The Cochlea, Transduction, and Auditory
advertisement

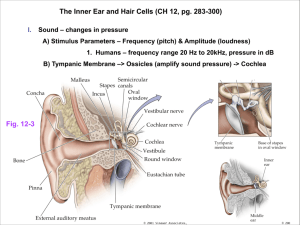
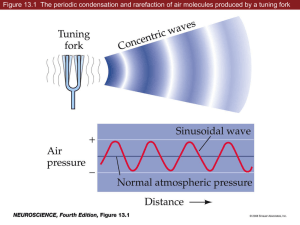
Review Resources Salamanca Study Abroad Program: Neurobiology of Hearing Must-See Websites: Journey into the world of Hearing (http://www.cochlea.org/en/spe) Auditory Animations, Univ. of Wisconsin (http://www.neurophys.wisc.edu/animations/) The cochlea, transduction, and auditory periphery The Cochlea, Fabio Mammano (http://147.162.36.50/cochlea/index.htm) R. Keith Duncan University of Michigan rkduncan@umich.edu HHMI lecture from Jim Hudspeth (http://media.hhmi.org/hl/97Lect3.html) Reviews: Gillespie et al. (2009) Cell, 139:33-44 Nouvian et al. (2006) J Membrane Biol, 209:153-165 Fettiplace and Hackney (2006) Nat Rev Neurosci, 7:19-29 Outline The auditory periphery Cochlear structure and function Cochlear fluids and endocochlear potentials Hair cell transduction Active cochlear mechanics Prestin and outer hair cell motility What does the ear have to do? Sound transmission Sound pressure transmits through incompressible fluids The identity of sounds is conveyed largely by their frequency content. The cochlea analyzes sound for frequency content, mapping frequency and energy level to a specific place and transmitting this along with timing to the CNS. spectra of the sounds coming out of the mouth spectrum of the sound produced by the glottis Overview of the cochlea Helicotrema 1. 2. 3. 4. 5. Cochlear Partition Scala Vestibuli Scala Tympani Spiral Ganglion Auditory Nerve Fibers 1 Sensory cells reside in the organ of Corti along the reticular lamina TM 1. Scala media 2. Scala vestibuli 3. Scala tympani 4. Reissner’s membrane 5. Basilar membrane 6. Tectorial membrane 7. Stria Vascularis 8. Auditory Nerve 9. Bony Spiral Lamina HeC PC DC BM Reticular Lamina Cochlear Fluids and the Endocochlear Potential Perilymph Driving Force For Potassium Organ of Corti structure hints at a division of labor among sensory cells TM: tectorial membrane BM: basilar membrane PC: Pillar cells DC: Dieter’s cells HeC: Hensen’s cells How do we know that hair cells are the transducer elements for hearing? (1) innervated, (2) loss associated with deafness Inner hair cells are primarily responsible for transmitting sound stimuli because these receive 95% of primary afferents and are more tightly associated with profound deafness. Passive cochlear mechanics Sound transmission into the inner ear sets the cochlear partition into motion. (more on this later) In mM: 145 Na+ 3 K+ 130 Cl1 Ca2+ This stimulates the sensory hair cells. Endolymph +80 mV 1: Inner hair cell 3,500 in human cochlea Primarily afferently innerv. 2: Outer hair cells 12,000 in human cochlea Primarily efferently innerv. In mM: 5 Na+ 160 K+ 130 Cl~ 0.02 Ca2+ -60 mV 0 mV General Hair Cell Structure Hair Cell & Hair Bundle Examples Hair Bundle: • Staircase (pipe-organ) of actin-filled microvilli called stereocilia • Bilateral symmetry • Stereocilia tapered at base • Tilted inward • Stereocilia interconnected • Kinocilium (true cilium) not present in mature cochlea of mammal IHC OHC Turtle VHC Bullfrog VHC Turtle VHC From Geisler text Functional differences in bundle shape? 2 Stereocilium Structure Tilney et al. (1983) Stereocilia are interconnected At least 3 types of linkages: • Tip links are arranged along staircase. • Lateral-links (or top connectors) and ankle links interconnect all neighbors (rows and staircase). • Ankle links appear transiently during development • Dense actin core • ~3000 filaments per • 18-30 form rootlet (pivot) • Hexagonally packed • Incorporation at tips • Support myosin motility • Mutations in actin assembly can cause thin/thick, tall/short, fused, or cytocauds • Links are differentially susceptible to enzyme treatment and noise trauma. • Links are important for cohesion and angled geometry of stereocilia arrangement. Breakage causes splaying of the bundle. The hair bundle moves as a unit Transduction: Methods Semi-intact preparations are used for whole-cell patch-clamp studies. Hair bundles stimulated with stiff probes or water-jets. Chick Tall Hair Cell Water-jet Stimulation 500 Hz 15º displacement Stroboscopic Lamp Transduction: Asymmetric, Saturating, Highly Sensitive Hudspeth and Corey, 1977 Bullfrog sacculus; Two-electrode recording (current-clamp) • 10 Hz triangle wave (Why use a triangle wave?) • +displ = toward tallest stereocilia • Astonishing sensitivity: threshold for change in receptor potential is ~1 pm. That corresponds to a 10 cm movement of the tip of the Eiffel Tower. • Intensity of sound (displacement of bundle) coded by a change in receptor potential…NOT in spike rate like for a neuron. Voltage-clamp: • Constrain voltage • Measure current Probe • Used to determine voltage dependence Patch Pipette Current-clamp: • Inject current • Measure voltage • Used to measure action potentials and receptor potentials Transduction: Directional Shotwell et al., 1981 3 Transduction: Fast Gating Transduction channels are located at the tip of the hair bundle Bullfrog sacculus, Voltage-clamp Activation latency: 25 ms 2 orders of magnitude smaller than latency in photoreceptors. Excludes involvement of 2nd messengers. More like mechanotransduction channels. Relaxation slower than activation, requires two exponential components, and is sensitive to Ca2+. There is an as yet unknown viscous or damping element involved in deactivation of the transduction channel. Specializations at Hair Bundle Tip Gentamicin (aminoglycoside antibiotic) used as open channel blocker of transduction channel. Jaramillo & Hudspeth, 1991 Tip-links are sensitive to Ca2+ chelators. Tip-links regenerate! Freeze Etch EM 50 nm Surface Rendering Kachar et al., 2000 Transduction channels are specifically associated with the shorter rows of stereocilia The Gating-Spring Hypothesis Beurg et al., 2009 Note: At this point, it is still unclear whether the tip-link can be the stretchy element.. Note: Transduction channel now known to be at the other end of the link. 4 The Gating-Spring Model Predicts Nonlinear Stiffness Molecular composition of the mechanotransduction apparatus • Tip-link = Cadherin23 and protocadherin15 • Both form dimers to give helical structure • Mutations in CDH23 and PCDH15 lead to deafness and tip-link loss • Binding of may be Ca2+ dependent (explains why BAPTA breaks links) • Combined structure would be VERY stiff • What then is the gating-spring? What then is the “gating spring?” Two hypothetical mechanisms have been proposed. What is the molecular nature of the transduction channel? What is the elastic element? Adaptation in transducer currents TRP? Unclear…best candidate was TRPA1; KO still transduces New possibilities? P2X? No…not right localization and not ATP gated Classic mechanically gated channels. No…ENaC KOs still transduce Blocked by tubocurarine, PPADS, Suramin. And how is it tied to channel gating? Relatively non-selective though prefers Ca2+ (200:1). Passes some large inorganic molecules (FM1-43). High single channel conductance (100 pS). Not voltage-gated. Blocked by amiloride and aminoglycosides. ENaC? Tmc1/2? Promising…Double KO eliminates transduction. Expression matches development and localization. Much work remains. Adaptation Models Displacement Stimulus fast ~ 0.1-1 ms (slow, ~ 20 ms) (fast, <1ms) Schwander et al., JCB, 2010 slow ~ 10-100 ms Fast adaptation: due to a Ca2+ dependent channel closure ( ~ 0.1 ms) Slow adaptation: depends on mysoin, ATP, and Ca2+ ( ~ 10 ms) Motor models of slow adaptation include myosin-dependent climbing/slipping of the upper tip-link density. Tension established by this process sets the resting open state of MET channels (Po ~ 0.1) 5 Hair Cell Receptor Potential Shaped by Transduction Current and Basolateral Conductances The receptor potential is governed by a variety of ion channel conductances, many of which are illustrated here. Open Questions • Mechanoelectrical transduction initiates a change in receptor potential. • Basolateral condutances set the resting potential, shape the receptor potential, and govern synaptic transmission. Lecture 2: Passive and active cochlear mechanics A place code is a general principle of many sensory systems (somatotopic, retinotopic, others?) Damage (hair cell loss) at particular locations along the cochlea is correlated with hearing loss at a particular frequency. But how is this frequency “tuning” established? What is the molecular composition of the transduction channel? Is the channel directly coupled to tip-links or is it gated by membrane stretch? In both cases, what is the equivalent of the gating-spring? Does membrane composition alter adaptation, gating-spring stiffness? Do tip-link proteins treadmill as connected side-links or form at stereocilium tip? What role does slow adaptation have for highfrequency stimuli? “Traveling Wave Era” Bekesy Cochlea Models 1 kHz Georg von Békésy (1899-1972) Awarded a Nobel prize in 1961 for studies on cochlear mechanics. See “Experiments in hearing” compiled by Glen Weaver. “Traveling Wave Era” “In situ” Measurements Tonotopic Distribution of Frequency • Improve visualization with silver grains and strobe illumination The cochlea is a frequency analyzer, breaking complex waves into frequency components. Tonos = tone; Topia = place Tonotopic = frequency place code 4 kHz • Measure amp. of motion for constant input pressure at various frequencies. 1 kHz 250 Hz Passive mechanics largely due to gradual changes in the stiffness of the basilar membrane. Base: thick and narrow Apex: thin and wide 6 A (Nonlinear) Amplifier in the Ear Gain = Amplitude of basilar membrane Amplitude of stapes motion Nonlinear Cochlear Mechanics: Compression Displacement (nm) Use laser to measure membrane at one tonotopic position. If you double the input to the basilar membrane, the output less than doubles…at some stimulus levels. Compare with stapes motion to get gain. Vary frequency of sound stimulus while keeping input (stapes motion) constant. Linear growth 1 dB/dB Sound pressure level (dB) Passive mechanics gives the “dead or high level shape” (like with Békésy). Something else happens at low sound levels in an alive animal (like with psychoacoustics). Although the cochlea is extremely sensitive, it can withstand sound pressures that are 1,000,000 times greater than sounds at the threshold for detection (120 dB). It survives by compressing the output of the cochlea for a large range of mid to high levels of input (~40 to 100 dB SPL) Cochlear mechanics is highly nonlinear. Linear vs Nonlinear Systems Nonlinear Cochlear Mechanics: Distortion Product Otoacoustic Emissions Acoustic Basilar membrane Distortion is a key feature of nonlinear systems. All combinations are generated (n*f1 ± m*f2) but cubic (2f1 – f2) is largest. Cubic Distortion Distortion products can also be measured directly on the basilar membrane! Therefore, it cannot be some strange effect of the speakers, microphones, or middle ear. OHC motility is a major contributor to the cochlear amplifer Loss of OHCs +50 mV OHC Motility -150 mV gain looks like a passive (dead) cochlea. nonlinearities (DPOAEs) lost. Bill Brownell causes a paradigm shift in 1980’s when he describes OHC motility (Scanning Electron Microscopy, 1984; Science, 1985) Change in voltage across the OHC membrane induces change in OHC length. Holley and Ashmore, 1988 • Constant volume (not osmotic effect). • Produces gating current without ion flux (not conventional ion channel). 7 OHC Motor Units OHC Nonlinear Capacitance Motor unit must be: • in the membrane, • tied to the cytoskeleton, • capable of generating large forces, • able to sense membrane voltage. IHC OHC1 OHC2 Freeze fracture electron microscopy shows densely packed particles, up to 3,000 per mm2….or about 300,000 to 6,000,000 particles per cell. OHC3 Block dominant ionic currents (K+, Ca2+). Voltage-clamp. Measure membrane capacitance (like in exocytosis). But now the change in capacitance is because of charged particles in the membrane, not fusion. (A) results from a mouse OHC. (B) Cm-Vm curves in an OHC compared with IHC. To sense membrane voltage, some part of the motor must be charged. When 6 million charges move in concert, they produce a measurable effect. We measure this as a nonlinear capacitance. The Prevailing View of the Cochlear Amplifier: Positive Feedback from OHC Motility What is the molecular nature of the OHC motor? Motility does not require ATP. Not a traditional motor. Phase-locked responses over 70 kHz. No 2nd messengers. Voltage-dependent and nonlinear. Requires extracellular anions (Cl-). Prestin, an anion sulfate transporter Active Hair Bundle Motion: Prestin is capable of motility and NLC Another Candidate for Cochlear Amplifier Motility in TSA201 cells Resonant frequency, transduction kinetics, and hair bundle stiffness changes with hair cell position along cochlea. NLC in HEK293 cells prestin Mutant and Non-transfected controls Crawford and Fettiplace, 1985 8 Active hair bundle movement involves calcium and “fast” adaptation Attempts at synergy Stimulus Added Energy Prestin knockout and knock-in models have established that this molecule is necessary for amplification, but they cannot address whether Prestin is sufficient. Current models suggest that hair bundle amplification acts as a pre-filter for somatic motility, but this issue is hotly debated. Hudspeth et al., 2000 Supplementary Information Open questions Membrane capacitance in OHCs acts like a low-pass filter with a cut-off around 4 kHz. How can the cochlear amplifier work above this frequency? Prestin is found in lower vertebrates. Are their hair cells motile? What role does it play there? External Ear Function Pinna, ear canal, and outer surface of tympanic membrane Charles Darwin (1907): the human pinna is vestigial and of no functional significance. Functions: Adornment Protect eardrum from foreign objects Cerumen (antimicrobial moisturizer) Collect sound Resonator (20 dB gain at 2.5 kHz) Sound localization cue Middle Ear Function Consists of inner surface of tympanic membrane plus ossicular chain (maleus, incus, and stapes) Conducts sound energy to inner ear. Eustachian tube equalizes pressure in tympanic cavity with atmospheric pressure (in children it’s a path for infection, leading to otitis media) 9 A model of K+ flux and electrogenics in stria vascularis and endolymph Middle Ear Function: Impedance Mismatch Ossicles arranged to pivot around a fulcrum. Cochlear fluids are denser than air. The impedance mismatch causes a loss of ~36 dB In Humans… Lever Ratio (1.3:1) Area Ratio (20:1) Another 2:1 from geometry of eardrum Lever Ratio malleus incus Area Ratio Oval Window sound waves Area = A Ear Drum Area = 20A Total 52:1 gain or 34.3 dB Electrocochleaography Cl Marginal Cell R.P. is about +90 mV! The high K+ in intermediate cells and extremely low K+ in intrastrial space gives rise to the +90 mV E.P. Auditory Brainstem Response (ABR) Cochlear microphonic What are the single-channel properties of the transduction channel? OHC Motility and Gating Charge • Pre-incubate with BAPTA. Often, single channel events could be seen. • Open probability increased when stimulated (positive displacement). • Single channel conductance is about 100 pS. • Knowing the maximum conductance of an intact hair cell gives about 1-2 channels per stereocilium. Crawford et al., 1991 10 Gating charge as a nonlinear capacitor? Is the capacitance of a cell dependent on membrane voltage? Capacitance is defined as dQ/dV. We typically ignore gating charges from the few thousand ion channels in a membrane. Therefore, membrane capacitance is generally given by: Extrinsic voltage sensor? C = eA/4pd. I- = Br- > NO3- > Cl- > HCO3- > F-. But if there are 300,000 to 6,000,000 charges all moving in concert, we must go back to C = dQ/dV. We often describe this gating current as a nonlinear capacitance. Q(Vm ) Cm dQ dVm Cytoplasmic monovalent anions are required for NLC Hypothesis: Voltage sensor and conformation change linked to anion translocation across membrane. The hypothesis (and model below) turned out to be false when Bai et al. (2009) identified and mutated the Cl- binding site, negating anion binding but preserving motility and nonlinear capacitance. Qmax 1 e (Vm V1 / 2 ) / ) Plot the shape of the derivative. Intrinsic voltage sensor? Shown: Only 1 of 4 suggested topologies Bai et al. (2009) neutralized alone or in combination many charged residues within putative transmembrane domains, causing changes in voltage response. 11