023 rs1532357 rs2306374 rs1945085 rs4793501 rs3827414 rs10424480 rs9946041 rs10791993 rs2948998 rs1746048 rs9449444 rs4365558 rs173700 rs8055236 rs2844479 rs3118914 rs886374 rs17034592 rs1037124 rs882300 rs12076129 rs7318731 rs894558 rs972869 rs891835 rs7525553 rs888663 rs10499480 rs3766793 rs3743200 rs896076 rs10505879 rs3916765 rs7171517 rs9487094 rs2273194 rs12199222 rs7678436 rs1858943 rs10505031 rs3752823 rs7138792 rs10486771 rs2162256 rs17141444 rs1524976 rs3805647 rs1077165 rs813164 rs3743157 rs300483 rs4653061 rs2079918 rs2822557 rs4640244 rs1805015 rs1802295 rs10184275 rs284489 rs11782819 rs9557082 rs10814916 rs1251588 rs3825214 rs4721923 rs989488 rs4371861 rs353121 rs2782934 rs17706439 rs1771428 rs4455882 rs4313471 rs4897336 rs3748069 rs11669012 rs12517906 rs2281680 rs1816002 rs10848958 rs2286036 rs10502372 rs189798 rs1396477 rs10509779 rs6663404 rs7172432 rs2171 rs9472138 rs10508288 rs9935794 rs3745060 rs11154022 rs1570211 rs6996971 rs1504749 rs11664670 rs1220025 rs17103138 rs5015480 rs891088 rs892267 rs12502295 rs923375 rs2853552 rs7336332 rs150724 rs2732494 rs2449222 rs6918956 rs4722613 rs2605463 rs9349200 rs11714343 rs7914674 rs391300 rs12741973 rs4743034 rs1035025 rs1040046 rs6885224 rs2149998 rs7191006 rs10518996 rs739981 rs7017184 rs4951536 rs11981985 rs710841 rs2849460 rs16872571 rs11108037 rs10237118 rs2291256 rs282544 rs551719 rs7178902 rs930716 rs334213 rs1245531 rs4537554 rs11856676 rs2580816 rs1270837 rs1436900 rs7209435 rs13438327 rs4503747 rs10468542 rs3924153 rs1533971 rs11580979 rs188019 rs11211081 rs1058793 rs2783169 rs4242252 rs2106913 rs1491192 rs2522422 rs2522425 rs2829588 rs535343 rs1401471 rs6982250 rs7542900 rs1553847 rs538827 rs16852525 rs1875206 rs10506546 rs7128948 rs3125050 rs756199 rs688391 rs4121677 rs569688 rs1490388 rs9568793 rs1901103 rs7403531 rs17171705 rs344907 rs4771591 rs7025486 rs10906115 rs17084051 rs1568482 rs13097792 rs6725887 rs7069291 rs1874326 rs2621469 rs597425 rs11708189 rs10014970 rs7812590 rs1944866 rs1949009 rs270504 rs1367158 rs2470984 rs11730157 rs2015454 rs10502032 rs300493 rs10922273 rs10834309 rs10496091 rs13077378 rs517871 rs499818 rs1017307 rs1007000 rs1525361 rs10085496 rs1880781 rs7743494 rs11122547 rs11599750 rs1029973 rs4842838 rs10895547 rs1111233 rs2146758 rs11123849 rs2024341 rs10014145 rs7669668 rs11090754 rs498872 rs6780569 rs1275419 rs804125 rs11131055 rs322239 rs6020814 rs12592821 rs745557 rs1052131 rs2670321 rs1293151 rs7092929 rs9825379 rs11926571 rs12356233 rs1865640 rs9326439 rs3825569 rs11170631 rs9626074 rs7954465 rs1545552 rs974110 rs4672150 rs6585827 rs4794665 rs10829156 rs10863936 rs7756992 rs944260 rs792747 rs785422 rs2074404 rs9318225 rs16886072 rs10518527 rs4379368 rs7833986 rs7660418 rs655487 rs17356672 rs11807640 rs2478830 rs11127920 rs1229542 rs7873584 rs540937 rs8048899 rs11900940 rs6564651 rs274917 rs1438342 rs1410518 rs10518622 rs2319517 rs943855 rs1137 rs6764363 rs12150284 rs4982323 rs989548 rs3847375 rs13389923 rs1406961 rs2876303 rs212062 rs6435396 rs6929078 rs6480314 rs4072683 rs2376999 rs1733724 rs13012046 rs2385576 rs11241936 rs248471 rs343079 rs17364464 rs1410811 rs925019 rs11223996 rs2347824 rs17291650 rs2853676 rs1399004 rs7314446 rs9391221 rs6563943 rs2390024 rs2794467 rs3797235 rs1407149 rs941567 rs913170 rs11782634 rs10915424 rs1435066 rs10430923 rs7809017 rs8000245 rs7810842 rs2383208 rs6476642 rs4527850 rs4977574 rs7000183 rs979976 rs3774937 rs1847118 rs16907259 rs13150558 rs6036896 rs1549318 rs11062784 rs11861962 rs3129055 rs2547416 rs928952 rs6905922 rs1281317 rs17288067 rs1898162 rs2206734 rs1293470 rs1549250 rs7003283 rs952361 rs13167932 rs12682841 rs9644059 rs964184 rs11859036 rs10483727 rs9317297 rs1467242 rs668204 rs16893890 rs7916697 rs1022141 rs1572072 rs2188857 rs7669856 rs2767000 rs9386234 rs9296824 rs16897288 rs6792584 rs11656696 rs706080 rs1870301 rs6856061 rs6671793 rs10282118 rs4129959 rs1889151 rs6499640 rs7754614 rs919129 rs12446047 rs10496166 rs9328448 rs140174 rs557962 rs12940030 rs6570507 rs9325777 rs10511904 rs10497323 rs9897551 rs1260836 rs463235 rs1444439 rs10504130 rs1888814 rs2717128 rs6670655 rs642773 rs1998038 rs10758892 rs6572380 rs12358475 rs3786800 rs758099 rs7963889 rs12931996 rs11763147 rs13123790 rs2750024 rs1267043 rs6667140 rs756202 rs7283906 rs6507838 rs1363258 rs6765739 rs7027110 rs6981943 rs12651137 rs1541010 rs7665764 rs1412444 rs10491472 rs7741993 rs10514431 rs4648011 rs2495699 rs914981 rs7013518 rs1476882 rs4130328 rs9848261 rs17117825 rs342169 rs8042680 rs6708562 rs1230545 rs4666636 rs1315134 rs9304456 rs1532358 rs2753268 rs7606754 rs6773931 rs1935681 rs565659 rs2967055 rs1333040 rs7698623 rs12579680 rs2305707 rs1053049 rs2839670 rs4687161 rs9359617 rs10754458 rs1412829 rs10502182 rs1968011 rs7742369 rs7332115 rs12583288 rs7488278 rs7404645 rs10514995 rs4405822 rs295208 rs8111071 rs12679004 rs12970134 rs280171 rs745324 rs968008 rs6284 rs2501968 rs1019156 rs10505784 rs17565975 rs9323914 rs788178 rs1965072 rs7856675 rs1192415 rs1076673 rs7651039 rs12338076 rs7183263 rs17550532 rs9358118 rs587847 rs10520520 rs834485 rs831983 rs2367563 rs5751614 rs17553657 rs3134904 rs704744 rs7045593 rs1044581 rs1105778 rs2236691 rs10504592 rs1376486 rs3027322 rs5215 rs1933988 rs6010620 rs4380028 rs167275 rs831571 rs472265 rs17584499 rs1741344 rs7084584 rs6699417 rs3111828 rs7112925 rs1635281 rs2171963 rs10510189 rs7823896 rs297367 rs705384 rs1145796 rs1324034 rs1012997 rs11918414 rs494459 rs1324982 rs10929060 rs10509680 rs7084752 rs6107955 rs7031748 rs779388 rs10932055 rs7029757 rs284495 rs7669522 rs4978434 rs4778818 rs1039524 rs2142219 rs803422 rs7028544 rs1719079 rs1490331 rs2221618 rs798489 rs26438 rs10165941 rs10777084 rs1320288 rs3752591 rs5219 rs747175 rs2274557 rs7249094 rs13201929 rs9563035 rs956627 rs1935881 rs7466269 rs2277912 rs2277142 rs2462140 rs245136 rs9941233 rs10777845 rs738494 rs992014 rs9366399 rs9374274 rs10010325 rs10008860 rs5768690 rs13266634 rs953259 rs6571228 rs4369779 rs9841585 Fig. 3. The SEN of Glaucoma. The network includes 713 SNPs (vertices) and 789 pairwise interactions (edges). The size of a vertex represents the strength of the main effect of its corresponding SNP, with the disease association ranging from 0.001% to 1.124%. The width of an edge indicates the strength of its corresponding interaction, with the disease association ranging from 0.693% to 1.163%. 250 200 120 150 100 frequency 400 frequency frequency 140 500 300 200 100 80 60 40 50 100 0 0 1 2 3 4 5 degree (a) 6 7 8 20 0 0.0 0.1 0.2 0.3 betweenness (b) 0.4 0.5 0.03 0.04 0.05 0.06 0.07 0.08 closeness (c) Fig. 4. The distributions of (a) degree, (b) betweenness, and (c) closeness centralities of vertices in the SEN of Glaucoma. large connected SNP network indicated a complex genetic basis of glaucoma. HPN is an computational model that systematically organizes and clusters various phenotypes and diseases based on their shared genetic association factors or related basic functional units. It also serves a powerful knowledge-based filter for GWAS data pre-screening and prioritization. Indeed, when compared to established data-driven filters, including the family of Relief algorithms, the HPN filtering performed equally well. This was evaluated by quantifying all the pairwise interactions of filtered sets of SNPs using different data-driven filters (data not shown). SEN is able to detect and analyze genetic interactions among a large set of attributes in genetic association studies. The derived SNP interaction network of glaucoma in this study had a significant connected structure and may serve as a map for identifying key genetic factors associated with the disease with further biological validations. The significant negative main effect assortativity of such a network indicated that epistatic interactions were likely to happen between SNPs with negatively correlated main effects. This finding suggests that most existing main-effect-based GWAS data filtering algorithms may overlook potential important interactions since some SNPs with low main effects would not be pre-selected using such filters. The set of SNPs with highest ranked centralities were annotated using Biofilter33 to their associated genes and, in turn, the genes to pathways and functional groups. We used the annotation to identify other complex diseases possibly sharing biology with glaucoma and suggest novel hypotheses for undocumented shared genetic interactions. It is known that glaucoma and type II diabetes (T2D) are associated, but direct genetic relationship remains unknown. We found SNPs connecting T2D to glaucoma: rs1333040 (CD = 5, CB = 0.043) and rs1053049 (CD = 5). The genes CDKN2B-AS1 and PPARD, which these two SNPs are respectively located in, are known to be associated with diabetes. Peng et al. observed that CDKN2A/B gene had a 1.17-fold (95% CI: 1.1-1.23) risk estimate for T2D.34 PPARD has been found to be associated with higher fasting glucose, glycated hemoglobin (HbA1c), and increased risk of T2D and combined impaired fasting glucose/T2D in a population-based Chinese Han sample.35 SNP rs9540221 (CD = 5) is also intergenic between NFYAP1 and LGMNP1, of which the former has also been associated with diabetes and obesity. Indeed, population-based studies suggest that persons with T2D have an increased risk of developing open-angle glaucoma (OAG): the Beaver Dam Eye Study,36 the Framingham Eye Study,37 and the Los Angeles Latino Eye Study.38 Chopra et al. has noted that there is a positive association between T2D and OAG in a sample Latino population study in Los Angeles, USA.38 The Rotterdam Study39 has also previously reported a positive association between DM and OAG, with a relative risk of 3.11 (95% CI, 1.12-8.66). Laboratory evidence for T2D and glaucoma linkages also exists. Some have observed that there is an inner retinal cell death, determined by the presence of hyaline bodies in the ganglion cell and retinal nerve fiber layer of the eyes of post-mortem eyes of patients with T2D.40 Possible hypotheses of T2D to glaucoma direct linkage include how altered biochemical pathways and vascular changes in T2D reduce blood flow and impaired oxygen diffusion, thus increasing oxidative stress and endothelial cell injury.41 Excessive glial cell activation may contribute to chronic inflammation, making the retinal ganglion cells more susceptible to glaucomatous damage.42 Connective tissue remodeling might also promote increased intraocular pressure (IOP) and greater optic nerve head mechanical stress, which can result in glaucomatous optic neuropathy.43 Diabetes is known to cause microvascular damage and may affect the vascular autoregulation of the optic nerve and retina. Thus a longer duration of T2D would be associated with a higher risk of OAG. Obesity, like T2D, is a trait often associated with glaucoma, but whose connection remains elusive. We found associations between obesity and glaucoma in SNPs rs12970134 (CD = 5, CC = 0.062), rs11807640 (CD = 8, CB = 0.516, CC = 0.071), rs9540221 (CD = 5), rs10777845 (CD = 5), and rs4365558 (CD = 5, CC = 0.063), all of which showed significance in our glaucoma study. SNP rs12970134 is located upstream of gene MC4R, the defects of which have been identified as a cause for autosomal dominant obesity.44 SNP rs11807640 is located downstream to gene CDK4PS, which has been associated with obesity traits among some postmenuposal women.45 Both rs9540221, which is intergenic between NFYAP1 and LGMNP1 and rs4365558, which is intergenic between A4GALT and RPL5P34, are SNPs situated between genes that have been associated with obesity as well. Finally, rs10777845 is upstream to gene RMST, which has been observed to be associated with severe early-onset obesity.46 In the investigation to determine direct linkage between glaucoma and obesity, several hypotheses exist. NewmanCasey et al. observed that not only was the frequency of OAG was higher among obese individuals (3.1%) than among non-obese individuals (2.5%), but that the relationship between OAG and obesity had significant correlations with gender.47 Obese women had a 6% increased hazard of developing OAG when compared to non-obese women, while there was no significant effect in men. Similar findings were made by Zang and Wynder, in which they observed OAG diagnosis as twice as likely in women with a body mass index (BMI) above 27.5, but no impact of BMI in men.48 Some hypothesize that increased orbital pressure as a result of excess fat tissue may cause a rise in venous pressure and a consequent increase in intraocular pressure (IOP).47 Our study also show links between Alzheimer’s disease (AD) and glaucoma. The SNP rs803422 (CD = 5, CC = 0.060), which we found to affect glaucoma is also associated with AD. In addition, the two genes SORCS3 and MTHFD1L which contain two of the SNPs with the strongest association are also known to be associated with AD. Patients with AD have a significantly increased rate of glaucoma occurrence. In a study in four nursing homes in Germany, 112 Alzheimer’s patients were taken as a case group. 29 of those 112 were found to have Glaucoma, a rate of 25.9% as opposed to a 5.2% rate in the control group.49 However, in spite of significant research on this topic, there have still been no clear clinical or genetic relationships found between the two diseases.50 Perhaps unsurprisingly, our research has also led to us connecting glaucoma with other phenotypes relating to the eye, particularly cataracts and myopia. The association between cataracts and glaucoma can be seen in the similar genes CDK4PS , NFYAP1, and LGMNP1, all of which contain SNPs found to be highly associated with glaucoma. Myopia and glaucoma are both directly related to the SNP rs569688 (CB = 0.415, CC = 0.074). There have been many recent studies about the connection between glaucoma and myopia, particularly primary openangle glaucoma (POAG) and high myopia. Though the connection has still not been found, it is believed the high myopia is another risk factor of POAG, though it is not as important as intraocular pressure. However, it is recommended that those with high myopia be screen for glaucoma more frequently.51 Of the SNPs and genes associated to glaucoma in our study, the majority that are not directly eye-related, are heart related. Glaucoma is connected therefore probably connected to cardiovascular disease, coronary artery disease, and coronary restenosis. SNPs rs17034592 (CD = 7, CC = 0.060) and loci LDB2, CDKN2B and TRNAQ46P are all associated with coronary artery disease. SNPs rs499818 (CD = 5, CC = 0.052), rs1333040 (CD = 5, CC = 0.052), and loci A4GALT, RPL5P34, and CST7 are all associated with myocardial infarction, and A4GALT and RPL5P34 are associated with cardiovascular disease. Studies have shown that cardiovascular disease may play an important role in the development and progression of glaucoma. However, it is also hypothesized that this relationship may be indirect, due to the variable of age.52 Additional studies about the relationship between intraocular pressure, the main risk factor for glaucoma, and cardiovascular disease exist. However, any genetic relationship between these two diseases is yet to be found. An undocumented interaction found in this study is the link between glaucoma and colorectal carcinoma. Relevant SNPs that were significant were rs972869 (CD = 7, CC = 0.052), located near the EPHB1 gene, and rs300493 (CD = 6, CB = 0.311, CC = 0.066), located in the gene NAV3, both of which are linked to colorectal carcinoma. Studies have shown that obesity and T2D are associated risks of colorectal cancer, which could lead to the hypothesis that colorectal carcinoma and glaucoma may be co-morbidities and are affected by similar pathways. In summary, the successful application of our methodology on GLAUGEN GWAS data proves the effectiveness of our bioinformatics framework, and suggests its great potential in detecting and characterizing gene-gene interactions in GWAS data. In future studies, we expect to extend the interaction analysis beyond the order of pairs. The increased computational demand would, however, require a stricter data pre-filtering. The more stringent SNP filtering can be achieved using a HPN based on SNP overlap to restrict the interactions to the most significant ones. Other avenues of research would include using eye-specific tissue genotype data to further refine the analysis and the drug response of the nodes of interest. Acknowledgements This work was supported by the National Institute of Health (NIH) grants R01-EY022300, R01-LM010098, R01-LM009012, R01-AI59694, P20-GM103506, P20-GM103534. References 1. R. Sachidanandam, D. Weissman, S. C. Schmidt, J. M. Kakol, L. D. Stein and et al., Nature 409, 928 (2001). 2. W. Y. S. Wang, B. J. Barratt, D. G. Clayton and J. A. Todd, Nature Review Genetics 6, 109 (2005). 3. H. J. Cordell, Human Molecular Genetics 11, 2463 (2002). 4. J. H. Moore, Nature Genetics 37, 13 (2005). 5. N. A. Davis, J. E. Crowe Jr., N. M. Pajewski and B. A. McKinney, Genes and Immunity 11, 630 (2010). 6. T. Hu and J. H. Moore, Network modeling of statistical epistasis, in Biological Knowledge Discovery Handbook: Preprocessing, Mining, and Postprocessing of Biological Data, eds. M. Elloumi and A. Y. Zomaya (Wiley, 2013) pp. 175–190. 7. A. Pandey, N. A. Davis, B. C. White, N. M. Pajewski, J. Savitz, W. C. Drevets and B. A. McKinney, Translational Psychiatry 2, p. e154 (2012). 8. J. L. Wiggs, M. A. Hauser, W. Abdrabou, R. R. Allingham, D. L. Budenz, E. Delbono, D. S. Friedman, J. H. Kang, D. Gaasterland, T. Gaasterland, R. K. Lee, P. R. Lichter, S. Loomis, Y. Liu, C. McCarty, F. A. Medeiros, S. E. Moroi, L. M. Olson, A. Realini, J. E. Richards, F. W. Rozsa, J. S. Schuman, K. Singh, J. D. Stein, D. Vollrath, R. N. Weinreb, G. Wollstein, B. L. Yaspan, S. Yoneyama, D. Zack, K. Zhang, M. Pericak-Vance, L. R. Pasquale and J. L. Haines, J Glaucoma 22, 517 (Sep 2013). 9. M. Takamoto and M. Araie, Ophthalmology Journal 58, 1 (2014). 10. J. H. Fingert, Eye (Lond) 25, 587 (May 2011). 11. K.-I. Goh, M. E. Cusick, D. Valle, B. Childs, M. Vidal and A.-L. Barabasi, Proceedings of the National Academy of Sciences 104, 8685 (2007). 12. C. Darabos, K. Desai, R. Cowper-Sallari, M. Giacobini, B. Graham, M. Lupien and J. Moore, Lecture Notes in Computer Science 7833, 23 (2013). 13. C. Darabos, M. J. White, B. E. Graham, D. N. Leung, S. Williams and J. H. Moore, BioData Mining 7, p. 1 (Jan 2014). 14. H. Li, Y. Lee, J. L. Chen, E. Rebman, J. Li and Y. A. Lussier, Journal of the American Medical Informatics Association : JAMIA 19, 295 (January 2012). 15. D. Welter, J. MacArthur, J. Morales, T. Burdett, P. Hall, H. Junkins, A. Klemm, P. Flicek, T. Manolio, L. Hindorff and H. Parkinson, Nucleic Acids Res 42, D1001 (Jan 2014). 16. T. Hu, N. A. Sinnott-Armstrong, J. W. Kiralis, A. S. Andrew, M. R. Karagas and J. H. Moore, BMC Bioinformatics 12, p. 364 (2011). 17. T. Hu, Y. Chen, J. W. Kiralis and J. H. Moore, Genetic Epidemiology 37, 283 (2013). 18. T. M. Cover and J. A. Thomas, Elements of Information Theory: Second Edition (Wiley, 2006). 19. A. Jakulin and I. Bratko, Analyzing attribute dependencies, in Proceedings of the 7th European Conference on Principles and Practice of Knowledge Discovery in Databases (PKDD 2003), , Lecture Notes in Artificial Intelligence Vol. 2838 (Springer-Verlag, 2003). 20. J. H. Moore, J. C. Gilbert, C.-T. Tsai, F.-T. Chiang, T. Holden, N. Barney and B. C. White, Journal of Theoretical Biology 241, 252 (2006). 21. D. Anastassiou, Molecular Systems Biology 3, p. 83 (2007). 22. J. H. Moore, N. Barney, C.-T. Tsai, F.-T. Chiang, J. Gui and B. C. White, Human Heredity 63, 120 (2007). 23. B. A. McKinney, J. E. Crowe, J. Guo and D. Tian, PLoS Genetics 5, p. e1000432 (2009). 24. M. E. J. Newman, Networks: An Introduction (Oxford University Press, 2010). 25. M. E. J. Newman, Physical Review Letters 89, p. 208701 (2002). 26. L. C. Freeman, Sociometry 40, 35 (1977). 27. A. Bavelas, Journal of the Acoustical Society of America 22, 725 (1950). 28. G. Sabidussi, Psychometrika 31, 581 (1966). 29. J. H. Moore and B. C. White, Lecture Notes in Computer Science 4447, 166 (2007). 30. C. S. Greene, N. M. Penrod, J. Kiralis and J. H. Moore, BioData Min 2, p. 5 (2009). 31. X. Sun, Q. Lu, S. Mukheerjee, P. K. Crane, R. Elston and M. D. Ritchie, Front Genet 5, p. 106 (2014). 32. C. International HapMap, Nature 437, 1299 (Oct 2005). 33. S. A. Pendergrass, A. Frase, J. Wallace, D. Wolfe, N. Katiyar, C. Moore and M. D. Ritchie, BioData Min 6, p. 25 (2013). 34. F. Peng, D. Hu, C. Gu, X. Li, Y. Li, N. Jia, S. Chu, J. Lin and W. Niu, Gene 531, 435 (Dec 2013). 35. L. Lu, Y. Wu, Q. Qi, C. Liu, W. Gan, J. Zhu, H. Li and X. Lin, PLoS One 7, p. e34895 (2012). 36. B. E. Klein, R. Klein and S. C. Jensen, Ophthalmology 101, 1173 (Jul 1994). 37. P. Mitchell, W. Smith, T. Chey and P. R. Healey, Ophthalmology 104, 712 (Apr 1997). 38. V. Chopra, R. Varma, B. A. Francis, J. Wu, M. Torres and S. P. Azen, Ophthalmology 115, 227 (Feb 2008). 39. S. de Voogd, M. K. Ikram, R. C. W. Wolfs, N. M. Jansonius, J. C. M. Witteman, A. Hofman and P. T. V. M. de Jong, Ophthalmology 113, 1827 (Oct 2006). 40. J. R. Wolter, Am J Ophthalmol 51, 1123 (May 1961). 41. V. H. Y. Wong, B. V. Bui and A. J. Vingrys, Clin Exp Optom 94, 4 (Jan 2011). 42. M. Nakamura, A. Kanamori and A. Negi, Ophthalmologica 219, 1 (Jan-Feb 2005). 43. V. C. Lima, T. S. Prata, C. G. V. De Moraes, J. Kim, W. Seiple, R. B. Rosen, J. M. Liebmann and R. Ritch, Br J Ophthalmol 94, 64 (Jan 2010). 44. K. M. Ling Tan, S. Q. D. Ooi, S. G. Ong, C. S. Kwan, R. M. E. Chan, L. K. Seng Poh, J. Mendoza, C. K. Heng, K. Y. Loke and Y. S. Lee, J Clin Endocrinol Metab 99, E931 (May 2014). 45. P. Coveney and R. Highfield, Frontiers of Complexity: The Search for Order in a Chaotic World (Faber and Faber, London, 1995). 46. E. Wheeler, N. Huang, E. G. Bochukova, J. M. Keogh, S. Lindsay, S. Garg, E. Henning, H. Blackburn, R. J. F. Loos, N. J. Wareham, S. O’Rahilly, M. E. Hurles, I. Barroso and I. S. Farooqi, Nat Genet 45, 513 (May 2013). 47. P. A. Newman-Casey, N. Talwar, B. Nan, D. C. Musch and J. D. Stein, Ophthalmology 118, 1318 (Jul 2011). 48. E. A. Zang and E. L. Wynder, Nutr Cancer 21, 247 (1994). 49. A. U. Bayer, F. Ferrari and C. Erb, Eur Neurol 47, 165 (2002). 50. P. Wostyn, K. Audenaert and P. P. De Deyn, Br J Ophthalmol 93, 1557 (Dec 2009). 51. S.-J. Chen, P. Lu, W.-F. Zhang and J.-H. Lu, Int J Ophthalmol 5, 750 (2012). 52. S. S. Hayreh, Survey of Ophthalmology 43, Supplement 1, S27 (1999).