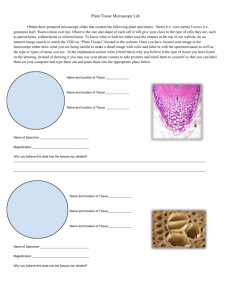
general biology ii laboratory manual
advertisement

GENERAL BIOLOGY II LABORATORY MANUAL Third Edition BREVARD COMMUNITY COLLEGE PALM BAY CAMPUS 1 TABLE OF CONTENTS Lab 1: Orientation and Safety ..............................................................................3 Lab 2: Microscopy ...............................................................................................7 Lab 3: Natural Selection and Classification ......................................................17 Lab 4: Bacterial Identification ...........................................................................29 Lab 5: Protists.....................................................................................................32 Lab 6: Fungi .......................................................................................................53 Lab 7: Plant Diversity Part 1..............................................................................61 Lab 8: Plant Diversity Part 2..............................................................................57 Lab 9: Animal Diversity Part 1..........................................................................68 Lab 10: Animal Diversity Part 2........................................................................82 Lab 11: Animal Diversity Part 3........................................................................87 Lab 12: Turkey Creek Field Trip.......................................................................90 Lab 13: Samsons Island Field Trip....................................................................93 Lab 14: Geocaching with GPS.........................................................................102 Lab 15: Field Ecology......................................................................................109 Lab Practical 1 Review ....................................................................................111 Lab Practical 2 Review ....................................................................................112 2 LAB 1: GENERAL LABORATORY PRACTICES AND LAB SAFETY PROCEDURES INTRODUCTION This laboratory manual has been developed to accompany the Biology II course. The coursework, lecture and lab, are designed to provide the student with a wide range of information about living organisms and systems. The experiments contained in this lab manual accompany the lecture information in such a way so as to illustrate and demonstrate. The lab portion of the course is a hands-on experience that will enhance the course material. Working in a laboratory environment requires that the student observe certain housekeeping procedures and safety precautions. These procedure and precautions are outlined below. The student needs to review and follow the procedure so as to ensure the safety of himself/herself, the instructor, fellow students, the equipment and the facilities. Safety in the lab demands that lab directions be followed carefully. Please read these 'General Laboratory Practices' carefully and be sure you understand each one. If you aren't sure, ASK!!! GENERAL LABORATROY PRACTICES 1. No food or drinks in the Lab. 2. No smoking. 3. Wear all safety equipment as required by the lab procedure or your instructor. Learn the location of all lab safety equipment. Wear safety glasses or goggles at all times. DO NOT WEAR CONTACT LENS AT ANY TIME. 4. Do not mix any chemicals except as instructed. Do not do unauthorized experiments. 5. Use fume hoods when required. 7. Tie long hair back to keep it out of flames or harmful liquids. 8. Wear shoes that cover all of your feet. No open toed shoes or sandals. 9. Do not taste any chemical. 10. Do not smell chemicals directly. Use your hand to waft the odor to your nose. 11. Do not pipet solutions by mouth. Use a rubber suction bulb or special pipet filler. 12. Do not work in the laboratory in the absence of your instructor or his authorized representative. 13. Handle glass tubing and thermometers with care. 3 14. Wash your hands before leaving the laboratory. 15. NO chemicals are to be flushed down a drain unless specifically instructed to do so by the lab procedure. 16. Wastes are to be poured into the appropriately labeled waste container (e.g., solvent waste, halogenated solvent waste, etc.). 17. DO NOT mix wastes from different categories. 18. Clean up broken glass immediately. DISPOSE OF IN SPECIFIED "BROKEN GLASS" CONTAINER ONLY. 19. Clean up solid and liquid spills immediately, but only after checking with your laboratory instructor about possible safety hazards. 20. Take containers to the stock of chemicals. Do not bring stock chemicals to your laboratory table. 21. Read the label on chemical bottles carefully. Insure that you have the correct chemical. 22. Do not insert a pipet or medicine dropper into a stock bottle. Avoid contamination by pouring a small quantity into a flask or beaker before taking a sample. 23. Use special care when handling stoppers or tops of bottles so as not to pick up contamination. 24. Take no more of a chemical than an experiment requires. 25. Never return as unused chemical to a stock bottle. Dispose of it as waste. 26. Set up your glassware and apparatus away from the front edge of your laboratory bench. 27. Follow any other housekeeping, safety, or disposal rules given by your instructor. Federal and State regulations require compliance with strict guidelines for handling, storage, and disposal of hazardous chemicals wastes. Because of the wide variety of chemicals used in a teaching laboratory, it is imperative that all students follow proper disposal procedures so as to not pollute our environment and groundwater supply. Willful violations of these rules can result in a zero being given for that lab. Repeated willful violations can result in an "F" being given for the course. Every effort has been made to minimize the hazardous chemicals used in the Lab. All chemicals provided can be used safely by following correct procedures. However, any chemical can be dangerous if used improperly. Material Safety Data Sheets (MSDS) will be provided for any chemical on request. 4 LABORATORY SAFETY PROCEDURES Safety is one of the concerns in any lab. Safety for humans is always a primary concern. The location of safety equipment should be common knowledge to anyone working in the lab. In order to become familiar with the safety equipment in the lab, make a map on the reverse side of this sheet showing the location of the following safety items. Indicate the fire escape route with an arrow. 1. Emergency shower and eye wash station 2. Fire extinguisher(s) 3. Fire blanket 4. Exits from the room 5. Fire escape route 6. Fire alarm boxes 7. Container for broken glass 8. Eletrical power cut off switch(es) 9. First aid box 10. Biohazardous Waste Container After you have read the 'General Laboratory Practices' and completed the map assignment, please complete this page and turn it into your lab instructor. ________________________________________________________________________ I have read and understand the general lab practices and procedures and am familiar with the location and operation of safety items in the lab. ________________________________ Printed Name _________________________________ Lab Instructor ________________________________ Signature _________________________________ Class Section 5 NAME_________________________ DATE:__________ SECTION________ LAB 1: MICROSCOPE TUTORIAL QUIZ. DIRECTIONS: Before using the microscopes in the lab, the Microscope Tutorial Program (installed on the desktop in the computer lab under Biology Tutorials) and the following quiz must be completed. While running the tutorial, match the following. Words may be used more than once or not at all. A. B. C. D. E. F. G. H. I. J. Limit of Resolution Numerical Aperture Light Microscopy Scanning Electron Microscopy Total Magnification Working Distance Depth of Field Field of View Field Size Electron Gun K. L. M. N. O. P. Q. R. S. T. Condenser Lens Electromagnetic Lenses Ion Pump Specimen Stage EDS Detector Sputter Coater Secondary Electron Detector Photomicrograph Micrometer Objective Lens ______ 1. Relies on the bending, or refraction of light rays. ______ 2. The visual circle of light. ______ 3. The amount of enlargement of the specimen. ______ 4. One millionth of a meter. ______ 5. Deposits a thin layer of metal over the specimen. ______ 6. Produces the primary electron beam. ______ 7. Influences intensity or brightness of light beam. ______ 8. Use magnetic fields to control primary electron beams. ______ 9. Detects x-rays emitted from an SEM specimen. ______10. The space between the objective lens and the specimen. ______11. The smallest detail which we may clearly see. ______12. A measure of the quality of the lens. ______13. A platform upon which the specimen rests. ______14. A picture of a magnified specimen or feature. ______15. The thickness of specimen that is in focus. 6 LAB 2: MICROSCOPY Microscopy Materials List • • • • • • Light or compound microscope Prepared slides: Letter “e” Colored threads Clean microscope slides Cover slips • • • • • Knife Toothpicks Iodine solution Onion Cutting Board INTRODUCTION The microscope is a precise piece of equipment that should be handled with special care. A microscope may be seriously damaged if dropped or bumped against a hard object. The student should report immediately to the instructor any defects that might occur to his or her microscope. The microscope should always be carried with both hands, one under the base and the other on the arm. THE LIGHT MICROSCOPE The following parts are the parts of the microscope that the student should be familiar with. Refer to Figure 1 to aid in locating these parts on your microscope. 1. The OCULAR (eyepiece) contains the upper most lenses of the microscope. Its function is to magnify. The part may be loose, but it should never be removed from the microscope as such practice will allow dust to get inside the instrument. As you look through the ocular, you may notice a solid line; this is a POINTER. Never attempt to clean the inner part of the ocular for you will remove the pointer. 2. The BODY TUBE connects the ocular to the nosepiece. This is a tube through which light rays pass between the upper and lower lenses. 3. The NOSEPIECE is a rotating disc on which the objectives are mounted. When moving the nosepiece, the fingers should be placed on the disc and not the objectives. 4. There may be three or four OBJECTIVES of different lengths and magnifying powers attached to the nosepiece of your microscope. These objectives, together with the ocular, magnify the size of the objects that you are observing. Remember, the shorter the objective the lower the power of magnification. 7 Figure 1 5. The ARM supports the above parts. This is one of two structures that should be held when carrying the microscope. 6. The STAGE is the platform with a mechanical stage for holding the slides in place. Note the circular opening in the center of the stage which allows light to pass through. The object which is to be viewed should be centered over this opening. 7. The ILLUMNATOR is a small lamp located directly beneath the stage. Electrical outlets are located on the tables. 8 8. The DIAPHRAGM may be an iris or rotating disc, depending on the kind of microscope. It is located below the stage. The diameter of the diaphragm may be controlled by either a lever on the iris or by rotation of the disc. Various objects can be seen better under certain light conditions. When using the highest magnification, more light is needed than when using lower magnifications. 9. The CONDENSER is a group of lenses beneath the stage. The condenser causes light rays from the illuminator to converge on the surface of the microscope slide. For most microscopic work, it is best to keep the condenser at its highest level. Only rarely is it desirable to lower it slightly. When the condenser is used at a lowered position, the resolving power is greatly reduced. There is a small milled wheel just under the stage that is used to control the position of the condenser. 10. The BASE is the heavy, horseshoe-shaped structure upon which the microscope rests. This is the other part of the microscope that is held when the microscope is being carried. 11. The COARSE ADJUSTMENT is the large milled wheel on the microscope, which is used in focusing the lenses. 12. The FINE ADJUSTMENT is the smaller milled wheel on the microscope. The wheel may be separate from the coarse adjustment wheel on some microscopes, where on others it is the smaller, outermost portion of a dual wheel assembly. Resolution and Magnification The resolution limit or resolving power of a microscope lens is a function of the wavelength of light, the design of the condenser, and the use of immersion oil with the 100X objective. The shortest wavelengths of visible light provide the maximum resolution. It is for this reason that all microscopes use blue filters over the light source. The best compound microscope lenses have a resolving power of approximately 0.2 microns. This means that two small objects that are 0.2 microns apart will be seen as separate entities under the oil immersion lens. If they are closer than this, a single object is seen due to the fusion of the images. Magnification of an object seen through the microscope is a function of the power of the ocular and objective. If the 10X objective is used with a 10X ocular, the magnification is 100X. When the oil immersion lens is used, the magnification is 10X x 100X, of 1000X. 9 THE SCANNING ELECTRON MICROSCOPE Scanning electron microscopy provides a three-dimensional view of surface features. With a scanning electron microscope (SEM), a narrow electron beam is played back and forth across a specimen's surface, which is either conductive itself or has been coated with a thin metal layer. Electron energy triggers the emission of secondary electrons in the metal. Equipment similar to a television camera detects the emission patterns, and an image is formed. In contrast to the light microscope, the scanning electron microscope has a depth of field at all magnifications up to 500 times greater than the light microscope. SEM Parts and Functions 1. Electron Gun Assembly: The electron gun assembly produces the primary electron beam. A voltage difference is established between the negatively charged tungsten filament (cathode) and a grounded anode plate. The filament is heated in order to generate a flow of electrons. Then the negatively charged electrons accelerate toward the anode. (This is referred to as "accelerating voltage".) 2. Apertures: Depending on the design of the SEM, one or more aperatures may be used to reduce and exclude extraneous electrons in the lenses. The accelerated electrons first pass through a hole in the anode which acts as a crude aperture. This creates a smaller, more cohesive electron beam. Decreasing the aperture size will result in an increase in depth of field, however, a loss of relative brightness. 3. Electromagnetic Lenses: The electromagnetic lenses focus the primary beam on the specimen and consist of two genral types: a. The Condenser Lens: This is the first lens that influences the electron beam. As the condenser lens current is changed, the position of the focal point in the column (focal length) is altered. This, in part, influences intensity of the electron beam when it strikes a given specimen. As a result, it directly affects the brightness of the image signal from that specimen. b. The Final Lens: A final lens is used to bring the beam into focus at the specimen by demagnifying (converging) it to a focal point at the specimen surface. This determines the diameter or spot size of the electron beam at the specimen. Specimen resolution is determined by this beam diameter. 10 4. The Vacuum System: The vacuum system allows passage of the electron beam through the column without interference. This is necessary because (1) a hot filament will oxidize and burn out in the presence of air, (2) the column must be kept clean if the beam is to be well-focused (the vacuum prevents corrosive moisture and chargeable dust particles which can deflect the beam), and (3) air molecules will scatter electrons. 5. The Specimen Stage: The specimen stage is the platform upon which the specimen rests in the column of the SEM. It is located directly below the final lens and represents a significant portion of the specimen chamber. Since, while viewing the specimen, the chamber must be under vacuum, so all manipulations of the stage are accomplished by delicate micrometers which can move the specimen in the x, y, or z directions (figure). Further, the specimen may be tilted at least 550 or rotated 3600. 6. The Signal Detection and Display Components: The signal detector forms an image by drawing electrons toward positive charges via a collector and transferring the energy of electrons to photons. This is accomplished by using a scintillator which contains phosphors that will produce small flashes of light (photons) when struck by electrons. The photons are carried by a light pipe and converted outside the microscope column to an amplified electronic signal. The signal can then be displayed on a cathode ray tube on the display console. PROCEDURE 1: Using the Microscope When properly used, the microscope should cause no eye strain. Try to keep both eyes open when working the monocular microscope and use the dominant eye to look through the ocular. If you wear glasses, it will not be necessary to use them with the microscope, since the microscope automatically corrects for this. 1. Obtain the microscope to which you were assigned from the microscope cabinet. When carrying the microscope, remember to place one hand under the base and the other on the arm. 2. Before the microscope is place on the desk, ample space must be provided for it. All books, purses and other unneeded paraphernalia should be put away. 3. Place your microscope in front of you in a comfortable working position about one inch from the table’s edge. The nosepiece should have the low-power (4X) objective in position over the opening in the stage. Make sure the switch is in the off position and plug the power cord into a suitable grounded electrical outlet. Turn on the illuminator. 4. Obtain a prepared microscope slide. Notice the label which describes the material mounted on the center of the slide. Make a gross examination of the slide. (If the slide is dirty, clean it by rubbing lightly with a soft cloth or paper towel; do not use expensive lens paper for this purpose.) 11 5. Secure the slide firmly in the mechanical stage. Be sure to wedge the slide BETWEEN the stage clips and NOT UNDERNEATH them. Rotate the coarse adjustment knob so that the low-power objective is about one inch above the stage. While observing from one side of the microscope (not through the ocular), adjust the slide so that the embedded material is in the approximate center of the opening. Make sure the side with the cover slip is up. Move the low-power objective down as close to the cover glass as possible without actually touching it. 6. While looking through the eyepiece, move the body tube or stage by turning the coarse adjustment until you can see the mounted material clearly. If you do not see the material, re-center the slide and repeat the procedure. If you turn the coarse adjustment too rapidly you may go past the point of focus without realizing it. 7. Bring the mounted material into the sharpest possible focus by turning the fine adjustment wheel about one quarter of a turn. Prepare the slide so the magnification may be increased by placing the material to be observed in the center of the field of view. If the material is not in the center, it may be lost when switching to a higher power because the field gets smaller. 8. Adjust the interpupillary distance. a. Pull the oculars apart the maximum distance. b. While looking through the oculars slowly move the oculars together until the two images form into one. c. Pull the oculars apart until the images splits into two parts again. Return to the best position. NOTE: Errors in this adjustment generally produce eye strain in one eye within 10 minutes. 9. Adjust the diopter. a. With the interpupillary adjustment properly adjusted, the diopter adjustment becomes much easier. While the left eye is closed, carefully use the fine focus to provide the best possible images to your right eye. b. Without touching the fine focus, open the left eye, close the right eye and rotate the diopter adjustment slowly from one extreme to the other. Somewhere between the extremes there will be a “best place”. c. Students generally notice no difference when first attempting the adjustment. Be assured there is a “best place”. NOTE: Errors in diopter adjustments generally produce nebulous headaches within 20 to 30 minutes. 10. Progress from the 4X to the 10X objective. Rotate the nosepiece until the 10X objective clicks into place. To focus, ONLY the fine adjustment wheel needs to be moved. 11. Follow the same procedure when shifting from the 10X to the 40X objective. 12 Changing to another Slide When you have finished viewing a slide and wish to view another, use the following procedure. 1. Be sure the low power (4X) objective is in position. 2. Loosen stage clips. 3. Remove the slide by slipping it horizontally forward (never up). 4. Return the used slide to the proper tray. 5. Put the new slide in place on top of the stage. 6. Proceed with the proper focusing technique. PROCEDURE 2: Colored Thread Slide 1. Using the “thread” slide, determine the spatial relationships such as the depth of field. The depth of field is the thickness of the specimen that may be seen in focus at any one time. a. Rotate the 4X objective into position and place the thread slide on the stage. Center the slide so that the region where the threads cross is in the center of the stage opening. Try to focus all threads at the same time. b. Rotate the 10X objective into position and focus on the cross. Can you focus on all the threads at the same time? c. Focus upward until the threads are just out of focus. Slowly focus down using the fine adjustment. Which thread comes into focus first? d. Is this thread lying under or over the other threads? Record your findings on the lab report. PROCEDURE 3: Letter “e” Slide 1. Look at the letter “e” slide. Move the slide slowly to the right. In what direction does the image in the ocular move? Is the image in the ocular inverted relative to the specimen on the stage? 13 PROCEDURE 4: Preparing Wet Mounts Wet mounts specimens are those that you make fresh in lab. The specimen is placed in a drop of water or stain on a microscope slide and then covered by a thin piece of glass or plastic called a cover slip. Wet mounts are useful for speed and easy preparation, but they do not usually allow great detail to be observed. 1. Make a wet mount of some onion cells using iodine stain. a. Obtain a clean slide and cover slip. b. Place a drop of iodine on the center of the slide, then place a single layer of onion skin (skin between the onion layers) in the iodine drop. Be sure to spread it out evenly so there is no overlap or double layering. c. Touch the cover slip to one edge of the drop, and gently lower it. (If you drop the cover slip too quickly, air bubbles will be trapped. You cannot see through an air bubble). d. Observe these cells first at 4X, then 10X and make a sketch of a few cells in the space provided on the data sheet. Be careful when working with the stain! 2. Make a wet mount of some cheek cells using iodine stain. a. Obtain a clean slide and cover slip. b. Place a drop of iodine stain on the center of the slide. c. Using a clean unused toothpick, gently scrape the inside of your cheek and mix it into iodine drop. d. Observe these cells first at 4X, 10X, and then 40X and make a sketch of a few cells in the space provided on the lab report. PROCEDURE 5: Comparing Light v. Electron Microscopy 1. Compare the "Thread" scanning electron micrograph to the image you saw using the light microscope. Using the scale provided, record the diameter of the bottom thread on your lab report. 2. Compare the "Cheek Cell" v. the "Onion Cell" micrographs. Note the differences between the two and compare them to the images you saw using the light microscope. 14 PROCEDURE 6: Storing the Microscope 1. Turn off the power. 2. Rotate the 4X objective into position. 3. Remove the slide from the stage and clean the stage. 4. Unplug the cord and wrap it around the base of the scope. 5. Replace the dust cover. 6. Return the scope to its numerical cabinet space using two hands; one hand should hold the arm and the other should support the base. Make sure the oculars face the wall so they will not be bumped and damaged. 7. Wash wet mount slides and throw away cover slips 15 Name: ___________________________ Section: ____________ Date:___________ LAB 2 LAB REPORT: MICROSCOPY 1. What is the total (highest) magnification of your microscope? 2. Which image of the onion cells is three dimensional, the light microscope image or the SEM image? 3. Which image of the cheek cells shows the external parts only, the light microscope image or the SEM image? 4. What cell type, plant or animal, are square shaped and in connected rows? 5. What cell type, plant or animal, are round or oval shaped with a loose unconnected arrangement? 6. Does the letter e slide appear to face the same direction to the naked eye as it does when viewed through the microscope or is the image inverted? 7. What color thread was on the top? 8. What color thread was on the bottom? 9. Estimate the diameter of the thread in micrometers using the scale on the SEM thread micrograph. 10. Sketch the cheek cells and onion cells below Cheek Cells 40 x Onion Cells 10 x 16 LAB 3: NATURAL SELECTION AND CLASSIFICATION A simple genetic trait in humans is the ability to taste the chemical phenylthiocarbamide (PTC). The trait is determined by one gene with 2 alleles; taster (dominant) and nontaster (recessive). If a person expresses the ability to taste PTC (has TT or Tt genotype) the taste is bitter. However, non-tasters (tt genotype) taste nothing. In the United States about 70% are tasters and 30% nontasters. Population genetics is concerned with the frequency of genes throughout a population. In this case we may ask how common is the T allele or the t allele? The question can be answered by applying the mathematical relationship expressed by the Hardy-Weinberg Law as shown below. (p + q)2 = 1 where: p = frequency of the dominant allele q = frequency of the recessive allele By expanding this expression it becomes more readily seen how simple it really is to determine gene frequencies. When expanded the expression becomes: (p + q)2 = p2 + 2pq + q2 = 1 Since q = the frequency of the recessive allele, q2 must be the same as tt. You can easily determine how many individuals have the tt genotype because these are non-tasters. In the U.S. population for example we expect 30% to be tt. Since tt is the same as q2, t must be equal to the square root of q or the square root of 30% (in order to actually perform the calculation this should be expressed in its decimal form, or .30). When the calculation is performed we find that the actual gene frequency for t is .548. Once you have determined q all you need to do to find p is to subtract: 1 -q = p, or 1 -.548 = .452 = p. Now let's see if this really is the correct answer. p2 = TT = (.452) (.452) = .204 2pq = Tt = 2(.452)(.548) = .495 q2 = tt = (.548)(.548) = .300 .999 = 1 17 PROCEDURE 1: Hardy-Weinberg Principle (To be completed as a class) a. Obtain a piece of plain filter paper and chew it for a few moments so that you will know what paper tastes like. b. Obtain a piece of filter impregnated with PTC and chew for a few moments to see if you can taste the chemical. c. Record your results on the table on the blackboard and fill in the class data table below. d. Use the Hardy-Weinberg principle to determine the frequency of T and t in your class sample. Total number of tasters in the class: ________ Total number of non-tasters in the class: ________ Decimal fraction of non-tasters in the class (number of non-tasters ÷ total number of students) = ________ = tt = q2 __ t = √q2 = ________ T = 1 -q = ________ p2 = TT = ( )( 2pq = Tt = 2( q2 = tt = ( )( ) )( ) ) = ________ = ________ = ________ List 3 factors that may make your class different from the general U.S. population PTC frequencies? 18 Procedure 2: Natural Selection Computer Assisted Lab (To be completed individually.) Go to the computer lab and run the Natural Selection program. This program shows how natural selection affects gene frequency in a population. Since gene frequency affects the characteristics expressed in a population, selection influences the traits passed on to future generations. Before running the program, make sure you understand Punnett squares and the principles of heredity. See GLOSSARY - last page. Using the Natural Selection Computer Program, complete the following Natural Selection work sheet. ACTIVITY 1: GENE FREQUENCIES Run Part A and then answer the following questions: A. If there is never any selection pressure, what will happen to the gene frequencies in the future generations of DD, Dd and dd? B. With selection, what are the possible genotypic frequencies in the 2nd generation? DD = _______ Dd = _______ dd = _______ 20th generation? DD = _______ Dd = _______ dd = _______ 50th generation? DD = _______ Dd = _______ dd = _______ C. What happens to the genotypic frequency of dd with selection as the generations continue? D. Will the d allele ever disappear? 19 ACTIVITY 2: GENE FREQUENCIES II (This part can be done without the computer simulation.) A. In a population of clams, 75% of the offspring have blue shells. What is the gene frequency of blue shells? _______ B. Assuming blue (B) is dominant over white (b) for shell color, set up a Punnett square to predict the color of the offspring's shells when a Bb (blue shell) and a bb (white shell) clam reproduce. C. In your Punnett square, list the gene frequencies of the blue (BB or Bb) and white (bb) shelled clams. BB = _______ D. Bb = _______ bb = _______ If a white shell is lethal to the clam, what would be the gene frequency of the Bb genotype in the offspring? Bb = _______ *Hint: Your gene frequencies must always add up to 100% or 1. E. What would happen to the white-shell trait in future generations? F. Would it ever disappear? 20 ACTIVITY 3: NATURAL SELECTION Run part B to answer the following questions: A. Light color in the moth Biston betularia before 1850 was a form of protective coloration. Explain why. B. What was the selection pressure that caused an increase in the black moth population? C. If pollution had not occurred, what would have happened to the gene frequency of the dark moths genotype? D. The insecticide DDT, used extensively during the 1940's and 1950's, virtually eliminated the common housefly, Musca domestica. However, it was noticed that some flies were unaffected by DDT, even when it was sprayed directly on their bodies. A DDT resistant for of Musca domestica had appeared in the population. Describe what probably happened to the frequency of the gene for DDT resistance in the common housefly. Draw a graph to support your hypothesis. 21 ACTIVITY 4: NATURAL SELECTION Use part C to investigate the following problems: A. Describe the change in coloration of the moth population after 20 generations of pollution that steadily increases from 50% to 80%. Use gene frequencies of 0.8 light/0.2 dark and 5 years between reports. B. What happens to the percentage of black moths in the population over 25 generations when pollution increases from 50% to 70% over 15 years, then decreases abruptly to 0 for 10 years? Use gene frequencies of 0.8 light/0.2 dark and 5 years between reports. C. New anti-pollution laws in Manchester, England are expected to reduce pollution from the current value of 65% to: 60% in 10 years 30% in 40 years 50% in 20 years 20% in 50 years 40% in 30 years With gene frequencies of 0.9 light/0.1 dark, use the computer model to predict when the percentage of dark moths will peak and what the percentages of light and dark moths will be after 50 years. D. How are pollution and the color of the moth population related? 22 GLOSSARY Allele: One or more forms of a gene for a given trait. Each individual carries a pair of alleles for one trait. Deleterious Gene: A gene that, if expressed, causes harm to the individual carrying it. Dominant: A gene that masks the expression of a recessive gene. It is shown in genetic notation as an upper-case letter. Gamete: A reproductive cell. Eggs are female gametes; sperm are male gametes. Gene: A unit of DNA that determines a hereditary characteristic. Gene Frequency: How often a gene occurs in a population. It is expressed mathematically as a decimal between 0 and 1. Genotype: Sum total of the genes that make up an individual. Heterozygous: Carrying a pair of genes for a specific trait that are not identical. Natural Selection: Darwin/Wallace theory that individuals in a population vary and struggle for existence. Those individuals having characteristics better adapted for survival live and reproduce. Population: One kind of organism existing together as a group at the same time and in the same area. Predator: An organism that feeds upon another (the prey) Punnett Square: A grid system used to compute results of genetic crosses. Recessive: A gene that is expressed in the absence of a dominant gene. It is shown in genetic notation as a lower-case letter. 23 INTRODUCTION TO CLASSIFICATION I. Classification and Variations Systematics, or systematic biology, is a broad area of study that deals with such topics as classification, evolution, individual variation, distribution, and naming of organisms. The term taxonomy is used when referring specifically to the process of naming. Nomenclature refers to the actual names or word structure used in names. The naming system, or classification system, employed in biology involves a series of categories in a hierarchy so that each level includes several sublevels. Below is an example of a commonly used hierarchical classification system. country state county city street number In biology the categories in the hierarchy are different, of course, but the same relationship exists among the various levels in the system. kingdom phylum class order family genus species (Note that the term phylum is used mostly for animal groups whereas botanists tend to use the term division to indicate the same level.) Most biologists agree that 5 kingdoms are required to adequately classify all the known organisms on earth. These kingdoms are: Monera, Protista, Fungi, Plantae, Animalia. Your textbook has detailed descriptions of each kingdom and the various groups within each. Consider the following example. kingdom: Animalia phylum: Chordata class: Mammalia order: Carnivora family: Felidae genus: Felis species: Felis concolor (panther) 24 Notice that the genus name is underlined and is used as part of the species name. The species name is a binomial; that is, it has 2 parts or words: the genus name and a modifier word. Both parts of the species name are underlined (or italicized in print). The first word of the scientific name is always capitalized and the second word is always lower case. The advantage of scientific names should be clear when you consider the variety of common names that most species have in various places. The panther, for example, is called mountain lion or cougar in some parts of its range, yet all three names refer to the same species. When trying to decide the placement of a given individual into the proper group, systematists must compare the various anatomical or other characteristics of the individual to those of other similar organisms. Anatomy is relied on more that other characters since it is readily evident. Don't we always ask: "What does it look like?" when trying to identify an unknown quantity of any kind? It is easy to recognize broadly different anatomical patterns that distinguish such species as horses and cats, yet when the organisms in question are more similar the distinction may be more difficult. In fact, often distinct and repeating differences occur within a species, especially between members of different sexes. This sexual dimorphism is readily observed in humans, for example. In order to know how much variation to expect when comparing 2 different individuals or groups of individual biologists resort to statistical procedures. Dichotomous keys are used to classify organisms. It is a systematic progression. You must first place the species in the largest of the categories and then work your way down to the lowest of the classification levels. To use the key, start at level 1. There will be 2 or more options. Select the option that most closely resembles the structures mentioned. That option will then indicate what level to go to next. That level will also have options. Select the appropriate option and proceed until a name is given. PROCEDURE 1: Keying Out Norms Below is a cute sample of a dichotomous key. Test your skills by keying out the different Norms. 25 Dichotomous Key Name ______________________________Section______ 1. Has pointed ears .................................... go to 3 Has rounded ears ....................................go to 2 2. Has no tail ............................................. Kentuckyus Has tail .................................................. Dakotus Norns belong to the genus Norno and can be divided into eight species that are generally located in specific regions of the world. Use the dichotomos key to identify the norns below. Write their complete scientific name (genus + species) in the blank. 3. Ears point upward .................................... go to 5 Ears point downward ..............go to 4 4. Engages in waving behavior ............................. Dallus Has hairy tufts on ears ..........................................Californius 5. Engages in waving behavior ............................. WalaWala Does not engage in waving behavior ....................go to 6 6. Has hair on head ............................................. 26 Beverlus Has no hair on head (may have ear tufts) .......go to 7 7. Has a tail ............................................. Yorkio Has no tail, aggressive ............................ Rajus Norm 2 Norm 1 _________________________________________ Norm 3 ________________________________________ ___________________________________________ Norm 4 __________________________________________ 27 _____ Norm 5 Norm 6 ________________________________________ __________________________________________ Norm 7 ________________________________________ Norm 8 28 LAB 4: BACTERIAL IDENTIFICATION INTRODUCTION Bacteria are small, relatively simple, single-celled organisms. They are members of the phylum Eubacteria in the kingdom Monera. they have existed on earth longer and are more widely distributed than any other organism. They are found in almost every imaginable habitat: in air, soil, and water and in extreme temperatures and harsh chemical environments. They can be photosynthetic, using H2S rather than H2O as the source of electrons, but most are heterotrophic, absorbing nutrients from the surrounding environment. Bacteria are called prokaryotes, from the Greek for "prenucleus." Their genetic material is not bound by a nuclear envelope. Bacteria do not have chromosomes; their genetic material is a single circular molecule of DNA. They reproduce by a process called binary fission, where the cell duplicates its components and divides into two cells. These cells usually become independent, but they may remain attached in linear chains or grape like clusters. In favorable environments, individual bacterial cells rapidly proliferate, forming colonies consisting of millions of cells. Because of the small size and similarity of cell structure, techniques used to identify bacteria are different from those used to identify macroscopic organisms. Staining reactions and properties of growth, nutrition, and physiology are usually used to make final identification of species. However, the structure and arrangement of cells and the morphology of colonies can contribute preliminary information that can help us determine the appropriate test necessary to make final identification. In this exercise, you will be identifying bacteria using a variety of techniques. You will use unaided visual observations as well as chemical reactions and computer data banks to learn some characteristics of bacterial cells and colonies. ASEPTIC TECHNIQUES When working with bacteria, it is very important to practice the following aseptic techniques to make sure that cultures being studied are not contaminated by organisms from the environment or that organisms are not released into the environment. 1. Wipe the lab bench with disinfectant (alcohol) before and after the lab activities. Let the alcohol evaporate rather than wiping it off. The evaporative process kills microbes. 29 2. Wash your hands before and after performing an experiment. 3. Flame all non-flammable instruments used to manipulate bacteria or fungi before and after use. If using a pre-sterilized loop, dispose of it in an autoclavable plastic bag provided after use. 4. Place swabs and toothpicks in the autoclavable plastic bag provided. 5. Wear a lab coat, a lab apron, or a clean old shirt over your clothes to lessen chances of staining or contamination accidents. The bacteria used in these exercises are not pathogenic (disease-producing); nevertheless, use proper aseptic techniques and work with care! If a spill occurs, notify the instructor. If no instructor is available, wearing disposable gloves, wipe up the spill with paper towels, followed by soap and water and a disinfectant. Dispose of the gloves and soiled towels in the autoclavable plastic bag provided. BACTERIAL IDENTIFICATION USING COLONY MORPHOLOGY A bacterial colony grows from a single bacterium and is composed of millions of cells. Each colony has a characteristic size, shape, consistency, texture, and color (colony morphology), all of which may be useful in preliminary species identification. Bacteriologists use specific terms to describe colony characteristics. Use Figure 1 to become familiar with this terminology and describe the bacterial species provided. Occasionally, one or more fungal colonies will contaminant the bacterial plates. Fungi may be distinguished from bacteria by the fuzzy appearance of the colony. The body of a fungus is a mass of filaments called hyphae in a network called a mycelium. Learn to distinguish fungi from bacteria. Differences in colony morphology and the shape of individual bacterial cells are important distinguishing characteristics of bacterial species. In this exercise, you will culture bacteria and observe and describe the morphology of colonies of several bacterial species. 30 Name:______________________ Date:__________ Section:_______ LAB 4: BACTERIA IDENTIFICATION LAB REPORT PROCEDURE 1. Choose an area which you are going to obtain a bacterial culture from. Obtain an agar plate and write the initials of your team members, the date and a description of your culture on the bottom of the Petri dish. 2. Using a sterile swab, pick up organisms from the area of your choosing and culture your plate by lightly rolling the swab over the top of the agar. 3. Place your plate in the area indicated by the instructor for incubation. 4. Incubating the culture 24 - 48 hours. 5. Observe several bacterial plates under a stereoscopic microscope until you find at least 4 colonies that differ in at least one aspect – colony shape, margin, surface and/or color – and record your observations below. Bacteria Name/ID Shape Margin Surface Color 1. 2. 3. 4. 31 LAB 5: PROTISTS OVERVIEW Protists are eukaryotic organisms. The ancestors of this group were the first to have a true nucleus, chromosomes, organelles such as chloroplasts, mitochondria, endoplasmic reticulum, cilia, and cell division by mitosis and/or meiosis. The ancestral protists not only gave rise to the modern protists but also to three other kingdoms of life: fungi, plants, and animals. Most protist are unicellular, although several of the algae are colonial or truly multicellular. Although protists are often described as being simple organisms, their cellular organization and metabolism is every bit as complex as those found in the so-called higher organisms. In fact, higher organisms are often much simpler at the cellular level because their cells are specialized to perform all functions necessary for life as independent organisms. Protists live everywhere there is water: in the ocean, in fresh water, in puddles, in damp soils, and, as symbiotes in the body fluids and cells of multi-cellular hosts. Some are autotrophic, making their own food materials through photosynthesis, while others are heterotrophic, absorbing organic molecules or ingesting larger food particles. All protists can reproduce asexually by mitosis while some are also capable of sexual reproduction by meiosis and nuclear exchange. A cyst stage is found in the life cycle of many protists; it allows the species to lie dormant and escape the harsh temporary conditions. The boundaries of the kingdom Protista are not well understood. When first proposed in 1969 by Robert Whitaker, the kingdom was defined as containing unicellular organisms, but subsequent studies have indicated that some fungi and plants are more similar to protists than they are to other fungi and plants. Consequently, some multi-cellular algae, such as kelps, and some fungi, such as the slime molds and water molds, are considered by some to be protists. About 60,000 species of living protists are known and a similar number have been described from the fossil record. 32 SLIME MOLDS Plasmodial Slime Mold: Phylum Myxomycota Physarum polycephalum is a true slime mold that grows in areas having cool temperatures, high humidity, and dead organic matter. Physarum is studied for its streaming protoplasm and for fundamental life processes. The Physarum life cycle involves an alternation of haploid and diploid phases. Slime molds have always been difficult to classify because they exhibit both animal like and fungus like characteristics. The creeping somatic phase is animal like, while the spore-producing reproductive structures are fungus like. The yellow, active phase of the diploid plasmodium resembles a giant mass of naked protoplasm surrounded by a thin plasma membrane. The plasmodium moves across the agar surface in a fanlike manner, ingesting its food by secreting enzymes ito the food vacuoles created as the plasmodium surrounds food particles in its path. Within the plasmodium is a mass of veinlike strands (gel) that carry the fluid (sol). The fluid protoplasm within the veinlike strands moves in one direction for about a minute and then reverses its flow. The streaming in the veins is probably assiciated with metabolism more than with movement. As the plasmodium travels, it sheds its outer sheath, leaving a slime track behind. After a period of time, the plasmodium may produce a number of stalked fruiting structures (sporocarps or sporangia) containing masses of spores. Meiosis occurs within the sporangium. The fragile sproangial wall confining the spores is called the peridium. In nature the spores are generally dispersed by air. If proper environmental conditions exist, germination occurs. The spore walls crack open, and one flagellated swarm cell or one myxamoeba usually emerges from each spore, grows, and multiplies. The presence of water favors the formation of swarm cells, whereas drier conditions induce myxamoeba formation. Pairs of these haploid swarm cells or myxamoebae fuse within 24 hours to form zygotes, which grow into plasmodia. If moisture is insufficient in the plasmoidial stage, the plasmodium will form a multinucleated, hardened mass of tissue called a sclerotium. this dry, resting stage can again form a plasmodium when favorable conditions for growth return. 11 12 PROCEDURE 1: Subculturing Plasmodial Slime Mold from Sclerotia Physarum polycephalum 1. Sterilize work area with bleach. 2. Label a petri dish with your initials, date and culture name. 3. Place a filter paper in the bottom of a petri dish. Slowly add a few drops of distilled water until the entire paper is barely wet. 4. Place the Physarum paper with the sclerotial tissue face dwon in the center of the filter paper and cover the petri dish. 5. Feed the Physarum oat flakes and place it in the designated area. 6. Observe daily changes in your culture over several weeks’ time and record your observations. 7. Using a low-power objective, observe the streaming of protoplasm in the veins of the plasmodium on the petri dish at the edges of the growth that extends beyond the filter paper. Cellular Slime Mold: Phylum Acriasiomycota Dictyostelium discoideum is a cellular slime mold. Upon spore germination, a single amoeboid cell emerges through a split in the wall of each spore. After 24 hours, many minute myxamoebae begin to appear. The myxamoebae are strictly parasitic on bacteria. the cells have food vacuoles that ingest and digest bacterial cells. After 48 hours, clear circular patches appear over the agar surface where digestion of bacteria has occurred. Once the population of myxamoebae reaches a certain point, the process of feeding and dividing stop. Some of the cells start attracting other cells by use of a chemotactic substance called acrasin (a nucleotide cyclic 3',5'-adenosine monophosphate, commonly known as cyclic AMP). Aggregations begin forming and a protective slime sheath (the slug or pseudoplasmodium) is secreted around the mass of naked cells. The slug leaves slime behind as it travels over the agar. When the slug stops migrating, it heaps itself up to start the formation of the fruiting body (sorocarp), a stalk with a head at the tip composed of rounded cells. These round cells become spores in a slimy drop. 13 PROCEDURE 2: Subculturing Plasmodial Slime Mold 14 Dictyostelium discoideum 1. Sterilize work area with bleach. 2. Label a plate of Lactose Agar with your initials, date and culture name. 3. Obtain a plate culture of Dictyostelium 4. Using a sterile loop, inoculate the Lactose Agar with a thin suspension of nonmucoid Eschericheria coli in a north-south streak. Dispose of the loop in an autoclave bag. 5. Using a sterile loop, pick up a Dictyostelium sorocarp (fruiting structure) and innoculate media/E. coli dish in an east-west orientation, perpendicular to the E. coli streaks. 6. Place the Dictyostelium in the designated area. 7. Observe daily changes in your culture over a week’s time and record your observations below. 8. Using a low-power objective, observe the sorocarps and/or migrating slugs present in the culture and record your observations. PHYLUM EUGLENOPHYTA Euglenas are a genus of autotrophic protists characterized by a generally elongated, spindle-shaped form and usually possess one or two flagella to facilitate movement in their aquatic environments. The group Euglenophyta is a small group, containing only about 800 species. Euglenophytae do not possess cell walls, but instead have a flexible outer covering called a pellicle, which is a type of plasma membrane. There is actually some debate regarding the classification of these organisms, with botanists classifying euglenids as algae because of their photosynthetic properties, and zoologists insisting on the classification as protozoa, due to euglena's motility and lack of cell wall. PROCEDURE 3: 1. Obtain a prepared slide of Euglena and observe with the medium-power (40X) objective of your microscope. Look within the cytoplasm and try to identify the chloroplasts and the centrally located nucleus. By closing the microscopes diaphragm to increase the contrast, you may be able to make out the flagellum at one end of the cell. 2. Sketch and label the structure of the euglena you observe under your microscope in the space provided. PHYLUM CHRYSOPHYTA (DIATOMS) 15 The 10,000 species of diatoms share a common characteristic: a cell wall consisting of two valves made of silica. They are often golden yellow in color because of an excess of carotenoid and xanthophyll pigments, which tend to mask the green of the chlorophylls that are also present. Diatoms are important as primary producers in the food chain of aquatic environments and their cell walls are used for a wide variety of industrial purposes, ranging from the polishing agent in toothpaste to reflective roadway paint additive. When the diatom cell dies, the siliceous valves do not disintegrate and accumulate as sediments. In California, some deposits of diatoms are 300 feet deep. These accumulations are known as diatomaceous earth. PROCEDURE 4: 1. Make a wet mount slide of diatomaceous earth available in the laboratory. Place a tiny drop of the diatomaceous earth mixture on the slide. Add a cover slip for viewing. Note the exceedingly delicate patterns of the diatom valves. These are the skeletal remains of cells that lived thousands of years ago. In a top-down view, some valves will be round, others triangular, ovoid, and irregular. When viewed from the side, these same valves will appear rectangular or ovoid. 2. Sketch a few different types of diatom valves below in the space provided. HETEROTROPHIC PROTISTS Phylum Rhizopoda: Ameboid Protozoans The best-known amoeboid protozoans are the amoebas, organisms that are continually changing shape through formation of projections called pseudopodia (the singular form is pseudopodium, meaning "false foot"). The amoeba are not capable of making their own food through photosynthesis like the other classes of protista mentioned earlier. Amoebas are heterotrophic, requiring an outside source of nutrients, which they must ingest. The amoeba accomplishes this through phagocytosis, in which pseudopodia are formed around food particles, and the pseudopodia fuse, creating a food vacuole within the cytoplasm. Enzymes are then emptied into the food vacuole, where the food particle is digested into a soluble form that can pass from the vacuole into the cytoplasm. PROCEDURE 5: Obtain a prepared slide of amoeba and examine it underneath your microscope. You will need to do this at medium power (40X), with your diaphragm adjusted to provide greater contrast. Label the multiple pseudopods emanating from your amoeba. Also try to locate the nucleus and food vacuoles, if possible. Sketch the structures you observe in the space provided. Phylum Ciliophora: Ciliated Protozoans 16 Unlike the flagellated protists (such a euglena), which use flagella, or amoeboid protists, which use pseudopodium for movement, members of the phylum Ciliophora use short, hairlike locomotory structures called cilia. We will be making observations of one of the largest ciliates, Paramecium Caudatum, which possesses certain features worthy of discussion. Paramecium possess two nuclei. One is a larger, centrally located macronucleus, which is associated with non-reproductive functions (i.e., synthesis of RNA coding for digestive enzymes). The other is a smaller micronucleus, which is located right next to the macronucleus. The micronucleus regulates reproductive functions. A paramecium's cilia serve a dual purpose. Aside from providing a means of locomotion, the cilia help to direct food particles into an opening of the digestive tract. This opening is located at the base of a depression called the oral groove. Under the microscope, the actual entrance (cytosome) will not be obvious, but the tubule leading from the oral groove, the gullet, may be identified. PROCEDURE 6: Obtain a prepared slide of Paramecium. Observe the organism under the microscope, and try to locate the position of the macronucleus, and if possible, the micronucleus. Also look for the oral groove or gullet, and other structures such as the food vacuoles. Make a sketch of the paramecium you observe, and try to label the structural components and organelles. PHYLUM CHLOROPHYTA: Green Algae The division of protists known as Chlorophycota , or green algae, include about 7000 or so species, which can be grouped together to create a natural progression from single cells to multi-cellular colonies. Three lines of evolutions are apparent: (1) the formation of colonies (2) the formation of multi-cellular filaments, and (3) the formation of definite multi-cellular organisms. Chlorphyta are very important phylogenetically because ancestral green algae are believed to have given rise to the land plants. Chlamydomonas: A Motile Unicell Chlamydomonas is a common motile, unicellular green alga or chlorophyte. The type of sexual reproduction in Chlamydomonas is species dependent. Some are isogamous, with no visual differentiation between male and female. Others are oogamous, in which relatively large, non-motile egg cells are produced within gametegangia (cells or organs that produces sex cells) known as oogonia. The numerous small motile sperm are produced in gametegangia known as antheridia. 17 Volvox: A Motile Colony Like Chlamydomonas, Volvox is a motile but, unlike Chlamydomonas, it is a colony rather than a unicel. A colony of Volvox consists of 500 to 50,000 cells. The coordinated stroking of two flagella in each cell allows the organism to move in a direction while spinning on its axis. The cells in the colony are held together by a gelatinous matrix and are connected to neighboring cells by thin strands of protoplasm. Young colonies of Volvox produced by either asexual or sexual reproduction may be contained inside the parent colony. In sexual reproduction, several cells in the colony differentiate into motile sperm and a few others become non-motile eggs. Sperm swim to the eggs, and fuse with them to form zygotes. Zygotes develop into daughter colonies inside the parent colony and are released when it dies. Because male and female gametes can be distinguished from one another, Volvox is described as being heterogamous. (having different male and female gametes). Because the egg is the larger of the two gametes, volvox is described as being oogamous. The cells that produce sperm are called antheridia and those that produce egg cells are called oogonia. Because of this differentiation of cell types and division of labor, the colony has some of the properties of a truly multicellular organism. PROCEDURE 7 1. Examine a prepared slide of Chlamydomonas under the microscope using the medium-power (40X) lens. Sketch your observations of Chlamydomonas in the space provided below. 2. Obtain prepared slides of Volvox and observe under medium power. Sketch your observations of Volvox in the space provided below. Spirogyra: A Non-motile Filament Spirogyra is a form of green algae that develops colonies in the shape of long filaments. Spirogyra is also commonly known as "green silk" or, a less complimentary moniker, "pond scum". The vegetative cells of Spirogyra are cylindrical in shape, containing a single, spiraling chloroplast. Spirogyra is isogamous, and does not produce motile gametes. In Spirogyra, gametes are transferred by a process called conjugation, in which an actual physical connection is made between gamete donor and gamete recipient cells. To reproduce sexually, two filaments of Spirogyra move close to each other and form conjugation tubes, which create a bridge for the transfer of contents from one cell to another. The cell donating the genetic material is known as the (+) mating type, and the recipient as the (-) mating type. The entire cytoplasmic contents serve as isogametes (gametes of equal size) in Spirogyra, with one isogamete moving through the conjugation tube into the other cell, where it fuses with the other isogamete to form a zygote. The zygote will later give rise to four haploid nuclei (nuclei containing only half of the chromosomal complement). Three of these will degenerate and the fourth will grow to produce a new filament. Asexual reproduction occurs by means of fragmentation, in which a small portion of the filament simply breaks off and continues to grow. Zoospores are not formed by spirogyra. 18 PROCEDURE 8: 1. Obtain the prepared slide of Spirogyra and look at it under your microscope using medium power. You will see Spirogyra represented in each of its stages of sexual reproduction. Compare your observations with the illustration provided. Try to identify the three major stages and draw what you see in the box provided below. Label the conjugation tubes. 2. Observe a prepared slide of Mixed Green Algae and identify at least three varieties of algae included on the slide. Sketch your observations below. PHAEPHYTA: Brown Algae This group includes the large kelps of the intertidal zones. Xanthophylls, chlorophylls, and other pigments provide the color. They are very plantlike in structure having leaf-like blades growing from a stem-like stipe, which may be attached to a root-like holdfasts. Some members of this group have gas-filled bladders, or floats. PROCEDURE 9: Observe live specimen of brown algae if available. Note the stipe, blades and bladder. Sketch your observations in the space provided below. RHODOPHYTA: Red Algae These algae possess phycobilin pigments, which can trap sunlight in deep marine waters. Some forms can aid in reef building (stone-like cell walls), others yield agar, and at least one is commercially grown for human food. The small, diploid sporophyte attached to the gametophyte produces spores (from a sporangium) that develop into a larger, independent sporophyte where meiosis leads to gametophytes and their gametes. PROCEDURE 10: Observe live specimen of red algae if available. Sketch your observations in the space provided below and record the information requested in the multi-celled algae table. SEARCHING FOR PROTISTS IN POND WATER PROCEDURE 11: Make a wet mount of pond water by placing a drop on a microscope slide and placing a cover slip over it. Include a small amount of green algae. Observe it under the microscope and try to identify some of the protists using the dichotomous keys and your previous experience. You may use the lab manual, the wall charts, your atlas and/or your text as a reference for other protists you may not have studied. Record the characteristics of four different phyla in the table below. 19 DICHOTOMOUS KEYS TO IDENTIFY COMMON PROTISTS Free-Living Protozoa 1. White or colorless………………………………………………………………..2 Colored……………………………………………………………………………8 2. Creeping (sliding) slowly or floating without apparent motion……………….3 Exhibits other motion…………………………………………………………….7 3. Spherically shaped with radiating “spines”…………………Actinosphaerium Not spherical in shape…………………………………………………………...4 4. Shape remains constant……………………………………………………..….5 Shape constantly changes………………………………………………………6 5. Possesses flatten test or shell with embedded or attached material; pale to brown in color……………………………………………………Aracella Possesses done-shaped test or shall with attached particles, usually of sand…………………………………………………………..Difflugia 6. Small; creeps using pseudopodia (false feet); single disc-shaped nucleus………………………………………………….Ameoba Large; creeps using pseudopodia; many (100’s) of small nuclei…………………………………………………..…..Pelomyxa 7. Cell has hair-like structures (cilia)……………………………………………16 Cell’s organ of locomotion is long whip-like flagella (no cilia)………………9 8. Green color…………………………………………………………………..…..9 Color not green…………………………………………………………………23 9. Colony of many cells…………………………………………………………..11 Single, motile cells……………………………………………………………..10 10. One observed locomotor flagella……………..………………………………15 Two observed locomotor flagella……………………………………………..14 11. Colony flat, disc-shaped, usually containing sixteen cells………………..Gonium 20 Colony spherical in shape……………………………………………………..12 12. Colony contains 32 cells or less………………………………………………13 Colony contains more than 32 cells……………………………………………Volvox 13. Colony contains 32 cells…………………………………………….……….Eudorina Colony contains 16 cells……………………………………………………Pandorina 14. Cell elongated with narrowed posterior…………………………………Chilomonas Cell oval-shaped………………………………………………………Chlamydomonas 15. Cell elongated, green in color…………………………………………………Euglena Cell elongated, colorless, with broad, rounded or truncate during locomotion; highly plastic when stationary, often appears to vibrate when in motion…………………………………………………………………………Peranema 16. Body has specialized groups of cilia, or cilia in specific areas………………...17 Body entirely covered with cilia……………………………………………………19 17. Cell not on stalk……………………………………………………….…………….18 Cell on stalk; cells contract (stalk appears to contract like spring)…….Vorticella 18. Cell oval-shaped with distinct point-like projections termed cirri (fused cilia); travels by “walking” using cirri…………………………………………….Euplotes Cell oval-shaped with two distinct ciliary bands, one anterior and one in the middle of the body; swims with spiral motion…………………………….Didinuium 19. Body trumpet-shaped or elongated…………………………………………………20 Body oval-shaped……………………………………………………………………..22 20. Body elongated; never attached to substrate………………………………………21 Body trumpet-shaped; usually attached to substrate………………………..Stentor 21 21. Large cell with elongated, flattened body with blunt ends; contracts to ¼ of its body length with stimulated……………………………………………..Spirostomum Small cell with elongated body, “cigar-shaped,” with rounded ends; swims rapidly in a corkscrew fashion……………………………………………………Paramecium 22. Small body, oval shaped, with small mouth; fast swimmer.....................Colpidium Extremely large body (visible with the naked eye), with large, wide mouth.......................................................................................Bursaria truncatella 23. Pink or rose-colored (ciliate)…………………………………………Blepharisma Dark bluish-green (ciliate)……………………………….…………………Stentor 22 23 24 Algal Survey Mixture 1. Cells contain chloroplasts, have green, yellow-green, golden yellow, or brownish pigments predominating………………………………………………………………3 Cells do not contain chloroplasts, but instead have blue-green pigments seemingly distributed and diffused throughout the cell……………………………2 2. Thread of large cells (trichome); composed of bead-like and barrel-shaped cells…………………………………………………………………………..Anabaena Trichome of very small cells in a gelatinous mass arranged in a macroscopic hollow ball……………………………………………………….……………..Nostoc 3. Cells arranged in filaments with distinctive green spiral-shaped chloroplasts in each cell………………………………………………………….………………….Spirogyra Cells not arranged in filaments……………………………………………………….4 4. Cells possessing flagella…………………………………………………………..….5 Cells not possessing flagella…………………………………………………………8 5. Single cells possessing flagella…………..………………………………………….6 Cells in a colony, each possessing flagella…………………………………………7 6. Cell is colored golden-brown and are differentiated into two distinct cones, each composed of plates…………………………………………………………Peridinium Cell is colored green with differentiation of regions. Cell has a large single chloroplast with two conspicuous flagella…………………………Chlamydomonas 7. Colony is spherical or oval; 8-32 truncated or pear-shaped bi-flagellated cells in the colony………………………………………………………………………….Pandorina Colony is spherical, hundreds of pear-shaped bi-flagellated cells in colony……………………………………………………………………………Volvox the 8. Cell is long and needlelike and colored golden brown. It possesses two distinct plates or valves, one overlapping the other………………………………….Synedra Cell is bow-shaped with pointed ends and colored green. Does not possess plates. Two semicells, each containing a chloroplast are divided by a distinct center band……………………………………………….………………Closterium 25 26 Lab 5: Protists Report: Name:_______________________________ Section:_________ Date:____________ Amoeba (label pseudopod) Paramecium Diatoms Euglena 27 Physarum Stages Observed Dictyostelium Stages Observed _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ __________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ Chlamydomonas Volvox 28 Spirogyra Single Filament Mixed Green Algae (Identify 3 Types) Spirogyra Early Conjugation Spirogyra Late Conjugation (Label Conjugation Tube) Brown Algae (Identify stipe, blade and bladder) 29 Pond Water Protists: Identify at least 4 different phyla of protists below. Common Name Phylum Cell Wall (+/-) Chloroplasts (+/-) Means of Locomotion 30 LAB 6: FUNGI Fungi Materials List • • • • Microscope Microscope slides Cover slips Scalpel • • • • Culture of Rhizopus Yeast culture Lichen specimens Fungi specimens INTRODUCTION FUNGI Fungi are everywhere. We've seen them in our yards -- curious white caps pushing their heads over blades of grass. While walking through the woods, these strange, almost alien life forms can be found plying their silent trade nearly everywhere our eyes wander -- in a pile of moist dead leaves, thriving on trees, both living and dead, on rocks, or in shallow pools of water. These opportunistic heterotrophs can grow and reproduce anywhere, breaking down the remains of those once living and using their scavenged resources to propagate new generations of mushrooms, yeasts, and molds. The fungi serve a valuable service in nature, tirelessly breaking down the remains of dead trees, leaves, and animals and returning their chemical components to the soil, completing the great circle of life. But fungi are important to us in many other ways as well. Penicillin, the antibiotic discovered in 1940, is derived from a common fungus often found on rotten fruit or cheese. Since that time, many other potent antibiotics, such as Streptomycin and Terramycin, have been isolated from various species of the lowly fungus. Also remember that we depend upon the fungus Yeast to make our baked goods rise, and to ferment fruits and grains to produce alcoholic beverages. Of course, not all fungi are benign. Various members of the Candida genus cause a variety of infections ranging from athletes foot, to thrush, to life-threatening, systemic infections. They can also ruin food supplies, sometimes even causing a dangerous type of food poisoning known as Ergotism, or St. Anthony's fire. During wet seasons, fungi can wipe out entire crops, as demonstrated by the great Irish Potato famine of 1845, in which nearly one million people died of starvation and poverty in a two year period. Fungi are classified into their own kingdom, the Kingdom Fungi. Once thought to be simple plants that had lost their chlorophyll, fungi are know understood to be an entirely different class of organisms, with properties all their own. Fungi are heterotrophic eukaryotes which digest their food externally and then absorb the small nutrient molecules that are released. Fungi can be classified either a saprobes (organisms that live on dead organic material) or as parasites (organisms that feed on living material). 31 Fungi have body structures and modes of reproduction dissimilar from those of most other organisms. Most fungi, excepting the yeast, are multi-cellular. The fungal cells come together to give rise to structures known as hyphae (hi-fay), which are a netlike masses of filaments which branch repetitively to give rise to mycelium (my-sill-ee-um). The fungal cells grow so rapidly that, within 24 hours, a single cell can give rise to more than a kilometer of new mycelium. Fungi reproduce by producing spores in a unique, three-phase sexual cycle (explained later in more detail). Besides lack of chloroplasts, another characteristic of fungi that help to distinguish them from plants is their cell wall. Plant cell walls are composed mainly of cellulose. The cell walls of fungi are composed of chitin (kite-tin), a protein also found in the hard shells of insects and crustaceans. Fungi are classified into three major phyla, listed below: GROUP COMMON NAME Phylum Zygomycota zygosporangium-forming fungi Phylum Ascomycota sac fungi Phylum Basidiomycota club fungi PHYLUM ZYGOMYCOTA: Conjugation Fungi All members of the Zygomycetes produce a thick-walled zygote, known as a zygosporangium. Most fungi in this division are saprophytes, including the common black bread mold, Rhizopus. Before the introduction of chemical preservatives into bread, Rhizopus was an almost certain invader, especially if the humidity was high. Growth of two different mycelia in close proximity is necessary before sexual reproduction will occur. (The difference in the mycelia is genetic rather than structural. Because they are impossible to distinguish, the mycelia are simply referred to as + and - strains). Sexual reproduction in Rhizopus occurs when two sexually compatible mycelia are in close proximity. As the hyphae from each mating type grow close, chemical messengers signal them to produce protuberances. When the protuberances make contact, gametangia are produced at their tips. Each gametangium contains many haploid nuclei of a single mating type. The wall between the two gametangia then dissolves, and the cytoplasms of the gametangia mix. Eventually, the many haploid nuclei from each gametangium fuse. The resulting cell contains many diploid nuclei resulting from the fusion of gamete nuclei of opposite mating types. Each diploid nucleus is considered a zygote. This multinucleate cell is called a zygosporangium. Eventually, a thick bumpy wall forms around this diploid cell. Meiosis occurs within the thick-walled zygosporangium, which then germinates to produce a sporangiophore and a sporangium. Some of the spores give rise to mycelia of one mating type, while others give rise to the opposite mating type. 32 PROCEDURE 1: 1. Observe a slide of Rhizopus containing the mycelium, which consists of many hyphae. Observe, sketch and label the numerous black dots called the sporangia. The sporangia are containers of spores, by which Rhizopus reproduce asexually. 2. From the culture, make a wet mount and look at the sporangia at 10x. If you can find a broken sporangium, count the spores released. Are there many or few spores in a single sporangium? Identify the rhizoids at the base of the sporangium bearing hypha. Rhizoids serve to anchor the mycelium to the substrate. Sketch the Rhizopus and label the sporangium, spores and hyphae in the space provided on your lab report. PROCEDURE 2: 1. Obtain a culture of (+) and (-) strains of Rhizopus containing the mycelium, which consists of many hyphae. The numerous black dots you will notice are the sporangia. The sporangia are containers of spores, by which Rhizopus reproduce asexually. 2. Obtain a plate of corn meal or starch agar. Initial the plate and label one side (+) and the other side (-) 3. Using a sterile swab, transfer the (+) strain to one side of an agar plate and the (-) strain to the other side. 4. Place in the culture drawer for a week and note the zygosporangium forming in the center. 5. On a prepared slides of Rhizopus, Conjugation, Rhizopus, Whole Mount and Rhizopus, Sporangia, Zygotes, find the stages of sexual reproduction in Rhizopus, including gametangia, zygotes, and zygospores. Use the 10X objective of your compound microscope to make your observations and sketch them below. 6. Using either + or - Rhizopus culture, make a wet mount and compare the sporangia to the prepared slides. If you can find a broken sporangium, count the spores released. Are there many or few spores in a single sporangium? Identify the rhizoids at the base of the sporangium bearing hypha. Rhizoids serve to anchor the mycelium to the substrate. Sketch and label your observations. PHYLUM ASCOMYCOTA: Sac Fungi Members of the Ascomycetes produce spores in a sac, the ascus, which develops as a result of sexual reproduction. Asexual reproduction takes place by means of production of asexual spores called conidia. The division includes organisms of considerable importance, such as the yeasts responsible for the baking and brewing industries, as well as numerous plant pathogens. A few members of the class Ascomycetes are highly prized for food, including morels and truffles. Peziza: A Cup Fungus 33 The fruit of some ascomycete fungi grow to are large size and are known as ascocarps. The edible morel or "sponge" mushroom is an ascomycete as well as are the cup fungi of the genus Peziza. Cup fungi are heterothallic, having two mating types. The apothecium contains three types of hyphae. Hyphae containing haploid nuclei grow from germinating spores of the + and types to form the bulk of the apothecium. In the center of the mass, a third hyphal type called an ascogenous hypha forms from the fusion of the + and - hyphae. Each cell of this hypha has two nuclei, one of the +type and one of the -type. These cells are dikaryons. The ascogenous hyphae grow upward to the surface of the cup. Here the + and - nuclei unite to produce a diploid zygote. It then divides by meiosis to produce four nuclei which each dividing by mitosis to form eight ascospores inside a saclike ascus. Asci should be visible on the inside of the cup in a layer called the hymenium. Baker’s Yeast: Unicellular Fungi Yeasts are unicellular fungi; they have no hyphae and they reproduce by budding. Yeast cells digest sugar and starch, forming glucose. The respiration of yeast changes glucose into alcohol and carbon dioxide, as you learned in the respiration lab. Baker’s yeast is in the Phylum Ascomycota. PROCEDURE 3: 1. Obtain a slide of a section of Peziza, and examine its structure. Compare your observations to the atlas. Can you see the hymenial layer on the slide? 2. Examine the slide of the ascocarp of the Peziza with the medium or high-dry power objectives. Label the elongate, fingerlike asci, which contain dark-colored, spherical ascospores. 3. Prepare a wet mount of living yeast cells (saccharomyces) and examine them under high-dry power. Find and label some yeast cells that are budding, a form of asexual reproduction. Yeast cells also have a sexual cycle in which meiosis leads to the formation of four haploid ascospores contained in an ascus. Sketch your observations below. PHYLUM BASIDIOMYCOTA: The Club Fungi Members of this group of fungi are probably what the average person thinks of as fungi because the division contains those organisms called mushrooms. Actually the mushroom is only a portion of the fungus. It's the "fruiting body", specifically a basidiocarp, containing the sexually produced haploid basidiospores. These basidiospores are produced by a club shaped basidium for which the group is named. Much (if not most) of the fungal mycelium grows out of sight, within the substrate upon which the basidiocarp is found. When a haploid basidiospore germinates, it produces a haploid primary mycelium. The primary mycelium is incapable of producing a fruiting body. Fusion between two sexually compatible mycelia must occur to continue the life cycle. Surprisingly, the nuclei of the two mycelia do not fuse immediately. Thus, each cell of this so called secondary mycelium 34 contains two genetically different nuclei. Such a cell is said to be dikaryotic. di- is Greek for "two", while karyon refers to the nucleus.) (The prefix The secondary mycelium forms an extensive network within the substrate. An environmental or genetic trigger eventually stimulates the formation of the aerial basidiocarp. Each cell of the basidiocarp is dikaryotic, including the basidia on the gills. Within the basidia, the two nuclei fuse; the basidia are now diploid. These diploid nuclei undergo meiosis, forming genetically distinct nuclei. Each nucleus flows with a small amount of cytoplasm through the hornlike projection at the tip of the basidium to form a basidiospore. Note that the gilled mushrooms do not reproduce by means of asexual conidia. PROCEDURE 4: 1. Study a prepared slide of Coprinus mushroom cap. Observe the slide first with the 4X objective of your compound microscope. In the center of the cap identify the stalk. The gills radiate from the stalk to the edge of the cap, much as the spokes of a bicycle wheel radiate from the hub to the rim. Identify the bas 2. Switch to the 40X objective to study a single gill. Note that the component hyphae produce club-shaped structures at the edge. These are the basidia. Each basidium produces for haploid basidiospores. Find and label them. (All four may not be in the same plane of the section.) Each basidiospore is attached to the basidium by a tiny hornlike projection. As the basidiospore matures, it is shot off the projection due to the buildup of turgor pressure within the basidium. Sketch your observations below. 3. Obtain a fresh mushroom and observe the inferior view. Note the pileus (cap). Cut the mushroom longitudinally to identify and label the longitudinal view of the pileus, gills, and stipe. LICHENS These composite organisms consist of and ascomycete fungus growing in close association with either blue-green or green algae. The fungus and algae together produce a unique superorganism, the lichen, can colonize environments that neither could colonize alone. The fungus contributes to this mutualistic association by absorbing minerals and moisture from the environment. The algae are nestled among the fungal hyphae, where they benefit from the absorptive process, and, in turn, produce carbohydrates and other organic molecules which are absorbed by the fungus. About 5 - 10% of the lichens dry weight is due to algal cells. Lichens are found from the Arctic to the tropics, growing on rocks, trees, and soils. About 25,000 kinds of lichens have been described. They range in color from black and white to delicate shades of green, yellow, brown, and red. 35 Lichens are able to live in harsh environments because they can survive long periods of desiccation. When it rains or fog rolls in, lichen can absorb 3 to 35 times its own weight in water. As water is absorbed, the algae become photosyntheticly active and remain so until a dry period begins. Because lichens are dry most of the time, they have very slow growth rates, increasing in diameter from less than 1 to 10 millimeters per year. On this basis, some large lichens may be thousands of years old. Lichens do not reproduce sexually, although the fungus or algal members may do so individually. Fragmentation of the lichen asexually propagates the association. Some lichens form special structures called soredia, which are minute fragments of fungus and algae that may be wind-carried to new locales. PROCEDURE 5: 1. Observe the collected specimens of lichens. Identify the three major types and sketch the different shapes in the space provided on your lab report. 2. Observe the collected specimens of fungi. List the common name, ID tag and phylum in the space provided on the lab report. 36 LAB 6: FUNGI LAB REPORT Name: _______________________________ Date:_______________Section: _______ Directions: Sketch and label the following Yeast, Budding Wet Mount (Identify a bud) Live Coprinus, Longitudinal Section (Label pileus, stipe and gills) Rhizopus, Wet Mount (Label Sporangium, Spores and Hyphae) 37 Lichen, fruticose Collected Specimen Lichen, foliose Collected Specimen Lichen, crustose Collected Specimen 38 Directions: Observe the collected specimens and identify them below. ID Tag Common Name/Type Phylum fruticose lichen foliose lichen crustose lichen rhizopus commercial mushroom shelf mushroom yeast sargassum truffles puffball 39 LAB 7: PLANT DIVERSITY PART 1 INTRODUCTION It's generally agreed that land plant arose from the green algae. Evidence includes identical food reserves (starch), the same photosynthetic pigments (chlorophylls a and b, carotenes, and xanthophylls), and similarities in the structure of their flagella. Some biologists have gone so far as to suggest that the land plant is nothing more than highly evolved green algae. One major mystery is the origin of a feature common to all land plants, alternation of generations. In alternation of generations, two distinct phases exist: A diploid sporophyte alternates with a haploid gametophyte. At first, alternation of generations is difficult to envision. As animals, we find this concept foreign. But think of it as the existence of two body forms of the same organism. The primary reproductive function of one body form, the gametophyte, is to produce gametes (eggs and/or sperm) by mitosis. The primary reproductive function of the other, the sporophyte, is to produce spores by meiosis. A fundamental distinction exists between the green algae and land plants in respect to alternation of generations. Although some green algae have alternation of generations, the sporophyte and gametophyte look identical. To the naked eye, they are indistinguishable. This is called alternation of isomorphic generations. (Iso - comes from a Greek word meaning "equal" and morph- is a Greek word for "form"). By contrast, the alternation of generations in land plants is heteromorphic (hetero- is Greek for "different"). The sporophytes and gametophytes of land plants, including the bryophytes, are distinctly different from one another. During the course of evolution, two major lines of divergence took place in the plant kingdom. The plants in one line had as the dominant phase the gametophytic generation, meaning that the sporophyte was never free living, but was permanently attached to and dependent upon the gametophytes for nutrition. Today, these plants are represented by the bryophytes, mosses, and their relatives. It seems that this line is an example of dead-end evolution, with no other group of plants present today arising from it. In the other line, the sporophyte led an independent existence, the gametophyte being quite small and inconspicuous. As you will see in the following exercises, liverworts and mosses are dissimilar in appearance. They do share similarities that result in placing the plants (Kingdom Plantae) into the division Bryophyta. These similarities include the following: 1. Both exhibit alternation of heteromorphic generations in which the gametophyte is the dominant organism. The sporophyte remains attached to the gametophyte, deriving most of its nutrition from the gametophyte. 2. Both are dependent on water for fertilization, since their sperm must swim to a nonmotile egg. 3. Both lack true vascular tissues, xylem, and phloem, and hence are relatively small organisms. 40 Step into almost any moist forest, look down, and what do you see ? More than likely, covering the bases of tree trunks, on decaying logs, and on rocks you will find mosses (and of course, fungi). Growing on rocks along streams you will find flattened liverworts. Life Cycle Comparison PART 1: LIVERWORTS We will be examining prepared slides containing cross-sections of the Thallose-type liverwort, Marchantia. This liverwort is commonly found on the surfaces of damp rocks and soil. Individual plants consist of a body called a thallus. The thallus has a lobed appearance due to the division of cells located at the notch of each tip of the thallus. The surface of the thallus appears to be divided into diamond-shaped areas, which correspond to air chambers located beneath the upper epidermis. Rhizoids are located beneath the surface as an adaptation for water and mineral absorption. The upper surface may contain tiny cuplike structures called gemmae cups. These produce asexual gemmae, which are small, multicellular disks of green tissue, which develop into new gametophytes when dispersed into suitable locations. Sexual reproduction involves separate male plants that bear antheridia and female plants that bear archegonia. The significant development here is the fact that gamete formation is 41 in a sex organ (gametangium) surrounded by a protective layer of non-reproductive tissue. Both types of sex organs are contained in receptacles, stalked structures that grow upward from the notches in the tips of thalli. Inside the sporangium, each spore mother cell undergoes meiosis, forming four spores. When the capsule dries, it ruptures, exposing a cottony mass of spores and elaters. The elaters, intertwined in this mass, are hydroscopic. Spiral thickenings in their cell walls cause the elaters to twist land coil as they dry or take up moisture, dislodging spores from the mass. The spores are disseminated by the wind and germinate to form a new gametophyte. PROCEDURE 1: 1. Obtain a prepared slide of a cross section of a thallus from Marchantia, and look at it under low power with your compound microscope. Sketch the cross section below. Indicate the upper and lower epidermis. Note the air pores on the upper surface, which open into air chambers. Beneath this layer is the lower storage tissue. Are the chloroplasts present in these cells? Projecting from the lower epidermis are the rhizoids which extend into the soil. Your slide may show ventral scales, which enclose longitudinal bundles of rhizoids that aid in capillary transport of water. What do you think is the function of the rhizoids. 2. Look at a slide of Marchantia antheridium (the male reproductive structure). Note the general shape and multicellular nature of the organ. The bi-flagellated sperm develop in the several antheridia on the upper surface of the male receptacle and are surrounded by sterile cells that protect the gametes. Because the receptacle is derived form the haploid thallus, sperm are produced by mitosis instead of meiosis. Several hundred sperm are produced in each antheridium. If you would like to view the sperm separately, a separate slide with Marchantia sperm will be made available. 3. Now look at a slide of an archegonium, the female reproductive structure. These inverted flasklike structures are in rows on the underside of female receptacles. At the base of each archegonium is a swollen area containing the egg. Extending downward from the egg is a neck canal. Fertilization requires water. Raindrops falling on the antheridia splash sperm onto the archegonium. Using their flagella, the sperm swim into the neck of the archegonium to fertilize the egg. It develops into the sporophyte stage. 4. Obtain a slide with maturing sporophytes on the archegonial receptacle. Following fertilization, the stalks of the archegonial receptacles elongate. The zygote divides repeatedly, forming the multicellular embryo of the sporophyte generation. Find the sporophyte stage on the slide and study it using low power on your compound microscope. The bulk of the cells in the sporophyte form a capsule, which surrounds other cells that differentiate into spore mother cells and elaters, the later being slender, elongate cells with spirally thickened walls. Together these cells make up the sporangium. Thus, the sporophyte generation of the thalloid liverwort is a small sporangium attached to the lower surface of the cap of a female receptacle growing from the gametophyte thallus. 42 PART 2: MOSSES (CLASS MUSCI) Many organisms commonly called "mosses"are not even plants. Reindeer moss is a lichen. Spanish moss is a vascular plant, and Irish moss is an algae. True mosses have the anatomical and reproductive features outlined below. Moss generally gets its start as moss spores, which are encapsulated haploid cells produced by meiosis. Within a couple of days, these spores germinate to produce protenema, algal-like filaments of cells. Moss Life Cycle 43 PROCEDURE 2: 1. Obtain a slide of Moss Protenema. Each protenema is a branching thread of single cells joined end to end. The colorless branches will develop into rhizoids, which are analogous to the roots of higher plants. Leafy green gametophytes will develop from buds on the protenema. As the buds develop, the protonema will continue to grow and form new buds. Protenema can spread to cover an area 40cm in diameter in a matter of months. Try to find a young gametophyte developing on the protenema. Because the gamete develops by mitotic division and differentiation, all tissues in the gametophyte are haploid. The leaves are usually a single cell thick, and are arranged in whorls of three around the central axis tissue. Sketch what you see on your slide. 2. Now obtain the slide labelled Moss Archegonia and Antheridia. You will notice that as the gametophyte stage matures, sex organs, or gametangia differentiate at the tip of the stem. The archegonia, the egg producing organs, will develop on one gametophyte and the antheridia (sperm producing organs) grow on another (sometimes, antheridia and archegonia will appear on the same gametophyte). Between the several gametangia at the tips are filaments of sterile cells called paraphyses. 3. Observe a small piece of sphagnum moss under low power. Identify the gametophyte leaves, and the capsule and operculum of the sporophyte. SEEDLESS VASCULAR PLANTS AND CONIFERS SEEDLESS VASCULAR PLANTS Seedless, terrestrial plants are analogous to the first terrestrial vertebrate animals, the amphibians, in their dependence on water for external fertilization and development of the unprotected, free living embryo. Both groups were important in the Paleozoic era, but have undergone a steady decline in importance since that time. Seedless plants were well suited for life in the vast swampy areas that covered large areas of the Earth in the Carboniforous, but were not suited for the drier areas of the Earth at that time or for later climactic changes that caused the vast swamps to decline and disappear. The fossilized remains of the swamp forests are the coal deposits of today. Although living representatives of the seedless vascular plants have survived for millions of years, their limited adaptations to the land environment have restricted their range. All seedless vascular plants have vascular tissue, which is specialized for conducting water, nutrients, and photosynthetic products. Their life cycle is a variation of alternation of generations, in which the sporophyte is the dominant plant; the gametophyte is usually independent of the sporophyte. These plants have stomata (openings in the epidermis of the leaves that allow gas exchange) and structural support tissue. However, they still retain the primitive feature of motile sperm that require water for fertilization; Therefore, the gametophyte is small and restricted to moist habitats. Economically, the only important members of this group are the ferns, a significant horticultural resource. The seedless vascular classes are broken down into the following four phyla: 44 Phylum Common Name • Psilophyta • Whisk Ferns • Lycophyta • Club Mosses • Sphenophyta • Horsetails • Pterophyta • Ferns PHYLUM PSILOPHYTA (WHISK FERNS) The psilophyta include two genera, Psilotum and Tmesipteris. Psilotum is tropical and subtropical in distribution, while Tmesipteris is restricted in distribution to Australia and islands of the South Pacific. Psilotum (see Atlas) is unique among vascular plants in that it does not possess roots or leaves. The sporophyte consists of a dichotomously branching aerial portion with small scale-like outgrowths and a branching underground portion, or series of rhizomes (underground stem that serves as a storage organ and means of vegetative propagation) with many rhizoids (hairlike structures that serve as roots). Psilotum is homosporous, with the spores being produced in sporangia borne on the ends of short, lateral branches (see Atlas). Upon germination, the spores give rise to bisexual gametophytes, which resemble portions of the rhizome. Like the rhizome, the subterranean gametophyte contains a symbiotic fungus. In addition, some gametophytes contain vascular tissue. The sperm of Psilotum are multiflagellated and require water to swim to the egg. Initially, the sporophyte is attached to the gametophyte by a foot, a structure that absorbs nutrients from the gametophyte. Eventually, the sporophyte become detached from the foot, which remains connected to the gametophyte. Tmesipteris grows as an epiphyte on tree ferns and other plants. The leaflike appendages of Tmesipteris are larger than the scalelike outgrowths (see Atlas) of Psilotum, but in other respects, Tmesipteris essentially similar to Psilotum. PHYLUM LYCOPHYTA (CLUB MOSSES) The four living genera and approximately 1000 living species of Lycophyta are the representatives of an evolutionary line that extends back to the Devonian Period (370 my ago). There are a number of orders of lycophytes, and at least three of the extinct orders included small to large trees. The three orders of living lycophytes, however, consist entirely of herbs. All lycophytes, living and fossil possess microphylls (tiny leaves with no vascular tissue) that are highly characteristic of the division. The tree lycophytes were among the dominant plants of the coal forming forests of the Carboniforous period (280 my ago), with some of these trees bearing seedlike structures similar to those of modern plants. 45 Perhaps the most familiar living lycophytes are the club mosses, Lycopodium (see Atlas). The approximately 200 species of this genus extend from the arctic regions into the tropics, but they rarely form conspicuous elements in any plant community. Most tropical species are epiphytes (plants that use other plants non-parasitically as a means of structural support) and thus rarely seen, but several of the temperate species form mats that may be evident on forest floors. The sporophyte of Lycopodium consists of a branching rhizome from which arial branches and adventitious roots (roots that grow laterally from the stem) rise. The microphylls of Lycopodium are usually spirally arranged. Lycopodium is homosporous; the sporangia occur singly on the upper surface of fertile microphylls called sporophylls (see Atlas) modified leaf or leaflike organs that bear sporangia. In some species, the sporophylls are similar to ordinary microphylls and are interspersed among the sterile microphylls. In others, the nonphotosynthetic sporophylls are grouped into strobili (see Atlas), or cones, at the ends of the arial branches. Upon germination, the spores of Lycopodium give rise to bisexual gametophytes that, depending on the species, are either green, irregularly lobed masses or branching, subterranean, nonphotosynthetic structures. Like the gametophytes of Psilotum and tmesipteris, those of the species lycopodium with subterranean gametophytes are associated with a symbiotic fungus. The development and maturation of antheridia and archegonia may require from 6 to 15 years, and they may even produce a series of sporophytes in successive archegonia as they continue to grow. Water is required for fertilization; the biflagellated sperm swim through water to the archegonium and then down its neck. Following fertilization, the zygote develops into an embryo, which grows within the venter of the archegonium. The young sporophyte may remain attached to the gametophyte for a long time, but it eventually becomes independent PHYLUM SPHENOPHYTA (HORSETAILS) Like the Lycophyta, the Sphenophyta extend back to the Devonian period. The sphenophytes reached their maximum abundance and diversity later in the Paleozoic era, about 300 million years ago. During the late Devonian and Carboniforous periods, they were represented by the calamites, a group of trees that reached 15 meters in height, with a trunk that could be more than 20 centimeters thick. Today, the Sphenophyta are represented by a single herbaceous genus, Equisetum, which consists of 15 species. The species of Equisetum (see Atlas) are known as the horsetails; they are widespread in moist of damp places, by streams, or along the edge of the woods. Horsetails are easily recognized because of their conspicuously jointed stems and rough texture. The small, scalelike leaves, which have a simple structure but are probably reduced megaphylls, are whorled at the nodes and alternate with the leaves. The internode (portion of the stems between the successive nodes) are ribbed, and the ribs are tough and strengthened with siliceous deposits in the epidermal cells. Horsetails have been used to scour pots and pans, particularly in colonial and frontier times, and have thus earned the name "scouring rushes". The roots are adventitious, arising at the nodes of the rhizomes. The aerial stems of Equesetum arise from branching underground rhizomes and, although they may die back during unfavorable seasons, the rhizomes are perennial. The Aerial stem is complex anatomically. At maturity, its internodes contain hollow pith, surrounded by a ring of smaller annals called carinal canals. Each of the smaller canals is associated with a strand of primary xylem and primary phloem. 46 Equisetum is homosporous. Sporangia are borne in groups of five to ten along the margins of small, umbrella like structures known as sporangiophores (sporangia bearing branches), which are clustered into strobili at the apex of the stem. The fertile stems of some species do not contain much chlorophyll, and in these species the fertile stems are sharply distinct from the vegetative stems, often appearing before them early in the spring. In other species of Equisetum, the strobili are borne at the tips of otherwise vegetative stems. When the spores are mature, the sporangia contract and split along their inner surface, releasing numerous spores. Elaters (see Atlas), which arise from the outer portion of the spore wall, coil when moist and uncoil when dry, thus presumably playing a role in spore dispersal. The gametophytes of Equisetum are green and free living, most being about the size of a pinhead; they become established mainly on mud that has recently been flooded and is rich in nutrients. The gametophytes which reach sexual maturity in three to five weeks are either bisexual or male. In bisexual gametophytes, the archegonia develop before the antheridia; this developmental pattern increases the probability of cross-fertilization. The sperm are multiflagellated and require water to swim to the eggs. The eggs of several archgonia and a single gametophyte may be fertilized and develop into embryos, or young sporophytes. PTEROPHYTA (FERNS) Ferns (see Atlas) have been relatively abundant in the fossil record from the Carboniferous period to the present, and closely related groups occurred as far back as the Devonian period. About two-thirds of the approximately 12,000 living species are found in tropical regions, with the other third inhabiting temperate regions of the globe, including desert areas. Ferns are by far the most abundant and diverse group of living, seedless vascular plants. Most garden and woodland ferns of temperate regions have flesh, siphonostelic rhizomes which produce new sets of leaves each year. The roots are adventitious, arising from the rhizomes near the bases of the leaves. The leaves or fronds are megaphylls and represent the most conspicuous part of the sporophyte. Their high surface to volume ratio marks them as more efficient photosynthetic organs than the microphylls of the lycophytes. The ferns are the only seedless vascular plants to possess megaphylls. Commonly, the fronds are compound; that is, the lamina is divided into leaflets, or pinnae (see Atlas), which are attached to the rachis, an extension of the leaf stalk, or petiole. In nearly all ferns, the young leaves are coiled in a bud and are commonly referred to as "fiddleheads." This type of leaf development is known as circinate vernation. It results from the more rapid growth on the lower than the upper surface of the leaf early in development and is mediated by the hormone auxin produced by the young pinnae on the inner side of the fiddle head. All but a few generations of ferns are homosporous. The sporangia are variously placed on the lower surface of the leaves, on specially modified leaves, or on separate stalks. The sporangia commonly occur in clusters called sori (see Atlas). In many genera, the sori are covered by specialized outgrowths on the leaf, the indusia which may shrivel when the sporangia are ripe. At this time, the mature spores -- the result of meiosis in the spore mother cells-- is affected through a crack in the so called lip cells of the sporangium. The sporangia are stalked, and each contains a special layer of unevenly thick-walled cells called an annulus. Contraction of the annulus caused tearing of the lip cells. Sudden expansion of the annulus then results in a catapult like discharge of spores. 47 Heterospory in living ferns is restricted to two specialized forms of water ferns. A number of extinct ferns were also heterosporous. The spores of most homosporous ferns give rise to free-living bisexual gametophytes. The gasmetophyte begins development as a small, pale green alga-like chain of cells called a germ filament, or protenema. It then develops into a flat, heart shaped membranous structure called the prothallus, (see Atlas) with numerous rhizoids on its central, lower surface. Both antheridia and archegonia develop on the ventral surface of the prothallus. The antheridia generally appear earlier than the archegonia, primarily among the rhizoids. The archedgonia then are formed near the notch, or indentation at the interior end of the gametophyte. The difference in timing or appearance of the two kinds of gametangia promotes outcrossing in ferns. Water is required for the multiflagellated sperm to swim to the eggs in both homosporous and heterosporous ferns. Early in its development, the embryo, or young sporophyte, received nutrients from the gametophyte via a foot. However, development is rapid and the sporophyte soon becomes an independent plant, at which time the gametophyte disintegrates. 48 PROCEDURE 3: FERN GAMETOPHYTE STAGE 1. Study the life cycle of a fern in your Atlas. Then observe the underside of a live fern specimen. Identify the pinnae and the sori (singular sorus). 2. In the sori are round structures called sporangia. Cells in the sporangia divide by meiosis to produce haploid cells that differentiate into spores. As the sporangia mature, they dry and split on the side opposite the annulus. The reinforced annulus snaps back explosively, releasing the spores. These spores are resistant to dehydration and aid in the dispersal of the ferns. They are often sold as fern "seed". How do spores differ from seed? ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 3. Obtain prepared slides of Fern Sori and Fern Sporangia. Identify and sketch as many of the structures as you can listed in the Atlas. Remember, the sori contain sporangia with spores. 4. Remove a sorus from a live fern specimen and observe it under low power using your compound microscope. Crush it up and make a wet mount. Try to identify the sporagium and spores. Sketch your observations in the space provided. 5. Obtain a prepared slide of fern gametegangia (Fern Antheridia and Archegonia). The sperm are produced by antheridia, located on the lower surface of the heartshaped leaf near the rhizoids. Eggs are produced in the archegonia, located on the lower surface near the cleft of the heart shaped leaf. A water film must cover the lower surface of the gametophyte (prothallus) for sexual reproduction to occur. The sperm swim through this film to the archegonium, where one sperm penetrates and fertilizes the egg. Identify and sketch as many of the structures as you can find listed in the Atlas (p. 61, fig. 6.53). Be sure you can tell the antheridia from the archegonia. 6. The fertilized egg is the start of the next generation, the sporophyte, in the alternation of generations. As the diploid sporophyte grows by mitotic division and photosynthesis, the gametophyte stage whithers and dies. Obtain the whole mount slide, Fern, Young Sporophyte , and observe it under low power with the compound microscope. Sketch what you see. 49 SEED PLANTS - GYMNOSPERMS During the evolution of vascular plants, the development of the seed was one of the most striking events to occur. Seeds have remarkable survival value and seem to be one of the reasons for the dominance of seed plants today: In examining the characteristics of seeds and seed plants, we find: 1. All seed plants produce pollen grains. Pollen grains serve as carriers for sperm. This characteristic is one factor accounting for the widespread distribution of seed plants. As a consequence of pollen production, the sperm of seed plants do not need free water to swim to the egg. Thus, seed plants are capable of reproducing in harsh climates where nonseed plants are much less successful. 2. Virtually all seeds have some type of stored food that the embryo uses as it emerges from the seed during germination. (The sole exception is orchid seeds, which rely on symbiotic association with a fungus to obtain nutrients.) 3. All seeds have a seed coat, a protective covering enclosing the embryo and its stored food. A seed coat and stored food are particularly important for survival. An embryo within a seed is protected from an inhospitable environment. Consider, for example, that a seed may be produced during a severe drought. Water is necessary for growth of the embryo. If none is available, the seed may remain dormant until growing conditions are favorable. When germination occurs, a ready food source is present to get things underway, providing nutrients until the developing plant can produce its own carbohydrates by photosynthesis. 4. As was the case with the bryophytes, fern allies, and ferns, seed plants exhibit alternation of generations. 5. All seed plants are heterosporous; that is, they produce two types of spores. Bryophytes and most fern allies and ferns are homosporous, producing only one spore type. Gymnosperms are one of two groups of seed plants. Gymnosperm translates literally as "naked seed," referring to the production of seeds on the surface of reproductive structures. This contrasts with the situation in angiosperms whose seeds are contained within a fruit. The general assemblage of plants known as gymnosperms contains plants in four separate phyla: 50 PHYLUM COMMON NAME Coniferophyta conifers Cycadophyta cycads Ginkgophyta ginkgos Gnetophyta gnetophytes By far the most commonly recognized gymnosperms are the conifers. Among the conifers, perhaps the most common is the pine (Pinus). Many people believe conifers and pines to be one and the same. However, while all members of the genus Pinus are conifers, not all conifers are pines. Give the name of a conifer that is not a pine: _______________________________ Anatomy of Pine In this exercise, you will study the anatomy and reproductive cycle of a pine as a representative gymnosperm in the phylum Coniferophyta. Leaves Look at the demonstration specimens of conifer needles (pines, firs, spruces, cedars, etc.) Note the shiny appearance to the needles. This is caused by the presence of a thick, waxy cuticle, which prevents evaporation of water, adapting the plants to dry environments. The wax coating and reduced surface area due to the needle shape allow coniferous leaves to remain on trees over winter in temperate and arctic climates when water freezes and cannot be translocated from roots to leaves. PROCEDURE 4: 1. Obtain a prepared slide of a cross section of Pine Leaf, Three-Needle and look at it with your compound microscope. The cuticle may not be visible because it was extracted by solvents used in the slide preparation process. Note the epidermis, which secretes the cuticle, and the underlying mesophyll. 2. Do stomata penetrate the surface of the leaf? ____________ 3. In the center of the mesophyll, note the endodermis surrounding one or two vascular bundles. Each bundle contains thick-walled xylem cells and thinner phloem cells. Beneath the upper surface of the leaf, two or more resin ducts should be visible. Using your Atlas, identify the stoma and sketch them in the space provided. 51 Stems The stems of pines and other conifers are woody, showing secondary growth. This leads to the formation of substantial amounts of secondary xylem toward the center of the stem and secondary phloem around the circumference. Obtain a cross section of a pine stem and look at it with your compound microscope and identify the following structures. Find the very center of the stem which contains the pith. It is surrounded primary xylem followed by secondary xylem. At the outer edge of the secondary xylem is the cambium layer, which divides to form new secondary xylem inwardly and secondary phloem outwardly. Resin ducts should be visible in the layers of xylem. The stem is surrounded by a thick bark or periderm, which originates from a bark cambium layer outside of the phloem. It protects the trunk from invasion by microorganisms, from desiccation, and from damage by fire, which is common in coniferous forests. PROCEDURE 5: Obtain a prepared slide of a cross section of Pine, Older Stem and look at it with your compound microscope. Using your Atlas, identify the xylem and phloem described above and sketch them in the space provided. Reproduction The familiar pine, fir, and spruce trees are all examples of the sprophyte generation of the gymnosperms. These mature sporophytes all originated from embryos in seeds. To locate the gametophytes, the reproductive structures must be examined. For pines, the reproductive structures are the young cones that develop in the spring of the year. Pine trees develop two types of cones: a small staminate or male pollen cone, which is on the tree for a few weeks, and the larger, more familiar ovulate female seed cone, which remains on the tree for two or more seasons. Examine the specimens of cones available in the lab. Identify the male versus female cone. The male staminate cones appear singly or in clusters near the bases of terminal branch buds in the spring of each year. Examine the specimens of pollen cones available in the lab. Each cone is composed of whorls of scalelike sporophylls, which bear two microsporangia on their lower surfaces. PROCEDURE 5: 1. Obtain a prepared slide of a longitudinal section of Pine Staminate (male) Cone and, using your Atlas look at it with your compound microscope. Scan the entire slide to determine which end represents the tip of the cone. Then locate the swollen microsporangia radiating from the cone axis and identify it on your sketch. Many microspore mother cells in each microsporangia divide by meiosis, each producing four microsporophylls or sperm cells. Identify these structures in a sketch below. 2. Obtain a prepared slide of a longitudinal section of Pine Young Ovulate (female) Cone and, using your Atlas, look at it with your compound microscope. After determining which end of the section is the tip of the cone and what structures are oviliferous scales, find the swollen ovules at the base of the scales and identify them on your sketch. The integument of the ovule surrounds the female gametophyte, which may be in any stage of development, depending on when the section was made. Identify these structures in a sketch below. 52 Name:_________________________ Section:___________ Date:___________ LAB 7: PLANT DIVERSITY PART 1 Directions: Using your Atlas, sketch your observations below. Marcantia, Thallus (Label the air pore) Marcantia, Antheridium (Label the spermatogenous tissue) Marcantia, Archegonium (Label the egg) Moss Protenema (Label the gametophytes) Sphagnum Moss live specimen (label the gametophyte and sporophyte) Moss Archegonia (Label the egg) Moss Antheridia (Label the spermatogenous tissue) 53 Fern Anththerida and Archegonia (Label egg and spermatogenous tissue) Fern Sporangia with Spores (Label sporangia with spores) Fern Sporophyte (Label sporophyte v. gametophyte) Fern Sori (Label sporangia with spores) Pine Leaf/Needle (Label stoma) Pine Stem (Label xylem and phloem) 54 Female Cone (Label egg) Male Cone (Label spermatogenous tissue) 55 ***TEST YOUR KNOWLEDGE*** Directions: Observe the collected specimens and identify them below. ID Tag Common Name/Type Phylum Juniper Staminate cone Ovulate cone fern sago horsetails moss monocot dicot 56 LAB 8: PLANT DIVERSITY PART 2 INTRODUCTION The angiosperms, seed plants that produce flowers, are placed in the division Anthophyta. The word angiosperm literally means "vessel seed," referring to the seeds being borne in a fruit. There are more flowering plants in the world today than any other group of plants. Assuming that numbers indicate success, it must be said that flowering plants are the most successful plants to have evolved. The most important characteristic that distinguishes the Anthophyta from other seed plants is the presence of flower parts that mature into a fruit, a container that protects the seeds, allowing them to be dispersed without coming into contact with the rigors of the external environment for some time. In many instances, the fruit also contributes to the dispersal of the seed. For example, some fruits stick to fur (or clothing) of animals and are brushed off some distance from the plant that produced them. Others are eaten by animals. the undigested seeds may pass out of the digestive tract, falling into an environment often far removed from the seeds' source. Our lives and diets revolve around flowering plants. Fruits enrich our lives and include such things as apples, oranges, tomatoes, beans, peas, corn, wheat, walnuts, pecans...the list goes on and on. Moreover, even when we are not eating fruits, we're eating flowering plant parts. Cauliflower, broccoli, potatoes, celery, and carrots are all parts of flowering plants. The number of different kinds of flowers is so large it's difficult to pick a single example to be representative of the entire division. Nonetheless, there is enough similarity among flowers that, once you've learned the structure of one representative, you'll be able to recognize the parts of most. Flower parts are believed to have originated as leaves modified during the course of evolution to increase the probability for fertilization. For instance, some flower parts are colorful, attracting animals that serve to transfer the sperm-producing pollen to the receptive female parts. Refer to your Atlas and study the life cycle of a typical flowering plant. Refer to it as you study the specimens in this exercise. 57 Flowering Plant Life Cycle 58 PROCEDURE 1: STRUCTURE OF THE FLOWER 1. Obtain a flower provided for dissection. The flower parts are arranged in whorls atop a swollen stem tip, the receptacle. The outermost whorl is frequently green (although not always) and is the calyx. Individual components of the calyx are called sepals. The calyx surrounds the rest of the flower in the bud stage. Use your Atlas to Identify these parts. 2. Moving inward, locate the next whorl of the flower, the usually colorful corolla made up of petals. Both the calyx and corolla are sterile; that is, they do not produce gametes. 3. The next whorl of flower parts consists of the male pollen-producing parts, the stamens (also called microsporophylls, "microspore-bearing leaves.") Examine a single stamen in greater detail. Each stamen consists of a stalklike filament and an anther. The anther consists of four microsporangia (also called pollen sacs). 4. Next locate the female portion of the flower, the pistil. A pistil consists of one or more carpels, also called megasporophylls, "megaspore-bearing leaves." If the pistil consists of more than one carpel, they are usually fused together, making it difficult to distinguish the individual components. 5. Identify the different parts of the pistil: at the top, the stigma, which serves as a receptive region on with pollen is deposited; a necklike style; and a swollen ovary. Note that the only members of the plant kingdom to have ovaries are angiosperms. 6. With a sharp razor blade, make a section of the ovary. (Some students should cut the pistil longitudinally; others should cut the ovary crosswise. Then compare the different section.) 7. Examine the sections with a dissecting microscope, finding the numerous small ovules within the ovary. Many flowers have more than one ovule per ovary. Make and label two sketches: one of the cross section and the other of a longitudinal section of the ovary. After fertilization, the ovules will develop into seeds, and the ovary will enlarge and mature into the fruit. Notice that the ovules are completely enclosed within the ovary. 59 8. There are two groups of flowering plants, monocotyledons and dicotyledons. the number of flower parts indicates to which group a plant belongs. Generally, monocots have the flower parts in threes or multiples of three. Dicots have their parts in fours or fives or multiples thereof. Count the number of petals or sepals in the flower you have been examining. Are you studying a monocot or dicot? ___________ PROCEDURE 2: MICROSPORANGIA AND THE MALE GAMETOPHYTE 1. Pollen grains are immature male gametophytes, and very small ones at that. The pollen grain consists of a large tube cell and a smaller, crescent-shaped generative cell that floats freely in the cytoplasm of the tube cell. When a pollen grain lands on the stigma of a compatible flower, it germinates, producing a pollen tube that grows down the style. The generative cell flows into the pollen tube, where it divides to form two sperm. Because it bears two gametes, the pollen grain is now considered to be a mature male gametophyte. Compare a prepared slide of Pine mature Pollen and Pine Germinated Pollen. Name at least one difference you see between the two: ___________________________________________________________ Using your Atlas, identify and sketch the pollen tubes located on the Pine Germinated Pollen slide in the space provided. 2. Tap some pollen from your flower onto a clean microslide. Add a drop of 0.5% sucrose, cover with a coverslip, and observe with the 10X objective of your compound microscope. Look for the pollen tube as it grows from the pollen grain. Set the slide aside for a bit and re-examine it 15 minutes later to see what's happened to the pollen tubes. Sketch your findings in the space provided. PROCEDURE: ROOTS, STEMS AND LEAVES 1. Obtain a prepared slide of Typical Monocot Root and, using the Atlas, identify and sketch as many structures as you can. 2. Obtain a prepared slide of Typical Monocot Stem and, using the Atlas, identify and sketch as many structures as you can. 3. Obtain a prepared slide of Typical Monocot and Dicot leaves and, using the Atlas, identify and sketch as many structures as you can. Be sure you can identify the monocot from the dicot. 4. Study the leaf type’s diagrams in your Atlas. Identify the collected specimens’ venation pattern, margin, and leaf arrangement on stems. 60 Use of Dichotomous Keys Florida has a wide variety of plant and animal life. Many species are hard to find because they are small, secretive, or confined to very small areas of the state. However, the woody plants, trees and shrubs, are usually conspicuous and several species are so widespread that most Floridians have an opportunity to encounter them often. The 14 plants described in this activity are just such species. Some, like the mangroves, are restricted to coastal areas, but others, like the sabal palm and palmetto, are found throughout the state. PROCEDURE 3 1. A series of diagrams of 14 native plants is provided. Each drawing has a blank under it. 2. Use the dichotomous key to identify each plant and write the name of the plant in the blank provided under the drawing. KEY TO PLANT DRAWINGS 1 2 3 a. Leaves large (a foot or more across), fan-like..................................... 2 b. Leaves not as above ............................................................................ 3 a. Leaves fan-like with distinct V-shaped structure from which the blade extends; plant grows as a tree in many parts of Florida........................ ...............................................................SABAL PALM (Sabal palmetto) b. Fan-like leave blade radiates from a flat point at the end of the petiole; plant grows as shrub....................SAW PALMETTO (Seronoa repens) a. Leaves needle-like ............................................................................... 4 b. Leaves not needle-like ......................................................................... 5 61 4 5 6 7 8 9 10 11 a. Needle-like leaves; needles over 6 inches long and bundled together in groups of 2 or more; fruit is a cone; tree wide spread in state.............. .....................................................................SLASH PINE (Pinus elliottii) b. Needle-like leaves short and soft; plant produces distinctive root structures called knees; grows in wet areas........................................ ..............................................BALD CYPRESS (Taxodium distichum) a. Leaves distinctly lobed......................................................................... 6 b. Leaves not lobed, although margin may be saw-toothed (serrate)..... 8 a. Lobes very deep, extending almost to midrib; leaf obviously longer than wide...................................................... TURKEY OAK (Quercus laevis) b. Leaves not as above ........................................................................... 7 a. Leaves with 3 prominent lobes; fruit winged; leaf petioles reddish...... .................................................................RED MAPLE (Acer rubrum) b. Leaves with 5 prominent lobes; fruit ball-like with sharp spike-like projections............................. SWEETGUM (Liquidambar styraciflua) a. Leaf margins serrate (saw-toothed) ................................................. 9 b. Leaf margins smooth ........................................................................11 a. Fruit consists of berries ...................................................................10 b. Fruit not berry-like .....................AMERICAN ELM (Ulmus americana) a. Leaves with large sharp-pointed serrations; berry-like fruit red in fall and winter................................................. AMERICAN HOLLY (Ilex opaca) b. Leaves only slightly serrated; fruit consists of waxy berries.............. ..........................................................WAX MYRTLE (Myrica cerifera) a. Leaves broad, at least as wide as 1/2 the length..............................12 62 12 13 b. Leaves narrow, with distinctly elongate appearance........................13 a. Leaves oval shaped; flowers large, white, and showy; fruit cone shaped with red berries..............................MAGNOLIA (Magnolia grandiflora) b. Leaves round; grape-like fruit; thick waxy stems.................................. ..........................................................SEA GRAPE (Coccoloba uvifera) a. Leaves waxy, leaf veins only slightly visible; seeds germinate while still on tree and grow into pencil-like structures before they fall off; prop roots grow from stems downward into mud; tree grows in coastal swamp forests in southern Florida........................................................................ .................................................RED MANGROVE (Rhizophora mangle) b. Leaves and fruit not as above; tree grows in coastal swamp in southern Florida; short erect "air roots" project up through the mud in a circular area surrounding tree..........BLACK MANGROVE (Avicennia germinans) 63 LAB 8: PLANT DIVERSITY 2 Name: _______________________________ Date:_______________Section: _______ 64 65 Directions: Sketch and label the following Ovary, Cross Section (Label ovary with ovules) (Label ovary with ovules) Pine Pollen Flower Pollen (Live specimen) Mature Pollen Germinated Pollen Monocot Leaf (lots of circles) Ovary, Long Section Before Sucrose After Sucrose Dicot Leaf (airplane wings) 66 Using the field notebook, choose 14 plants and list the venation and margin below. Plant Name Venation Margin 67 LAB 9: ANIMAL DIVERSITY PART 1 - INVERTEBRATES INTRODUCTION All animals are multicellular organisms and are heterotrophic, meaning that they obtain food by ingesting other organisms or their by-products. Careful study of comparative anatomy and embryology reveals many similarities in structure and development, implying an ancestral evolutionary relationship among all animals. Animals are thought to have arisen from one group of Protista (mostly unicellar and simple colonial organisms), although from which group no one knows. In this and the following lab topics, you will investigate body form and function in examples of eight major group of animals. You will use these investigations to ask and answer questions about the lifestyle of each animal and the phylogenetic, or evolutionary, relationships among the animals. In addition to phylogenetic relationships, many structural and functional adaptive themes related to the size, lifestyle, or environment of the animal appear over and over again in animal design. Thus, the study of the anatomy of animals can lead to predictions about their phylogenetic relationships and conclusions about their lifestyles and environment. In the next few laboratory periods, you will examine and dissect several animals, relating the morphology of these organisms to their body functions, lifestyles, and phylogenetic relationships. As you study each animal, relate your observations to the unifying themes of this lab: phylogenetic relationships and criteria that are the basis for animal classification, the relationship between form and function, and the relationship of the environment and lifestyle to form and function. In your comparative study of these organisms, you will investigate 13 characteristics. Before you begin the dissections, become familiar with the following characteristics and their descriptions: 1. 2. 3. Symmetry: Is the animal (a) radially symmetrical, (b) bilaterally symmetrical or (c) asymmetrical? Tissue Organization: Are cells organized into well-defined tissue layers and, if so, how many distinctive layers are present? Body Cavity: Is a body cavity present? A body cavity - the space between the gut and body wall - is present only in three-layered organism, that is, in organisms with the embryonic germ layers ectoderm, mesoderm and endoderm. There are three types of body forms related to the presence of a body cavity and its type. Use your text as a reference when determining body cavity types. (a) (b) (c) 4. Acoelomate, three-layered bodies without a body cavity. Tissue from the mesoderm fills the space where a cavity might be; therefore, the tissue layers closely pack on one another. Pseudocoelomate, three-layered bodies with a cavity between the endoderm (gut) and mesoderm (muscle). Eucoelomate (coelomate), three-layered bodies with the coelom, or cavity, within the mesoderm (completely surrounded by mesoderm). Mesodermal membranes suspend the gut within the body cavity. Openings into the Digestive Tract: Can you detect where food enters the body and digestive waste exits the body? Some animals have only one opening, which serves as both mouth and anus. Others have a body called a "tube within a tube," with an anterior mouth and a posterior anus. 68 5. 6. 7. 8. 9. 10. 11. 12. 13. Circulatory System: Does this animal have open circulation (no blood vessels) or does it have closed circulation (the blood flows entirely through vessels)? Habitat: Is the animal terrestrial or aquatic? Organs for Respiration: Can you detect the surface where oxygen enters the body and carbon dioxide leaves? Many animals use their skin for respiration. Others have special organs, including gills, lungs, spiracles and tracheae. Organs for Excretion: How does the animal rid its body of nitrogenous waste? In many animals, these wastes pass out of the body through the skin by diffusion. In others there are specialized structures, such as malpighian tubules, lateral excretory canals, lateral canals with flame cells, structures called nephridia, and kidneys. Type of Locomotion: Does the animal swim, crawl on its belly, walk on legs, burrow in the substrate, or fly? Does it use cellular structures, such as cilia, to glide the body over the substrate? Support Systems: Is their a skeleton present? Is it an endoskeleton or exoskeleton? Animals with no true skeleton may be supported by water; fluid within and between cells and in body chambers such as a gastrovacular cavity or coelom provides a "hydrostatic skeleton." Segmentation: Can you observe linear repetition of similar body parts? The repetition of similar units, or segments, is called segmentation. Segments can be more similar (as in the earthworm) or less similar (as in a lobster). Appendages: Are there appendages (organs or parts attached to a trunk or outer body wall)? If so, how many are present? Type of Nervous System: Do you see a brain and nerve cord? Is the nerve cord dorsal or ventral? As you carefully study or dissect each organism, refer to these thirteen characteristics, observe the animal, and record your observations in the Summary Table at the end of this section. You may find it helpful to make sketches of difficult structures or dissections in the margin of your lab manual for future reference. Before you begin this study, read and become thoroughly familiar with dissection techniques, orientation terms, and planes and sections of the body described in the following pages. ALWAYS WEAR GLOVES WHILE DISSECTING PRESERVED ANIMALS! 69 TERMINOLOGY AND TECHNIQUES FOR DISSECTION ORIENTATION TERMINOLOGY Orientation terminology used with quadrupeds (four-legged animals such as the fetal pig) differs from terminology used with bipeds (such as humans). Become familiar with the following terms, which refer to quadrupeds. 1. Right/left: Always refer to the animals right or left, not yours. 2. Anterior, cranial: Toward the head. 3. Posterior, caudal: Toward the tail. 4. Dorsal: Backside; from the Latin dorsum, meaning "back." 5. Ventral: Bellyside; from the Latin venter, meaning "belly." TERMS RELATING TO POSITION IN THE BODY 1. Proximal: Near the trunk, attached portion, or point of reference; for example, "The pig's elbow is proximal to its wrist". 2. Distal: Farther from the trunk, attached portion, or point of reference; for example, "The toes are distal to the ankle." 3. Superficial: Lying on top or near the body surface. 4. Deep: Lying under or below. PLANES AND SECTIONS A section is a cut through a structure. A plane is an imaginary line through which a section may be cut. Anatomists generally refer to three planes or sections. 1. Sagittal Section: Divides the body into left and right portions or halves. this is a longitudinal or lengthwise section form anterior to posterior. 2. Frontal Section: A longitudinal or lengthwise section from anterior to posterior, this divides the body into dorsal and ventral portions or halves. 3. Transverse Section: Also called a cross section, this divides the body into anterior and posterior portions or cuts a structure across its smallest diameter. 70 DISSECTION TECHNIQUES When studying the anatomy of an organism, the term dissection is perhaps a misnomer. Dissection literally means to cut apart piece by piece. In lab, however, it is usually more appropriate to expose structures rather than dissect them. Initial incisions do require that you cut into the body, but after the body cavities are opened, you will usually only separate and expose body parts, using dissection rarely. Accordingly, you will use the scalpel when you make initial incisions into the body wall of large animals, but seldom when studying small animals or organs of large animals. Scissors are used to deepen initial cuts made by the scalpel in large animals and to cut into the body of smaller animals. When using scissors, direct their tips upward to prevent gouging deeper organs. Once the animal’s body is open, use forceps and the blunt probe to carefully separate organs and to pick away connective tissue obstructing and binding organs and ducts. Needle probes are only minimally useful. Never cut away and organ or cut through a blood vessel, nerve, or duct unless given specific instructions to do so. Producing a good dissection takes time and cannot be rushed. As you study the anatomy of animals, your goal should be to expose all parts so that they may be easily studied and demonstrated to your lab partner or instructor. Before beginning your dissections, obtain a dissecting tray and cover the rubber mat with damp paper towels. It's helpful to use pins to hold body cavities open. Remember, always wear gloves and avoid directly inhaling preservative fumes. PHYLUM PORIFERA - SPONGES (SYPHA) INTRODUCTION Species of sponges in the phylum Porifera are among the simplest multicellular animals because they lack distinct organs. Phylogenetically the sponges are an offshoot of the main evolutionary patterns seen in the animal kingdom. They consist of cells surrounding a hollow central area called the spongocoel. The spongocoel is not a coelom nor a digestive system; it is part of the water movement system of the sponge. The body walls of sponges are perforated by numerous openings that connect to a canal system. The body walls of sponges are supported by a skeleton consisting of (1) calcium carbonate crystals, (2) silica crystals, or (3) fibers of a protein called spongin. These three types of skeletal elements form the basis for dividing sponges into classes. PROCEDURE 1: 1. Observe the collected sponge sample and compare your observations with Figure 7.4 p. 86 of your atlas. Sketch your observations below. 2. Observe the prepared slide of Commercial Sponge Fibers. Note the spongin fibers and sketch some spongin in the space provided. 3. Observe the prepared slide of Sponge Spicules and sketch below. 4. Complete the Summary Table, filling in all information for sponge characteristics in the appropriate row. 71 PHYLUM CNIDARIA - HYDRAS (HYDRA) INTRODUCTION The Cnidaria are animals with rudimentary organ development and are noted for their radial symmetry. The name Cnidaria comes from the term cnidocytes, which are special stinging cells found in these animals. Cnidarians have bodies consisting of two welldefined layers, the outer epidermis and an inner gastrodermis that lines the digestive system. Between these cell layers is a layer of gelatinous material called mesoglea. The rudiments of a neuromuscular system are also seen for the first time in this group. Most species in this phylum are marine; however, there are a few freshwater species, including the microscopic solitary organism Hydra. PROCEDURE 2: 1. Using LOW POWER, study a prepared slide of Hydra and compare your observations with your atlas. Sketch your observations in the space provided. 2. Study the preserved specimen of Common Jellyfish with a stereoscopic. 3. Compare the specimen to the atlas. The jelly like consistency of the medusa is due to a thick layer of mesoglea. The carbohydrates and proteins of this gel-like material are highly hydrated, and contribute most of the bulk of the jellyfish body. 4. Find the mouth at the end of the manubrium on the subumbrellar surface. Trace the digestive system into the gastric pouch to the four radial canals and to the circular canal with extensions into each of the tentacles. Differentiate between the gastric pouch and the gonads. 5. The medusa swims by slowly contracting neuromuscular cells in the body wall, which forces water out of the subumbrellar cavity. Locate the velum, the skirt-like membrane that narrows the area through with water escapes when the muscles contract, thus increasing the propulsive velocity. 6. Locate the tentacles of the jellyfish which contain batteries of cnidocytes and adhesive pads that sting and immobilize prey. 7. Observe the collected samples of coral, jellyfish and man-of-war. Complete the Summary Table for hydra characteristics in the appropriate row. 72 PHYLUM PLATYHELMINTHES - PLANARIANS (DUGESIA) INTRODUCTION Species in the phylum Platyhelminthes, the flatworms, exhibit several structural advances over the cnidarians. The nonparasitic forms contain well-developed organ systems including digestive, excretory, reproductive, nervous, and muscular systems. In the parasitic species, many of these systems have been reduced or modified through adaptation to a parasitic way of life. For example, the neuromuscular system is reduced in parasitic flatworms so that only limited movement is possible. In tapeworms, the digestive system is absent since nutrients are obtained from the host's intestine by absorption. the greatly enlarged reproductive system of parasitic species produces enormous numbers of eggs, insuring that the species will infect other hosts and continue to thrive. Planarians are free-living flatworms; that is, they are not parasitic and their body is dorsoventrally flattened. They are found under rocks, leaves, and debris in freshwater pods and creeks. They move over these surfaces using a combination of muscles in their body wall and cilia on their ventral sides. PROCEDURE 3: 1. Observe the prepared slide of Planaria, cross section through the pharyngeal region, and compare your observations with those in your atlas. Sketch your observations below. 2. Using the lowest power on the microscope, observe the prepared slide of a Taenia, gravid and compare your observations with those in your atlas. What does gravid mean?__________________________________________ 3. Sketch your observations below. 4. Complete the Summary Table row for Planarian characteristics. PHYLUM NEMATODA - ROUNDWORMS (ASCARIS) INTRODUCTION Species in this phylum show two characteristics not found in the previously studied three phyla: (1) a tube-within-a-tube body plan in which the digestive system has a separate mouth and anus, and (2) a body cavity in which the internal organs reside in a free space between the endoderm and ectoderm. Because this cavity is not lined with the mesoderm, it is called a pseudocoel. Ascaris is a roundworm, or nematode, that lives as a parasite in the intestines of mammals such as horses, pigs, and humans. Most often these parasites are introduced into the mammalian body when food contaminated with the eggs is eaten. Keep in mind the problem of adaptation to a parasitic habit as you study the structure of this animal. 73 PROCEDURE 4: 1. Observe a prepared slide of a cross section through the body of Ascaris lumbricoides, Adult Male. Compare your observations with those in your atlas and sketch the structures you can identify in the space provided. 2. Observe a prepared slide of a cross section through the body of Ascaris lumbricoides, Adult Female. Compare your observations with those in your atlas and sketch the structures you can identify in the space provided. 3. Complete the Summary Table for the roundworm characteristics in the appropriate row. PHYLUM MOLLUSCA INTRODUCTION Second only to the phylum Arthropoda in numbers of species, the phylum Mollusca includes thousands of species living in many diverse habitats. Most are marine. Others live in freshwater or on land. Many mollusks are of economical importance, being favorite human foods. The term "mollusk" means soft-bodied. Besides being soft-bodied, most mollusks share four characteristic features: (1) a hard external shell for protection; (2) a thin structure called the mantle, which secretes the shell; (3) a visceral mass in which most organs are located; and (4) a muscular foot used for locomotion. ANATOMY OF A SQUID In this exercise, you will dissect a squid, a highly active molluscan species. Although the squids do not have a hard shell, they do possess a mantle that forms a sheath around the visceral mass. Instead of a muscular foot, squids swim rapidly by jet propulsion using a siphon. The squids are marine predators. They have well-developed eyes to spot prey. 74 MATERIALS dissecting instruments dissecting pan with damp paper towel fresh squid disposable gloves PROCEDURE 5: PART I: EXTERNAL ANATOMY 1. 2. 3. 4. 5. 6. 7. 8. 9. Look at a whole specimen of a squid. Referencing your atlas, note the head, tentacles, and two large eyes. The orientation of the squid is unusual. The head and tentacles represent the ventral surface and the opposite end bearing two fins is the dorsal surface. The body is covered by a mantle that forms a sheath around the visceral mass. At the junction of the mantle and the head, on the posterior surface, find the siphon. Water is drawn into the mantle cavity through the siphon by contraction of muscles in the mantle. This allows the animal to swim rapidly by jet propulsion. Steering is accomplished by varying the direction of discharge from the funnel, by movement of the arms, and by movement of the fins. The squid's body is stiffened by a longitudinal skeleton, the quill/pen (gladius). It consists of chitin, a complex polysaccharide. Feel the quill embedded in the anterior mantle (side opposite that bearing the siphon). With your thumb index finger, remove the pen/quill (gladius) on the dorsal surface of the squid. Set aside for further usage at a later time. Locate the eight arms and two tentacles ("grasping arms") found attached to the head. Compare and contrast the two body parts. Locate the eyes. Describe their location. (Dorsal, ventral, lateral, anterior, posterior) Locate the Mouth. It is found in-between the arms of the squid. Take a metal probe and find the opening. Now with your thumb and your index finger on the ventral side, squeeze the mouth so the beak is pushed out through the opening. REMOVE THE BEAK. Locate the Chromatophores and rub them with your finger. What color do they appear? Where are they found on the body? What function do these chromatophores have and why is that useful for the squid? On the Ventral surface locate the siphon or funnel. What is this structure's function At the posterior end of the squid, locate the two fins. What are these used for? PART II: INTERNAL ANATOMY 1. On the Ventral surface directly behind the siphon take your scissors and begin to cut the mantle all the way to the posterior tip. REMEMBER TO LIFT AND CUT SO YOU DO NOT DAMAGE THE INTERNAL ORGANS. 2. Locate the muscles that attach to the siphon. 75 3. Locate the gills. They have a feather-like appearence. What is the function of the gills? Why do you think they are located inside the body? 4. At the bases of the gills, the brachial hearts should be visible. They pump blood through the gills. Squid have closed circulatory systems, unlike other mollusks that have open systems. 5. Is your squid male or female? Do you see any yellowish, translucent eggs found in the posterior end of the trunk? If yes, you have a female. If not, you have a male. 6. Finally, look centrally in the body cavity for the ink sac. It is black in color and has a tube leading to the siphon. . When disturbed, a pigment is released from the sac into the water in the mantle cavity. The cloudy suspension leaves the siphon, forming a confusing cloud that allows the squid to escape predators 7. Take the PEN or gladius that has been drying out and pole it into the ink sac. Be careful; do not get any ink on yourself. Take the tip of the pen and sign your name on a piece of paper. 8. When you are finished with your dissection, throw your squid in the trash. 9. Observed the collected specimen of the squid. 76 ANATOMY OF A CLAM (OR MUSCLE) In this exercise, you will dissect a clam (or muscle), a molluscan species with a shell made of two parts called valves. Most clams are marine, although many genera live in freshwater lakes and ponds. MATERIALS dissecting instruments dissecting pan with damp paper towel fresh clam disposable gloves PROCEDURE 6: 1. Obtain a fresh clam and microwave it for 2 minutes or until firm. 2. Observe the external anatomy of the preserved clam. Certain characteristics will become obvious immediately. Can you determine symmetry, support systems, and the presence or absence of appendages? Are there external signs of segmentation? Record observations in the Summary Table. 3. Before you continue making observations, determine the dorsal, ventral, anterior, posterior, right, and left regions of the animal. Identify the two valves. The valves are held together by a hinge near the umbo, a hump on the valves. The hinge and umbo are located dorsally, and the valves open ventrally. The umbo is displaced anteriorly. Hold the calm so that the umbo is away from your body, and cup one of your hands over each valve. The valve in your right hand is the right valve; the valve in your left hand is the left valve. The two valves are held together by two strong adductor muscles inside the shell. Compare your observations with your Atlas. 4. To study the internal anatomy of the clam, you must open it by prying open the valves. Insert the handle of your forceps or scalpel between the valves and twist it to pry the valves open further. Carefully insert a scalpel blade, directed toward the dorsal side of the animal, into the space between the left valve and a flap of tissue lining the valve. The blade edge should be just ventral to (below) the anterior adductor muscle. The flap of tissue is the left mantle. Keeping the scalpel blade pressed flat against the left valve, carefully loosen the mantle from the valve and press the blade dorsally. You will feel the tough anterior adductor muscle. Cut through this muscle near the valve. 5. Repeat the procedure at the posterior end and cut the posterior adductor muscle. Lay the clam on its right valve and carefully lift the left valve. As you do this, use your scalpel to loosen the mantle from the valve. If you have been successful, you should have the body of the clam lying in the right valve. It should be covered by the mantle. Look for pearls between the mantle and shell. How do you think pearls are formed? 77 6. Lift the mantle and identify the visceral mass and the muscular foot. Using your Atlas, locate the gills, which have a pleated appearance. Locate the mouth between two flaps of tissue just ventral to the anterior adductor muscle. Look just above the posterior adductor muscle and locate the anus. 7. Open the visceral mass by making an incision with the scalpel, dividing the mass into right and left halves. Begin this incision just above the foot and cut dorsally. You should be able to open the flap produced by this cut and see organs such as gonads and digestive gland. 8. When you are finished with the dissection, throw your clam in the trash. 9. Observe the collected specimen of a clam. 78 LAB 9: ANIMAL DIVERSITY 1 Name: _______________________________ Date:_______________Section: _______ SPONGE SPICULES HYDRA (ID tentacle, hypostome, basal disc) SPONGIN FIBERS TAENIA (ID proglottid) Ascaris lumbricoides, Adult Male (ID intestine, testis, cuticle and pseudocoel) PLANARIA (ID epidermis, pharyngeal cavity and pharynx) Ascaris lumbricoides, Adult Female (ID intestine, uterus, cuticle with eggs and pseudocoel) 79 SUMMARY TABLE Refer to your text book, course outline and atlas to complete the following ANIMAL SYMETRY TISSUE ORGANIZATION BODY CAVITY DIGESTIVE OPENINGS CIRCULATORY SYSTEM HABITAT Sponge Hydra Planarian Round worm Earth worm Clam Crayfish Grasshopper Lamprey Frog 80 SUMMARY TABLE (Continued) ANIMAL RESPIRATORY ORGANS EXCRETORY SYSTEM LOCOMOTION SUPPORT SYSTEM SEGMENTATION APPENDAGES Sponge Hydra Planarian Round worm Earth worm Clam Crayfish Grasshopper Lamprey Frog 81 LAB 10: ANIMAL DIVERSITY PART II - INVERTEBRATES PHYLUM ANNELIDA CLAMWORMS (Nereis) AND EARTHWORMS (Lumbricus terrestris) INTRODUCTION Annelids were the first animals to evolve the condition of segmentation - the division of the cylindrical trunk into a series of similar segments. Segmentation is internal as well as external, with segmentally arranged components of various organ systems and the body cavity. The coelom is more or less divided by septa into compartments within each segment, each lined by peritoneum. Unlike the proglottids of the cestodes, segments cannot function independently of each other. The circulatory system is closed, with the blood entirely contained in vessels. There is a well-developed nervous system with a central nervous system composed of two fused dorsal ganglia (brain) and ventral nerve cords. The circulatory system is not segmented. The evolutionary significance of segmentation is that each segment or group of segments can become specialized. Although this is an important characteristic of most of the phyla we have yet to study, it is barely evident in the earthworm. PROCEDURE: Observe the collected earthworm specimen and complete the Summary Table (Animal Diversity I), filling in all information for Earthworm characteristics in the appropriate row. PHYLUM ARTHROPODA INTRODUCTION Organisms in the phylum arthropoda have been very successful species. Evidence indicates that arthropods may have lived on Earth half a million years ago. They may be found in almost every imaginable habitat: marine waters, fresh water, and almost every terrestrial niche. Many species are directly beneficial to humans, serving as a source of food. Others make humans miserable by eating their homes, infesting their domestic animals, eating their food, and biting their bodies. In this exercise, you will observe the morphology to two arthropods: the crayfish (an aquatic arthropod) and the grasshopper (a terrestrial arthropod). 82 ANATOMY OF A CRAYFISH Crayfish live in streams, ponds, and swamps, usually protected under rocks and vegetation. They may walk slowly over the substrate of their habitat, but they can also swim rapidly using their tails. The segmentation seen in annelids is seen again in crayfish and all arthropods; however, you will see that the segments are grouped into functional units. MATERIALS dissecting instruments dissecting pan with damp paper towel preserved crayfish disposable gloves PROCEDURE 1: 1. Obtain a preserved crayfish, study its external anatomy, and compare your observations with your Atlas. Determine if you have a male or female. Describe the body symmetry, supportive structures, appendages, and segmentation and record your observations in the Summary Table (Animal Diversity Lab - Part 1). 2. Identify the three regions of the crayfish body: the head, thorax fused with the head), and abdomen. Note the appendages associated with each region. 3. Feathery gills lie under the lateral extensions of a large, expanded exoskeletal plate called the carapace. To expose the gills, use scissors to cut away a portion of the plate on the left side of the animal. 4. Remove the dorsal portion of the carapace to observe other organs in the head and thorax. Compare your observations with your Atlas. a. Start on each side of the body at the posterior lateral edge of the carapace and make two lateral cuts extending along each side of the thorax and forward over the head, meeting just behind the eyes. This should create a dorsal flap in the carapace. b. Carefully insert a needle under this flap and separate the underlying tissues as you lift the flap. 5. Locate the stomach in the head region. It is a large, saclike structure. It may be obscured by the large, white digestive glands that fill the body cavity inside the body wall. Leading posteriorly from the stomach is the intestine. Make longitudinal cuts through the exoskeleton on either side of the dorsal midline of the abdomen. Lift the exoskeleton and trace the intestine to the anus. (When shrimp are "deveined" in preparation for eating, the intestine is removed.) 6. Turn your attention to the anterior end of the specimen again. Pull the stomach posteriorly (this will tear the esophagus) and look inside the most anterior portion of the head. Find two green glands (they do not look green), the animal's excretory organs. 7. Observe the brain just anterior to the green glands 83 ANATOMY OF A GRASSHOPPER The grasshopper, an insect, is an example of a terrestrial arthropod. Insects are the most successful and abundant of all land animals. they are the principal invertebrates in dry environments, and they can survive extreme temperatures. They are the only invertebrates that can fly. As you study the grasshopper, compare the anatomy of this terrestrial animal with the aquatic crayfish just studied. This comparison should suggest ways that terrestrial animals have solved the problems of life out of water. MATERIALS dissecting instruments dissecting pan with damp paper towel preserved grasshopper disposable gloves PROCEDURE 2: 1. Observe the external anatomy of the grasshopper. Compare your observations with your Atlas. a. b. c. Note the symmetry, supportive structures, appendages, and segmentation of the grasshopper. Observe the body parts. The body is divided into three regions: the head, the thorax (to which the legs and wings are attached), and the abdomen. Examine the appendages on the head. Speculate about their functions, and locate the mouth opening into the digestive tract. Turning your attention to the abdomen, locate small dots along each side. (You will need a dissecting microscope or magnifying glass to see these.) These dots are spiracles, small openings into elastic air tubes, or tracheae, which branch to all parts of the body and constitute the respiratory system of the grasshopper. This system of tubes brings oxygen directly to the cells of the body. 2. Remove the exoskeleton. First take off the wings and, starting at the posterior end, use scissors to make two lateral cuts toward the head. Remove the dorsal wall of the exoskeleton and note the segmented pattern in the muscles inside the body wall. Compare your observation with your Atlas as you work. 3. The heart of a grasshopper is an elongate, tubular structure lying just inside the middorsal body wall. This probably will not be visible. 4. Locate the digestive tract and again note the mouth. Along the length of the tract are specialized regions, such as the crop, stomach, and intestine. The enlarged rectum at the end of the intestine absorbs excess water from any undigested food. The rectum leads to the anus. 5. The excretory system is made up of numerous tiny tubules, the malpighian tubules, which empty their products into the anterior end of the intestine. These tubules remove urea and salts from the blood. Locate these tubules. *Observe the collected arthropod specimens and complete the Summary Table (Animal Diversity I), filling in all information for Crayfish and Grasshopper characteristics in the appropriate row. 84 PHYLUM ECHINODERMATA - ECHINODERMS INTRODUCTION Echinoderm means "spiny skin." Members of this phylum include the sea stars, brittle stars, sea urchins, sea cucumbers, sea lilies, and feather stars. These animals are all marine, living on the bottom of both shallow and deep seas. Their feeding methods range from trapping organic particles and plankton (sea lilies and feather stars) to scavenging (sea urchins)and predatory behavior (sea stars) The echinoderms exhibit five-part radial symmetry and a calcareous (containing calcium carbonate) endoskeleton (internal skeleton) composed of many small plates. Much of the coelom is taken up by the water-vascular system, important to movement, attachment, respiration, excretion, food handling, and sensory perception. MATERIALS dissecting instruments dissecting pan with damp paper towel preserved starfish disposable gloves PROCEDURE 3: 1. Obtain a preserved specimen of a sea star and keep your specimen moist with water in a dissection pan. Referencing your atlas, note the central disk on the aboral side - the side without the mouth - the five arms, and the madreporite, a light-colored calcareous sieve near the edge of the disk between two arms. The madreporite is the opening of the water-vascular system. 2. Note the many spines scattered over the surface of the body. Near the base of the spines are many small pincher-like structures, the pedicellariae. These structures grasp objects that land on the surface of the body. 3. Locate the mouth on the oral side. Note that an ambulacral groove extends from the mouth down the middle of the oral side of each arm. Numerous tube feet extend from the water-vascular system and occupy this groove. Each tube foot consists of a bulb-like structure attached to a sucker. The amount of water in the bulb of a tube foot determines whether it applies suction to or releases suction from the substratum. the animal moves by alternating the suction and release mechanisms of the tube feet. The suction created by the tube feet also is used to adhere to the shells of bivalves as the sea star uses muscular action to pry the shells open to get at their soft insides. the tube feet, along with the skin gills, also function in the exchange of gases and NH3 excretion 85 4. With your dissecting scissors, cut across the top of an arm about 1 cm from the tip. Next, cut out a rectangle of spiny skin by carefully cutting along each side of the arm to the central disk and then across the top of the arm at the edge of the disk. Observe the hard, calcareous plates of the endoskeleton as you cut. Remove the rectangle of skin to uncover the large coelom, which contains the internal organs. 5. Cut around the madreporite to remove the upper portion of the body wall of the central disk. the mouth connects with an extremely short esophagus, which leads to the pouch-like cardiac stomach. The cardiac stomach opens into the upper pyloric stomach. A slender, short intestine leads from the upper side of the stomach to the anus. Find the two green, finger-like digestive glands in each arm, which produce digestive enzymes and deliver them to the pyloric stomach. 6. Identify the dark gonads that are located near the base of each arm. the sexes are separate but are difficult to distinguish, except by microscopic examination. 7. The water-vascular system is unique to echinoderms. The madreporite leads to a short stone canal, which in turn leads to the circular canal surrounding the mouth. Five radial canals lead from the circular canal into the ambulacral grooves. Each radial canal connects by short side branches with many pairs of tube feet. 8. The nervous system (not shown in your atlas) is simple. A circular nerve ring surrounds the mouth, and a radial nerve extends from this into each arm, ending at a light-sensitive eyespot. There are no specific excretory organs. Dispose of your dissected specimen in the trash. 9. Observe the collected Echinoderm specimens (sea star, brittle star, and sea cucumber). 86 LAB 11: ANIMAL DIVERSITY PART III - VERTEBRATES PHYLUM CHORDATA CLASS AGNATHA: JAWLESS FISHES INTRODUCTION The agnathans were the first vertebrates to evolve. They include the presentday lampreys and hagfishes. Agnatha means "without jaws,: a condition characteristic of these jawless fishes. They have a cartilaginous (made of cartilage), primitive skeleton without a cranium and with incomplete vertebrae. The hagfishes are marine, and the lampreys are represented by both marine and freshwater species. Both hagfishes and lampreys feed on the blood and tissue of fishes, rasping wounds in their sides. Hagfish actually burrow into and often through the bodies of their prey. PROCEDURE 1: 1. Examine a collected specimen of a sea lamprey. Referencing your atlas, note it’s slender, rounded body. The skin of the lamprey is soft and lacks scales. Look at the round, sucker-like mouth, inside of which are circular rows of horny, rasping teeth and a deep, rasping tongue. Although there are no lateral, paired appendages, there are two dorsal fins and a caudal fin. Note the gill slits. 2. Complete the Summary Table for the Lamprey characteristics in the appropriate row. 87 CLASS AMPHIBIA: AMPHIBIANS iNTRODUCTION The amphibians were the first vertebrates to assume a terrestrial existence, having evolved from a group of lobe-finned fishes. The paired appendages are modified as legs, which support the individual during movement on land. Respiration is by lungs, gills, and the highly vascularized skin and lining of the mouth. There is a three-chambered heart, with a doubled circulation through it. Reproduction requires water, or at least moist conditions on land. The larvae generally live in water. The skeleton is more bony than that of the bony fishes, but a considerable proportion of it remains cartilaginous. the skin is usually smooth and moist, with mucous glands; scales are usually absent. This group of vertebrates includes the frogs, toads, and salamanders. MATERIALS dissecting instruments dissecting pan with damp paper towel preserved frog disposable gloves PROCEDURE 2: 1. The leopard frog illustrates well the general features of the vertebrates and the specific characteristics of the amphibians. Study a preserved specimen after rinsing it in fresh water. Examine the external anatomy of the frog and compare your observations to your atlas. 2. Find the two nostrils at the tip of the head. These are used for inspiration and expiration of air. Just behind the eye is a disk-like structure, the tympanum, the outer wall of the middle ear. There is no external ear. The tympanum is larger in the male than in the female. Examine the frogs of other students in your lab. Is your frog a male or female? 3. At the back end of the body locate the cloacal opening. 4. The forelimbs are divided into three main parts: the upper arm, forearm, and hand. The hand is divided into a wrist, palm, and fingers (digits). The three divisions of the hind-limbs are the thigh, shank (lower leg) and foot. The foot is further divided into three parts: the ankle, sole, and toes (digits). 88 5. Fasten the frog, ventral side up, with pins to the wax of a dissection pan. Lift the skin with forceps. Then make a superficial cut with your scissors from the end of the trunk forward and just left or right of center, to the tip of the lower jaw. Pin back the skin on both sides. 6. Note the white line along the midventral line and the large abdominal muscles you have exposed. Lift these muscles with your forceps, and cut through the body wall with your scissors from the end of the trunk to the tip of the lower jaw, cutting through the sternum (breastbone) but not damaging the internal organs. Pin back the body wall as you did the skin to expose the internal organs. Refer to your Atlas to study the internal anatomy. 7. Locate the spleen and the following organs of the digestive system: mouth, tongue, pharynx (throat), liver, gall bladder, stomach, small intestine, and large intestine (colon). Go back to the liver and look underneath it to find the lungs. 8. Amphibians have a three-chambered heart, with two thin-walled atria and a thick-walled ventricle. 9. In the female, the ovaries expel eggs into the oviducts which lead to the uterus. If you have a female frog, she may be filled with black eggs. 10. Locate the fat bodies, many yellowish branched structures just above the kidneys. Their function is to store food reserves for hibernation and reproduction. PROCEDURE 3: 1. Examine preserved specimens of a variety of Chordates. 2. Make a flash card for every specimen and phylum. 89 Lab 12: Turkey Creek Sanctuary Below is a list of a few of the animals and plants commonly found in the habitats of Turkey Creek Sanctuary. Circle anything you see on this trip. Then make a phylum/division list. BIRDS Mammals Invertebrates Black-throated Blue Blue jay Cardinal Carolina wren Cormorant Cranes Egrets Hawks Herons Mocking birds Osprey Owls Florida mouse Manatee Opossum Raccoon River otter Spotted skunk Squirrel Bee Butterfly Flies Mosquitoes Naval Orchid Spider Spiders Quail Redstart Vulture Warbler White pelican Reptiles Fish Bass Sea Trout Blue gill Bream Garfish Mullet Snook Tarpon Dog Fennel Flowers/Shrubs/ect Florida Elderberry Foliose lichen Ball moss Fruticose lichen Beauty berry Giant maiden cane Blazing Star Golden polyploidy Boston Fern Gopher Apple Bracken Fern Lantana Brazilian Pepper Mistletoe Butterfly Orchid Mulberry Cactus Orchids Cogan Grass Partridge Pea Conradina Grandiflora Persimmon Crustose lichen Poison ivy Deer tongue Alligator Anoles Box turtles Florida Cooters Frogs Gopher tortoise Indigo snake Lizards Rattlesnake Scarlet King Snake Soft shelled turtle Water moccasin Prickly pear cactus Puff Ball Rag weed Red Blanket Lichen Reindeer Lichen Resurrection Fern Rosary pea Rosemary Rouge plant Rusty Lionia Saw Palmetto Scrub paw paw Serpent Fern Shelf Mushroom Shiny Blueberry Trees American Elm Chapman Oak Dahoon holly Hercules Club Hickory Laurel Oaks Live Oak Long Leaf Pine Maple Myrtle Oak Red Bay Red Maple Red Mulberry Sabal (cabbage) palm Sand Live Oak Sand pines Saw palmetto Sugarberry Swamp dogwood Turkey oak Wild Olive Shoestring fern Silk Grass Sky Blue Lupine Smilax vine Spanish moss Spanish needle (Shepherds needle) St. John’s wart Sugar Berry Virginia creeper Wild bird grape vine Wild coffee Wild pine air plant Wild Pineapple Wire grass Yucca 90 Name:_______________________________ Date:________Section:_________ LAB 12: TURKEY CREEK FIELD TRIP Summary Paragraph: Critique: 91 Using your Turkey Creek list, complete the following phylum table: COMMON NAME PHYLUM 92 LAB 13: SAMPSONS ISLAND FIELD TRIP Below is a list of a few of the animals and plants commonly found in the habitats of the Indian River Lagoon and Sampsons Island. Circle anything you see on this trip. MAMMALS manatee marsh rabbit raccoon dolphin REPTILES American alligator common garter snake rat snake black racer snake lizard MISCELLANEOUS moss lichen TREES Australian pine (exotic) Brazilian pepper (exotic) black mangrove red mangrove white mangrove cabbage palm sand pine live oak FISH snook stingray mullet pinfish pipefish seahorse BIRDS pelican cormorant egret heron gull osprey roseate spoonbill ibis stork anhinga OTHER: FLOWERS/SHRUBS beach daisy morning glory Hurcules’ club sea grape persimmon wax myrtle/bayberry INVERTEBRATES jellyfish shrimp barnacle blue crab clam fiddler crab oyster 93 Name:____________________________ Section:_______Date:__________________ LAB 13: SAMSONS ISLAND FIELD TRIP DATA RECORD SHEET Site:_____________________ Section_________ Date:_______________ Recorded by________________________________ PHYSICAL PARAMETERS Temperature Record/Circle ____________0C Wind Direction Time:_______________ CHEMICAL/BIO PARAMETERS Record/Circle Dissolved Oxygen (DO) ________mg/l Nitrate (N03-) ________mg/l Relative Strength calm slight mod.strong Nitrite (N02-) ________mg/l Conditions (Circle ALL that apply) pH Reading: Rainfall sunny cloudy partly cloudy overcast dawn dusk dry rain _________ Clorine (Cl2) Acid (0-5.9) Neutral (6.0-7.9) Base (8.0>) ________mg/l Turbidity _______ Ammonia (NH3) ________mg/l Salinity __________0/00(ppt) Phosphate (H2PO4-) Water Depth ____________meter m NTU ________mg/l 94 Using your Samson’s Island list, complete the following phylum table: COMMON NAME PHYLUM 95 Garmin Etrex PAGE UP/DOWN Buttons ENTER 1. Hold the POWER button down to turn on. Note: After a few minutes a page will be displayed TROUBLING TRACKING SATELLITES ARE YOU INDOORS NOW? POWER After a few moments the WAIT….TRACKING SATELLITES screen should appear. Selct YES, the press ENTER. If this is not the screen that you see, clcik on the ENTER button and select NORMAL SKYVIEW (use the Up/Down button to highlight NORMAL SKYVIEW, then press ENTER). 96 2. Press PAGE four times, 3. Press the PAGE button until the SATELLITE page is visible. Press the ENTER BUTTON. Use the UP/Down keys to select ADVANCED SKYVIEW. NOTE the pages that come into view: 1. Satellite Page 2. Map page 3. Pointer page 4. Menu page The black squares with numbers in represents the position of the satellites: north is up etc.; the outer circle is the horizon, the inner circle is 45o elevation; the dot is directly overhead. The satellites that are in view are also listed at the bottom of the screen. 97 4. Press the PAGE button until you get to the MENU PAGE. Use the Up/Down buttons to highlight SETUP Press ENTER Use the Up/Down buttons to highlight TIME Press ENTER Use the Up/Down buttons to highlight TIME ZONE Press ENTER Use the Up/Down button to set the TIME ZONE to US-EASTERN Press PAGE to get back to the SETUP page. This ensure that the unit reads time in the correct time-zone. 5. From the SET UP page select UNITS. Set the POSITION FRMT as UTM/UPS. Set the MAP DATUM as NAD83 Set the UNITS to METRIC Press PAGE to get back to the SATELLITE page. 6. Take the unit outside to a place where you have a clear view of all of the sky. The unit will now start collecting the radio signals from the satellites. Note how the Black boxes change to normal numbers when a satellite’s signal is obtained. The black bar above the satellite number is the strength of the signal. 98 7. When you have collected 4 satellites, the SATELLITE page will state READY TO NAVIGATE. 8. Press PAGE until you get the MENU page. Select MARK. You current position is listed at the bottom of the page Write it down here: _______________ N _______________ E Plot your position on the attached map. Note: since you have set your unit to UTM./UPS, your coorediante will look different than the image opposite. You should see 17R UTM in addition to the Easting (top number) and Northing (bottom number). 9. Use the Up/Down keys to select the name (006 in the diagram opposite). Press ENTER Highlight each letter to write HOME. Then select OK and press ENTER. 99 10. Press Page until you get the Pointer Page. Try walking in a straight line for about a minute. Note the speed and direction. This unit is not a compass. To obtain a direction it calculates the direction traveled between the last two position recorded: IF YOU WANT TO KNOW THE DIRECTION YOU ARE FACING YOU MUST CONTINUE TO WALK IN THAT DIRECTION!!!! Note: if you press ENTER, the information at the bottom of the page changes. 11. Use the MARK Page to get you position now. Write it down here: _______________ N _______________ E Plot your position on the attached map. 12. Press the POWER button down for 3 seconds to turn the unit off. 100 101 Lab 14: GPS Made Easy with Geocaching Begin with a discussion on the concept of GPS. Explain how satellites are used to find a position on earth via triangulation – therefore, a minimum of three satellites is needed to obtain a position reading. A clear view of the sky is needed for reception. A GPSr is a global positioning system receiver – the device used to actually find your position on the ground. When seeking a predetermined point, the GPSr must be in motion (i.e., you can’t stand still in one spot) to obtain accurate positioning when tracking. This is also true when using the compass feature of a GPSr. Discuss how GPS is used in conjunction with GIS – how many layers of data can be used to paint a particular picture. Discuss the many applications for GIS in the natural and built environment (e.g., mark trails, pinpoint nests, identify property boundaries, map vegetation communities, etc. etc.) Describe the sport of geocaching – a scavenger hunt using a GPSr. Information is available at www.geocaching.com or in the following pages. Geocaching can be used to introduce people to using a GPSr. Points should be predetermined in advance. For the sake of this activity, we can use several pre-exising points at Turkey Creek Sanctuary – however please note that these caches may not always be there – and you might want to check the website to make sure they are active before you head out. Usually cachers trade items in caches. You can do one of two things – either tell them not to trade any items and only sign their name to the log. Or else tell them to bring 4 trade items each (a pencil, magnet, sticker, unique coin, etc.) to trade. No food in the caches!!! The cache called “Turkey Creek Library” is a book exchange cache, so people can trade paperbacks there. Divide the class into 4 groups. Have each group start at a different cache and rotate through in a sequence so that there aren’t too many people in one place at a time and there is no overlapping. The following sequence is roughly in a counter-clockwise circle around the Sanctuary. Group 1 sequence 1. Turkey Creek CITO cache 2. The Turkey Creek Library 3. Overlooking a Turkey 4. After a Morning on the Water Group 3 sequence 1. Overlooking a Turkey 2. After a Morning on the Water 3. Turkey Creek CITO cache 4. The Turkey Creek Library Group 2 sequence 1. The Turkey Creek Library 2. Overlooking a Turkey 3. After a Morning on the Water 4. Turkey Creek CITO cache Group 4 sequence 1. After a Morning on the Water 2. Turkey Creek CITO cache 3. The Turkey Creek Library 4. Overlooking a Turkey Some tips to remember: − GPSr will not work as well under tree cover – try and keep as clear a signal as possible. − Keep in motion when tracking to keep the arrow pointing in the right direction. − Triangulate to find an accurate position – you will often have to narrow down the location by approaching from a couple of different directions. − The BCC GPSrs will probably only have an accuracy of about 10-30 feet. When you get close and have triangulated into an area, put the device down and use your eyes! − Minimize trampling of any area that is off the trail. Do not litter. In fact, take trash out with you! Stress the concept of zero-impact hiking. 102 Turkey Creek geocaches These are the coordinates for 4 caches in the Sanctuary. They should plug in the coordinates for each cache and use the GPSr to find their way there. It might be a good idea to do a practice cache first, to bring someone experienced with you (several of the Sanctuary staff have done this at least once before), and/or to have practiced using a GPS. You might want to visit these sites and print out a hard copy of the full description for each one. This will give you some hints as well as details about what to look for. Turkey Creek CITO cache N 28° 00.991 W 080° 36.205 http://www.geocaching.com/seek/cache_details.aspx?guid=f3156461-1e9a-4ad4-8f74-e6bc9e1c46ea The Turkey Creek Library N 28° 00.862 W 080° 36.262 http://www.geocaching.com/seek/cache_details.aspx?guid=bb409e74-2dc2-4e94-bb4337c0ac1303dd Overlooking a Turkey N 28° 00.985 W 080° 36.052 http://www.geocaching.com/seek/cache_details.aspx?guid=e389a85b-65f4-47e4-9060-204ed40c5b12 After a Morning on the Water N 28° 01.069 W 080° 35.905 http://www.geocaching.com/seek/cache_details.aspx?guid=a4155d92-1630-40da-bc07-8f5982a0a28e If the students want to log their visits when they get home, the can visit www.geocaching.com to set up an account. But that should not necessarily be a part of the classroom activity – and I would only encourage it if the people would like to geocache elsewhere another time. You may want to get some real geocachers to help out with this – each team would benefit from having an experienced GPS user to give them some tips. You can contact Mark Petrillo at 848-3777 and he will try to get some assistants out to help you on the day of the field trip if possible. 103 From http://www.geocaching.com/faq/ What is Geocaching? Geocaching is an entertaining adventure game for gps users. Participating in a cache hunt is a good way to take advantage of the wonderful features and capability of a gps unit. The basic idea is to have individuals and organizations set up caches all over the world and share the locations of these caches on the internet. GPS users can then use the location coordinates to find the caches. Once found, a cache may provide the visitor with a wide variety of rewards. All the visitor is asked to do is if they get something they should try to leave something for the cache. How do you pronounce Geocaching? You pronounce it Geo-cashing, like cashing a check. Are there any other names for Geocaching? The GPS Stash Hunt, Global Positioning Stash hunt is interchangable. Geocaching has become the standard for the game, however. The word Geocaching broken out is GEO for geography, and CACHING for the process of hiding a cache. A cache in computer terms is information usually stored in memory to make it faster to retrieve, but the term is also used in hiking/camping as a hiding place for concealing and preserving provisions. So what's the big deal? You gave me the coordinates so I know where it is. Seems pretty easy. It is deceptively easy. It's one thing to see where an item is, it's a totally different story to actually get there. What is a GPS device? A GPS unit is a electronic device that can determine your approximate location (within around 6-20 feet) on the planet. Coordinates are normally given in Longitude and Latitude. You can use the unit to navigate from your current location to another location. Some units have their own maps, built-in electronic compasses, voice navigation, depending on the complexity of the device. You don't need to know all the technical mumbo jumbo about GPS units to play Geocaching. All you need to do is be able to enter what is called a "waypoint" where the geocache is hidden. What are the rules in Geocaching? Geocaching is a relatively new phenomenon. Therefore, the rules are very simple: 1. Take something from the cache 2. Leave something in the cache 3. Write about it in the logbook Where you place a cache is up to you. 104 What is usually in a cache? A cache can come in many forms but the first item should always be the logbook. In its simplest form a cache can be just a logbook and nothing else. The logbook contains information from the founder of the cache and notes from the cache's visitors. The logbook can contain much valuable, rewarding, and entertaining information. A logbook might contain information about nearby attractions, coordinates to other unpublished caches, and even jokes written by visitors. If you get some information from a logbook you should give some back. At the very least you can leave the date and time you visited the cache. Larger caches may consist of a waterproof plastic bucket placed tastefully within the local terrain. The bucket will contain the logbook and any number of more or less valuable items. These items turn the cache into a true treasure hunt. You never know what the founder or other visitors of the cache may have left there for you to enjoy. Remember, if you take something, its only fair for you to leave something in return. Items in a bucket cache could be: Maps, books, software, hardware, CD's, videos, pictures, money, jewelry, tickets, antiques, tools, games, etc. It is recommended that items in a bucket cache be individually packaged in a clear zipped plastic bag to protect them. What shouldn't be in a cache? Use your common sense in most cases. Explosives, ammo, knives, drugs, and alcohol shouldn't be placed in a cache. Respect the local laws. All ages of people hide and seek caches, so use some thought before placing an item into a cache. Food items are ALWAYS a BAD IDEA. Animals have better noses than humans, and in some cases caches have been chewed through and destroyed because of food items in a cache. Please do not put food in a cache. Where are caches found? The location of a cache can be very entertaining indeed. As many say, location, location, location! The location of a cache demonstrates the founder's skill and possibly even daring. A cache located on the side of a rocky cliff accessible only by rock climbing equipment may be hard to find. An underwater cache may only be accessed by scuba. Other caches may require long difficult hiking, orienteering, and special equipment to get to. Caches may be located in cities both above and below ground, inside and outside buildings. The skillful placement of a small logbook in an urban environment may be quite challenging to find even with the accuracy of a gps. That little logbook may have a hundred dollar bill in it or a map to greater treasure. It could even contain clues or riddles to solve that may lead to other caches. Rich people could have fun with their money by making lucrative caches that could be better than winning the lottery when you find it. Just hope that the person that found the cache just before you left a real big prize! Can I move a cache once I find it? Unless there's a note in the cache containing instructions on moving it to a new location, don't move the cache! Responsible cache owners check on their caches occasionally and would be alarmed to find theirs missing. Are there any variations in the game? YES! We strongly encourage it, actually. Geocaching is a game that constantly reinvents itself, and the rules are very flexible. If you have a new idea on how to place a cache, or a new game using GPS units, we'd love to hear about it. 105 Some examples • Offset Caches - They're not found by simply going to some coordinates and finding a cache there. With the Offset Cache the published coordinates are that of an existing historical monument, plaque, or even a benchmark that you would like to have your cache hunter visit. From this site the cache hunter must look around and find offset numbers stamped/written in or on some part of the marker site, or continue based on instructions posted to geocaching.com • Multi-caches - The first cache gives coordinates (or partial coordinates) to the next location, or multiple caches have hints to the final cache. • Virtual caches - A cache is actually an existing landmark, such as a tombstone or statue. You have to answer a question from the landmark and let the "cache" owner know as proof that you were there. How long do caches exist? It all depends on the location of the cache and its impact on the environment and the surrounding areas. Caches could be permanent, or temporary. It's up to the cache owner to periodically inspect the cache and the area to ensure that impact is minimal, if not nonexistant. When you find a cache, it's always a good idea to let the cache owner know the condition as well. Periodically, Geocaching.com will review each cache to ensure that everything is still current. We cannot guarantee that a cache will exist at any given time, but we'll do our best to ensure the list is as current as possible. If you do find that a cache is missing/defaced, please let the cache owner know as soon as possible! What do I do if I find out that a cache has gone missing? If you visit a cache location and the cache is missing, always make sure to log the cache as "not found" on the web site so the cache owner knows. If you notice that the logs show an unusual number of "not found" logs, please inform this web site so we can check on the cache page. The cache can be temporarily disabled so the cache owner can check in on it. Sometimes, though rarely, when the cache owner cannot be contacted we can either allow folks to adopt the cache or have the cache removed completely from the site. We rely a lot on the geocaching community to let us know the status of caches in their area. 106 From http://www.geocaching.com/about/finding.aspx Finding your first Geocache Step 1 – Researching the Cache Keep in mind that distances can be deceiving. When you’re using your GPS unit to find a cache, the unit only knows how close the site is as the crow flies (a direct line). You may be a mile from the cache, but there may be a river in the way, or a near-vertical climb involving 3 miles of switchbacks, or a mountain – you get the picture. 1. Buy a map of the area from your local camping store for those caches that are off a trail or too remote to drive close to. Topographical maps (which show features of the land like hills) are best, so you can get a good idea of the terrain you’ll be crossing. You’ll also know whether to bring your Honda Civic or rent a Land Rover. 2. For car geocaches (ones you can drive to and walk a short distance), use MapBlast. Geocaching.com provides you with a link to MapBlast so you can get directions to that location. Make sure to zoom in on the location to make sure it’s near a road. MapBlast can only get you so far! 3. If you have a good idea of the area, you can navigate via the GPS unit. This is best when the park is small. This is also the most challenging, and is not recommended for your first hunt. You’ll most likely need to do all three things to prepare and reach the geocache, though our experience has shown different combinations for each cache. I’d always start with one of the online map sites first to get an idea of the area, then decide on whether you need to buy a map or use what you have. Since this is your first time, it’s also ok to read the stash notes, look at a picture of the cache, or read other people’s experiences finding the cache. Some may be visible from 20 feet away, while others in more trafficked areas may be buried under some rocks (or in one case, in a World War II bunker!). Getting within a mile or two of the site isn’t usually too difficult – it’s the last mile that’ll get you every time. Step 2 – Preparation Preparation is key in any kind of outdoor activity, but you can never stress enough the importance of preparation and safety. Keep these tips in mind when searching for a cache: 1. Have a buddy with you! Never go off into the woods or remote locations without a partner, especially when Geocaching. We don’t want you focusing on your GPS unit and walking off a cliff. It’s great fun, so think about planning a camping trip around the stash hunt with your family or friends. 2. Many of the caches are off-trail, so make sure to be aware of your surroundings. If you’re concentrating on your GPS unit, look around you occasionally for holes, bears, etc. 3. Bring and drink plenty of water, and don’t drink directly from a stream! For some of the more difficult trips, bring a water filtration system. You can get them at most camping stores. 4. Let someone know where you’re going and when you’re coming back. Step 3 – The Hunt Now you’re ready for the hunt. 107 1. It should be pretty straightforward to get within a mile or so from the cache (unless it’s deep off-trail). If you’ve done your research, follow the map more than the GPS unit (although we keep ours on the whole time). It’s inevitable that you’ll lose signal from overhanging trees, mountains, etc. 2. If you’re using USFS roads (US Forestry service), the signs for each road can be pretty small in size. Instead of street signs, they’re brown signs that have white writing running vertically. Usually they’re close to the ground. Sometimes you may have to backtrack on the road to locate them. 3. It’s always good to have a compass on hand if your GPS unit doesn’t have one. 4. When you leave your car, mark its location as a waypoint! Sounds silly, but once you get deep into the cache hunt, it’s easy to get disoriented. We’ve learned this from experience! 5. When you get close to the Geocache (within 300 feet, which is the length of a football field), make sure to check your GPS unit signal. Sometimes the signal will have an error between 25-200 feet. Don’t concentrate as much on the arrow as the distance decreasing, as you get closer to the site. 6. For the last 30 feet, use a compass or direct your buddy in the direction of the cache. In some cases we’ve had good luck circling the site with the GPS unit to get a good area to search. 7. The final 30-100 feet is the hardest. It helps to think like the person who hid the cache. If there are stumps around, investigate around the base. Check for a pile of rocks. Some stashes, especially in people-trafficked areas, are pretty ingeniously hidden, so it helps to know the container they used. Step 4 – The Find Huzzah! You found the cache! Congratulations! Now what? • Usually you take an item and leave an item, and enter your name and experience you had into the log book. Some people prefer to just enter their name into the log book. It’s an accomplishment enough to locate the cache. • Make sure to seal the cache and place it back where you found it. If it had some rocks covering it, please replace them. It’s pretty straightforward. • Remember that waypoint we suggested you create where your car/trail was located? Use that now to get back! You’ll be glad you had it. • When you get home, email the person who hid the cache and let them know you found it! They’re always happy to know the condition of their cache and it’s nice to know that people are looking for them. Great work! After several trips to geocaches in your area, you’ll be ready to place your own. Welcome to the exciting world of Geocaching! 108 LAB 15: FIELD ECOLOGY INVESTIGATION (MAKE-UP/ALTERNATE LAB IN THE EVENT OF RAIN) This is an outdoor investigation. Take a slow walk through an ecosystem of your choice and make the following observations and conclusions. I. TYPE OF ECOSYSTEM:___________________________ II. LIMITING FACTORS AND HABITATS: A. Describe the physical environment: _______________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ B. Describe the biological environment: _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ C. Describe any instances of competition between species for light, space and/or water: _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ D. Find a moist, shady, cool habitat. Record the life forms you find there: _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ 109 E. Find a dry, sunny, warm habitat. Record the life forms you find there: _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ F. Are there different organisms for each habitat? Explain: _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ G. Was any organism found in both habitats? Explain: _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________________ III. FOOD RELATIONSHIPS: A. Observe and record three producers, three primary consumers and three secondary consumers: B. PRODUCERS 1ST CONSUMERS 2ND CONSUMERS 1._____________ 1._____________ 1._____________ 2._____________ 2._____________ 2._____________ 3._____________ 3._____________ 3._____________ Design a food web showing the food relationships between these life forms. 110 GENERAL BIOLOGY II LABORATORY PRACTICAL I REVIEW 1. Given titled prepared slides, identify the following as representatives of Kingdom Protista and: • a diatom as Phylum Chrysophyta. • an amoeba as Phylum Rhizopoda with pseudopod • a Euglena as Phylum Euglenophyta and as a flagellate. • a Paramecium as Phylum Ciliophora and as a cilliate. • Chlamydomonas (unicellular), Volvox (colony) and Spirogyra (filamentous) as Phylum Chlorophyta. • Spirogyra conjugation tube. 2. Given live specimens, identify the following as • types of lichen e.g. foliose, fruticose or crustose • Bread mold as Phylum Zygomycota • Baker's yeast as Phylum Ascomycota • Edible mushrooms as Phylum Basidiomycota; pileus, gills, & stipe • Physarum slime mold as Phylum Myxomycota • Dictystelum slime mold as Phylum Acrasiomycota 3. Given herbarium specimens, identify • the phyla (angiosperms are Phylum Anthophyta) • (d) pine cones as staminate vs. ovulate cone. • (e) flower pistil (stigma, style & ovary) and stamen (anther & filament) • Moss gametophyte v. sporophyte 4. Given titled prepared slides, identify the following as fungi or plants and • • • • • • • • • • • • Rhizopus as Phylum Zygomycota & sporangium, zygosporangium, hypha Peziza as Phylum Ascomycota and asci and ascospore Coprinus as Phylum Basidiomycota, basidia and basidiospore from gill Marcantia as Phylum Hepatophyta & air pore Lichen Thallas – algal v. fungal layers Moss archegonia v. antheridia; identify egg v. spermatogenous tissue Fern sori – sporangia and spores Fern – archegonia v. antheridia; identify egg v. spermatogenous tissue Pine stem – xylem v. phloem Pine leaf – stoma Pine cone – ovule v. microsporangium Monocot v. dicot 5. Given a dicotomous key, identify herbarium specimens. 6. Given herbarium specimens, identify leaf venation, margin and stem arrangement and phylum. 111 GENERAL BIOLOGY II LAB PRACTICAL II REVIEW 1. Identify the phylum of each collected specimen on display. 2. Identify the phylum to which the following slides belong and the structures listed: • Commercial Sponge Fibers: spongin vs. spicules and function • Hydra: tentacle, hypostome, basal disc • Planaria: epidermis, pharyngeal cavity, pharynx • Taenia: proglottid • Ascaris, male: intestine, pseudocoel, testis • Ascaris, female: intestine, pseudocoel, uterus, cuticle with eggs 3. From either a whole specimen or a dissected specimen, identify the parts listed: • Clam: hinge, foot, mouth, gonad, digestive gland, gills, mantle • Crayfish: external parts - Antenna, eye, cephalothorax, abdomen, carapace, swimmerets, walking legs, tail/telson; internal parts - gills, brain, green (excretory) glands, stomach, intestine, abdominal muscles • Grasshopper: external parts - Antenna, compound eye, mouthparts, spiracles, head, thorax abdomen, forewing, hindwing; internal parts crop, stomach, intestine • Starfish: arm, madreporite, central disk, spines, tube feet, mouth, ambulacral ridge, digestive gland, gonad • Frog: mouth, lungs, heart, gall bladder, stomach, spleen, small intestine, large intestine, kidneys, liver • Squid: head, arms, tentacles, eyes, mouth, mantle, fins, siphon/funnel, gills, brachial hearts, ink sac • Common Jellyfish: mouth, tentacles, ring/circular canal, radial canals, gonad in gastric pouch 112