ORG CHEM II LAB(MAJOR'S) KELLY IR Problem Set 1 ANSWER
advertisement

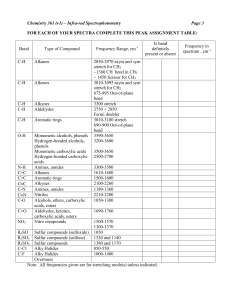
ORG CHEM II LAB(MAJOR’S) KELLY IR Problem Set 1 ANSWER KEY 1. K Allyl alcohol The allyl alcohol OH stretch is indicated by the large, broad absorption between 3200-3500 cm-1. The double bond is indicated by the C-H stretch in the region just to the left of 3,000 cm-1. This can be differentiated from benzyl alcohol by the small C=C stretch at about 1650 cm-1. Note that the C-O stretch absorption at about 1025 cm-1 indicates a 1˚ alcohol. C-C stretch Olefinic C-H stretch CH 2 bend Aliphatic C-H stretch C-H OOP O-H stretch C-O Stretch (1˚) 2. N. Cyclooctene Cyclooctene has an ir spectrum very similar to 1-heptene, but can be differentiated from it by close examination of the spectrum. Both cyclooctene and 1-heptene have very simple spectra, with olefinic C-H stretch absorptions above 3,000 cm-1. Both also have very small C=C stretch absorptions about 1650 cm-1. Cyclohexene has a fairly strong CH2 scissoring bend at about 1470cm-1. It does not, however, have a CH3 symmetric umbrella bend at 1375cm-1. There is a weak absorption (probably part of the fingerprint) at about 1350cm-1, but this is too low to be the CH3 bend. C-C stretch CH 2 bend Olefinic C-H stretch C-H OOP Aliphatic C-H stretch 3. D Heptaldehyde The intense carbonyl C=O stretch peak at ~1725 cm-1 suggests either an aldehyde, or possibly a ketone (although it is a little higher frequency than a standard ketone). The aldehyde is indicated by the two aldehyde C-H stretch absorptions which appear at 2750 & 2850 cm-1. Note that the peak at 2850 cm-1 appears as a shoulder on the much more intense absorption corresponding to the symmetric CH2 stretching vibration. Aldehyde C-H Stretch CH 2 Bending Aldehyde C=O stretch Aliphatic C-H stretching 4. M Benzyl Alcohol Note the broad O-H stretch absorption and the olefinic C-H stretch absorptions. This spectrum can be differentiated from that for allyl alcohol by observing the combination and overtone bands from 1600-2000cm-1, which are indicative of a monosubstituted benzene. In addition the C=C ring stretch at about 1600 + 1475cm-1 indicate that it is an aromatic ring. C-C stretch Aromatic ring breath Aromatic C-H stretch C-O Stretch (1˚) C-H OOP C-O Stretch (1˚) 5 L Butyl acetate Note the presence of a C=O stretch at about 1750-1760cm-1. This would tend to indicate the possibility of an ester functional group, or possibly a strained ring ketone. The broad and intense C-O stretch absorption at about 1250cm-1is a strong indicator of an ester group. This band is the C-O stretch corresponding to the bond of the ester oxygen and the carbonyl carbon. In addition, the C-O stretch at about 1050cm-1 is due to the other C-O stretch to a 1˚ carbon. C-O stretch 1˚ Aliphatic C-H stretch C-O stretch to carbonyl carbon C=O stretch 6 B 3-Pentanone Note the presence of a C=O stretch at about 1710-1720cm-1. This would tend to indicate the possibility of an ketone, aldehyde, or possibly a conjugated ester. There are no absorptions at 2850 and 2750cm-1 which would correspond to the aldehyde C-H stretch, thus it is not likely to be an aldehyde. There are no olefinic C-H stretch absorption bands above 3000cm-1, nor are there any C=C stretch absorptions around 1600 to 1650cm-1. Thus it is not likely a conjugated ester (also note the lack of strong C-O stretch absorptions at 1250cm-1 in the ester). The compound is likely a ketone. C=O stretch 7. F Propanoic Acid The broad O-H absorption from 2400 to 3400cm-1 is a dead giveaway for a carboxylic acid. Note also the relatively broad C=O at about 1710cm-1 and the C-O stretch at 1250cm-1. Broad O-H stretch of acid dimer C-H stretch C-O stretch C=O stretch 8 H 1-Butanol Note the broad alcohol O-H stretch at 3200-3500cm-1. More importantly, note the 1˚ alcohol CO stretch at ~1050cm-1. C-O Stretch (1˚) O-H stretch 9. I Cyclohexane Note the simplicity of the spectra, only C-H stretch and bend absorptions. The most important aspect of this spectrum is what is NOT present. Note the absence of a CH3 bend at 1375 cm-1 . This would suggest a ring structure. NO CH 3 SYMMETRIC BEND CH 2 Scissoring bend C-H stretch 10. E 2-Butanol Note the broad alcohol O-H stretch at 3200-3500cm-1. More importantly, note the 2˚ alcohol C-O stretch at ~1100cm-1. C-O Stretch (2˚) O-H stretch 11. C 1-Hexene 1-Hexene has an ir spectrum very similar to cyclooctene, but can be differentiated from it by close examination of the spectrum. 1-Hexene has a CH3 symmetric umbrella bend at 1375cm-1. Also note if you will, that the unsymmetric double bond C=C stretch of 1-heptene is more intense than the symmetric C=C stretch in cyclooctene. C=C stretch CH 3 bend Olefinic C-H stretch 12. J 2-Methyl-2-butanol Note the broad alcohol O-H stretch at 3200-3500cm-1. More importantly, note the 3˚ alcohol C-O stretch at ~1150cm-1. C-O Stretch (3˚) O-H stretch