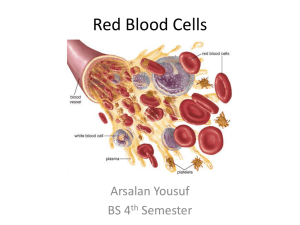
Hemoglobin: Structure, Function, and Metabolism
advertisement

Structure of hemoglobin • hemoprotein Structure, function, and metabolism of hemoglobin (complex protein: globin + prosthetic group) • quaternary structure: 4 subunits • prosthetic group of each of the subunit = heme Vladimíra Kvasnicová 4 polypetide chains 4 molecules of heme 4 ferrous (Fe2+) ions HEME hemoglobin MYOGLOBIN • it has not a quarternary structure: only 1 polypeptide chain • found in muscles: binds O2 „for storrage“ Mr = 64 500 The figure is found at http://dtc.pima.edu/~biology/202alpha/lesson1/hemoglobin.jpg (March 2007) • higher affinity to oxygen than hemoglobin The figures are found at http://www.virtuallaboratory.net/Biofundamentals/lectureNotes/AllGraphics/myoglobinSurface.jpg and http://courses.washington.edu/conj/protein/hemo.gif (March 2007) Types of hemoglobin and its subunits • adult hemoglobin: HbA1 = α2β2 HbA2 = α2δ2 (∼ 2% from total Hb of adults) • fetal hemoglobin HbF = α2γ2 ! higher affinity to O2 than HbA ! binds oxygen more firmly at lower pO2 (placenta!) The figure is found at http://www.labcorp.com/datasets/labcorp/html/img/fethgb.jpg (March 2007) Structure of heme Pyrrole • cyclic tetrapyrrole • the pyrrols has different substituents • belongs among porphyrins (heme = Fe-protoporphyrine IX) • it contains: conjugated double bonds → red color 4 nitrogen atoms (N) 1 ferrous ion (Fe2+) → in the middle of the tetrapyrrole structure by coordination-covalent bonds hemoglobin The figures are found at http://www.medical-definitions.net/images/hemoglobin.jpg and http://omlc.bme.ogi.edu/spectra/hemoglobin/hemestruct/heme-struct.gif (March 2007) Pyrrole Qiuz The figures are found at http://www.medical-definitions.net/images/hemoglobin.jpg and http://omlc.bme.ogi.edu/spectra/hemoglobin/hemestruct/heme-struct.gif (March 2007) Synthesis of hemoglobin • bone marrow • in erytroblasts, not in erythrocytes • 4 individual subunits are connected by noncovalent bonds to form tetramer of Hb • hemoglobin is an intracellular protein: within ery concentration of Hb in blood: female 120 – 162 g/l male 135 – 172 g/l 1. What is the concentration of Hb in blood? 2. Describe the structure of Hb 3. Where is oxygen bound into Hb? 4. How many O2 can be bound to Hb? 5. Draw the saturation curve of Hb Synthesis of hemoglobin Synthesis of heme - REPETITION Disorders: • THALASSEMIA = group of genetically determined disorders: absence or reduced synthesis of a globin chain (α or β thalassemia) • mainly in the bone marrow (Hb) and in the liver (cytochroms) • mitochondria / cytoplasm / mitochondria • substrates: succinyl-CoA + glycine • ANEMIA (= decreased oxygen-carrier capacity of blood) • important intermediates: sideropenic anemia – insufficient concentration of Fe δ-aminolevulinic acid (= 5-aminolevulinic, ALA) sickle cell anemia – point mutation in the β-globin gene forms abnormal HbS (Glu → Val) porphobilinogen (PBG = pyrrol derivative) uroporphyrinogen III (= 1st porphyrinogen – precursor of heme) protophorphyrine IX (= direct precursor of heme) Synthesis of heme - regulation ALA-synthase the key regulatory enzyme in all tissues pyridoxal phosphate dependent (vit. B6) ALA-synthase 1 (liver) inhibited by heme (feed back inhibition) regulation of transcription and by allosteric mechanism some drugs ↑ amount of ALA-synthase (↓ conc. of heme) ALA-synthase 2 (erythroblasts) neither feed back inhibition nor induction by drugs regulated on the level of iron availability The figure is from: Color Atlas of Biochemistry / J. Koolman, K.H.Röhm. Thieme 1996. ISBN 0-86577-584-2 Disorders of heme synthesis PORPHYRIAS • inborn or acquired • classification by defect enzyme • accumulation of heme precursors in the body (skin) and their excretion with urine or feaces (dark color) • neurogical symptomps, photosensitivity Degradation of hemoglobin • cells of reticulo-endothelial system (RES) of spleen, bone marrow, liver, and skin • Hb released from erythrocytes in blood vesels is bound by haptoglobin → RES • free heme is transported by hemopexin HEMOGLOBIN → 4x globin + 4x heme • globins chains → amino acids lead poisoning – accumulation of ALA (blood, urine) (inhibition of porphobilinogen synthase) • heme → Fe3+ + CO + biliverdin→bile pigments→feaces Qiuz The figure is from: Color Atlas of Biochemistry / J. Koolman, K.H.Röhm. Thieme 1996. ISBN 0-86577-584-2 1. Where is Hb synthesized? 2. What failures of Hb synthesis do you know? 3. What substrates are needed for the synthesis of heme? 4. What is the source of iron for the synthesis of heme? 5. What is the cause of jaundice during an excessive degradation of erytrocytes? Transport of blood gases Transport of blood gases Air composition: 78% N2 21% O2 1% water, inert gases, CO2 (0,04%) pO2 Air pressure: arterial blood venose blood 13,33 kPa 5,33 kPa 100 mmHg 40 mmHg 5,33 kPa 6,13 kPa 40 mmHg 46 mmHg 1 atm = 101 325 Pa (~ 101 kPa) = 760 Torr (= mmHg) pCO2 1 mmHg = 0,1333 kPa 1 kPa = 7,5 mmHg (alveols) Transport of blood gases - function of hemoglobin • it transports O2 and part of CO2 (and CO) • it binds H+ (reacts as a buffer) • O2 and CO: bound to Fe2+ in heme → 4 O2 / 1 Hb „oxyhemoglobin“ HbO2 /„carbonylhemoglobin“ COHb • CO2 is bound to globin! (-NH2 of side chains of amino acids) „carbaminohemoglobin“ HbCO2 • H+ is bound to residues of His „deoxyhemoglobin“ HHb The figure is found at http://people.eku.edu/ritchisong/RITCHISO//301notes6.htm (March 2007) Transport of blood gases - transport of CO2 1. largely in a form of HCO3- (~ 70%) CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+ enzyme: carbonic anhydrase spontaneous dissociation (in erytrocytes) 2. bound to hemoglobin (~ 23%) 3. freely dissolved (~ 7%) The figure is found at http://fig.cox.miami.edu/~cmallery/150/physiol/sf41x11.jpg (March 2007) Transport of blood gases - reactions in erytrocytes - O2 tissues: CO2 + H2O → H2CO3 → HCO3- + H+ H+ + HbO2 → HHb + O2 → aerobic metabolism lungs: HHb + O2 → HbO2 + O2 H+ H+ + HCO3- → H2CO3 → H2O + CO2 → excreted The figure is from http://science.kennesaw.edu/~jdirnber/Bio2108/Lecture/LecPhysio/42-29-BloodCO2Transport-AL.gif (March 07) Hemoglobin saturation curve - saturation with oxygen - The figure is found at http://employees.csbsju.edu/hjakubowski/classes/ch331/bind/MbHbbindcurve.gif (March 2007) The figure is found at http://dr-amy.com/rich/oxygen/fig1.gif (March 2007) HbF is left-shifted (it has higher affinity to oxygen) The figure is found at http://www.biocrawler.com/encyclopedia/Fetal_hemoglobin (March 2007) The figure is found at http://dr-amy.com/rich/oxygen/fig1.gif (March 2007) Saturation of hemoglobin by oxygen • quaternary structure of hemoglobin allosteric effect T-conformation: lower affinity to O2 (deoxy Hb) R-conformation: higher affinity to O2 (oxyHb) T ↔ R Hb + O2 ↔ HbO2 The figure is found at http://employees.csbsju.edu/hjakubowski/classes/ch331/bind/MbHbbindcurve.gif (March 2007) Saturation of hemoglobin with oxygen Factors affecting the saturation: alkaline pH and ↑ pO2 stabilize R-conformation (IN LUNGS) acidic pH, ↑ pCO2, ↑ temperature and 2,3-BPG stabilize T-conformation, i.e. deoxyHb (IN PERIPHERY) shift of the saturation curve toward right The animation is found at http://en.wikipedia.org/wiki/Image:Hb-animation2.gif (March 2007) Bohr´s effect = the saturation of Hb by O2 drops because lowering pH The figure is found at http://employees.csbsju.edu/hjakubowski/classes/ch331/bind/MbHbbindcurve.gif (March 2007) The figure is found at http://www.nd.edu/~aseriann/dpg.html (March 2007) Patological forms of hemoglobin Qiuz 1. methemoglobin (over 3%) 1. What is the % proportion of O2 and CO2 in air? 2. What is pO2 in arterial blood? 3. What is pCO2 in arterial blood? 4. How is CO2 transported in the human body? 5. Summarize factors decreasing the affinity of Hb to oxygen metHb Fe3+ instad of Fe2+ unable to transport oxygen !!! 2. glycohemoglobin (over 6%) HbA1c after long term increased glycemia 3. carbonylhemoglobin (over 2%) after CO poisoning 4. sulfhemoglobin, cyanhemoglobin poisoning by H2S, HCN or by cyanides COHb Qiuz 1. Compare the fetal and adult Hb 2. What is methemoglobin? 3. What is glycohemoglobin? 4. What is carbonylhemoglobin? 5. What is carboxyhemoglobin? Carbon monoxide poisoning • CO has 200x higher affinity to Hb than O2 • it forms COHb = carbonyl hemoglobin (formerly called carboxyhemoglobin) • max. allowed concentration in the air: 0.003% • intoxication by CO depends on pCO and a time of its exposition (0.04% ∼ strong headache, 2-3 hours: unconsciousness; 1% ∼ death after a few minutes) CO binds to Fe2+ instead of oxygen The figure is found at http://www.orthosmoke.org/index.php/pt/Carbon%20Monoxide (March 2007) The figure is found at http://dr-amy.com/rich/oxygen/fig1.gif (March 2007) Carbon monoxide poisoning Carbon monoxide poisoning CONSEQUENCES may result due to: • exposure to automobile exhaust • smoke inhalation • an improperly ventilated gas heater • or other appliance (incomplete burning) • decreased oxygen-carrying capacity of Hb • decreased delivery of oxygen to cells CO prevents reversible displacement of O2 on Hb CO shifts the O2-hemoglobin dissociation curve to the left CO inhibits the intracellular respiration CO may bind directly to cardiac and skeletal muscle to cause direct toxicity and to components of the nervous system to cause demyelination and neurologic symptoms Saturation of hemoglobin with CO physiological value: < 2% „cherry red coloration to the skin“ The figure is found at http://www.acsu.buffalo.edu/~lcscott/carbonmonoxide.html (March 2007) The figure is found at http://www.uhseast.com/134221.cfm (March 2007) COHb / total Hb (ratio in %) Qiuz Describe the first aid in case of an intoxication of a person by carbon monoxide The figure is from http://www.coheadquarters.com/CORisk/figco32x.htm (March 2007) Carbon monoxide poisoning TREATEMENT • fresh air • exposure to high concentrations of oxygen (the 100% oxygen is administered by a face mask) it is recommended in patients who have a history of loss of consciousness, carbonyl hemoglobin saturation greater than 25%, metabolic acidosis and cerebellar findings on neurologic exam