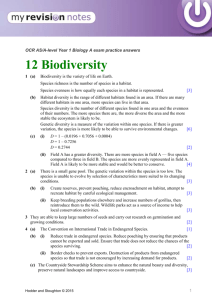
Evolution of Generalists and Specialist in Spatially Heterogeneous
advertisement

Evolution of Generalists and Specialist in Spatially Heterogeneous Environments Author(s): Peter H. Van Tienderen Source: Evolution, Vol. 45, No. 6 (Sep., 1991), pp. 1317-1331 Published by: Society for the Study of Evolution Stable URL: http://www.jstor.org/stable/2409882 Accessed: 13/09/2010 17:28 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=ssevol. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact support@jstor.org. Society for the Study of Evolution is collaborating with JSTOR to digitize, preserve and extend access to Evolution. http://www.jstor.org Evolution,45(6), 1991, pp. 1317-1331 EVOLUTION OF GENERALISTS AND SPECIALISTS IN SPATIALLY HETEROGENEOUS ENVIRONMENTS PETER H. VAN TIENDEREN' Departmentof Botany,Duke University, Durham NC 27706 USA and Institutefor Ecological Research,Heteren,THE NETHERLANDS Abstract.-Quantitative genetic models are used to investigatethe evolution of generalistsand specialistsin a coarse-grainedenvironmentwith two habitat typeswhen thereare costs attached to being a generalist.The outcomes forsoftand hard selectionmodels are qualitativelydifferent. Under softselection(e.g., forjuvenile or male-reproductivetraits)the population evolves towards the single peak in the adaptive landscape. At equilibrium,the population mean phenotypeis a compromise between the reaction that would be optimal in both habitats and the reactionwith the lowest cost. Furthermore,the equilibrium is closer to the optimal phenotypein the most frequenthabitat,or thehabitatin whichselectionon thefocaltraitis stronger.A specialistgenotype always has a lower fitnessthan a generalist,even when the costs are high. In contrast,under hard selection (e.g., for adult or female-reproductivetraits)the adaptive landscape can have one, two, or threepeaks; a peak representsa population specialized to one habitat,equally adapted to both habitats,or an intermediate.One peak is always foundwhen the reactionwiththe lowest cost is not much different fromthe optimal reaction,and this situationis similar to the soft selection case. However, multiple peaks are presentwhen the costs become higher,and the course of evolution is then determinedby initial conditions,and the region of attractionof each peak. This implies thatthe evolution of specializationand phenotypicplasticity may not onlydepend on selectionregimeswithinhabitats,but also on contingent,historicalevents (migration,mutation).Furthermore,the evolutionarydynamicsin changingenvironmentscan be widely different for populations under hard and soft selection. Approaches to measure costs in naturaland experimentalpopulations are discussed. Key words.-Cost of adaptation, genotype-environment interaction,phenotypicplasticity,quantitativegenetics. Received October 18, 1990. Accepted February 15, 1991. The adage "a jack-of-all-tradesis a masterofnone" regularlyappears in discussions on the evolution of specialization in heterogeneousenvironments(e.g., MacArthur and Connell, 1966). Yet, an organismdoes not necessarilyhave to be a master of all trades:afterall, a generalistcan exploitmultiple habitat typesor food sources,while a specialist is limited to only one or a few. The question to be addressed thenbecomes under what conditionsspecialization is expected to evolve (Levins, 1968; Futuyma and Moreno, 1988), forwhichthereappear to be two main categories(Van Tienderen, 1990). In the firstplace, individual adaptations may not be feasible;then we are in the realm of constraintson evolution,in all its possible manifestations(Maynard Smith et al., 1985; Gould, 1989). For instance,the bill length of birds may determine their feedingefficiencyon differentprey types. ' Correspondence:InstituteforEcological Research, P.O. Box 40, 6666 ZG Heteren,The Netherlands. Generalist birds with intermediate bill lengths may evolve when prey types are alike,specializationwhenmorediverseprey typesrequirehighlydissimilarbeaks (MacArthurand Connell, 1966). Furthermore, genetic polymorphism may occur under certain conditions (Hedrick, 1986; Hoekstraet al., 1985). Anothermanifestationof constraintsis a perfectgenetic correlation betweenthe expressionof a traitin two environmentsthat precludes an adaptive selectiveresponse(Via and Lande, 1985; Via, 1987). The secondreasonwhyspecializationmay evolve is thatthe costs involved in being a generalistare veryhigh.It probablycannot be assumed that,in general,adaptive phenotypic plasticityin response to environmental factorsis withoutsome cost (Bradshaw, 1965). Althoughgeneralismis often equated with plasticityin particulartraits, generalismand its costs may also be associated with the abilityto maintain homeostasisacross environments.This paper considers the evolution of a quantitativetrait 1317 1318 PETER H. VAN TIENDEREN optima different A 50 optima fitness equal B 1.0 CD3 / 20 10 10 0 1 C 12 - 08-generalist - specialist .. .specialist 1 2 0.4 ~~~~~~~~~~~~02 0.0 habitat habitat habitat in twohabitats.Optimaindicatedbysoliddots.Three Selectionfora plasticand a staticphenotype In (A), theoptimain thetwohabitatsaredifferent arepictured, twospecialists and a generalist. genotypes (10 In (B), optimalphenotypes arethesamein bothhabitats(25), and 40 respectively), i.e.,selectionforplasticity. i.e., selectionforstasis.In (C), fitness differences are associatedwithboth(A) and (B) forthethreegenotypes. The assumption of a costto simultaneous adaptationimpliesthatwithineach habitatthegeneralists have a lowerfitness thanthespecialists, despitetheirsimilarphenotypes in thefocalhabitat.The genotypes pictured arethreeexamplesoutofa potential arrayofgenotypes, ranging fromspecialists to thegeneralist. The outcome ofselectiondependsbothon thefitness withinthehabitats(C) and thewayin whichfitness within differences habitatscombinesto makefitness at thepopulationlevel. FIG. 1. in a coarse-grainedenvironmentwith two opmentcapable of producingdifferent phehabitat types,assuming that genotypesde- notypes,and mechanismsforgatheringand viating from a specific cost-freeresponse processingenvironmentalinformationmay experiencea reductionin fitness.Both se- be costly(Futuymaand Moreno, 1988). The lectionfora plastic (Fig. 1A) and a homeo- resourceinvestmentsneeded forthese two staticresponse(Fig. 1B) may lead to a sim- processes need not be the same and, moreilarpatternoffitnessdifferences betweenthe over, the two need not always be coupled. generalistand specialiststrategies(Fig. 1C). If costs are mainly due to accurate assessSelection for a homeostatic reaction (Fig. mentof the environment,a random choice 1B) may occur for example in the case of between alternativephenotypeswith fixed regulationof body temperaturein animals probabilities might be the best strategy in temperature. (Cooper and Kaplan, 1982; Lloyd, 1984). thatlive in habitatsdiffering A constant internal temperaturemay be Althoughmakingmistakescan be seen as a beneficialbecause it allows enzymesto react cost factorfora generalist,this possibility at theiroptimal temperature.However, the is not furtherconsidered;in our models aspotential to keep body temperaturecon- sessmentof theenvironmentmay be costly, stant can be achieved only by numerous but it is nonerratic. plastic anatomical, physiologicalor behavVan Alstyne(1988) studiedgrazingofthe ioral mechanisms,requiringresources,and brown alga Fucus distichus by the herbivsuch a generaliststrategymay pay offonly orous marinesnail Littorina sitkana.In the if organismsdo indeed encounterhabitats field,the phenolic concentrationsin the alin ambient temperature. differing gae varied with herbivore density,and a Fitness costs of adaptation to a hetero- herbivore-induceddefensemediatedby tisgeneous environmentmay have different sue damage was shown to exist. There is a origins.The developmentalmechanismbe- two-week time-lag in production of phehind a conditionalreactionhas two aspects: nolics after tissue damage, during which first, developmentalpathwaysmustbe pres- damaged plants are even more attractiveto ent that can resultin different phenotypes, the snails than undamaged plants (Van Aland second, the particular path followed styne, 1988). Thus, in habitats with high must depend on the environmentin which herbivoredensitiesalgae withthisinducible the genotypeoccurs. Both a flexibledeyel- systemmay be outcompetedby specialized EVOLUTION OF GENERALISTS AND SPECIALISTS 1319 algae with high phenolic concentrations, next generationis independentof the focal while in the absence of grazing they may trait (e.g., each subpopulation has a fixed have a lower fitnessthan the specialistthat "carryingcapacity"). Holsingerand Pacala cannot be induced (e.g., due to the costs of (1990) argue that this may pertain to jumale fitness, detectinggrazingand maintainingprecur- venile traitsand traitsaffecting sors,allowinga triggered response).Similar because thesetraitsmay have littleeffecton costs may occur in otherplantswithinduc- the total production of offspringin each ible defenses against pathogens or herbi- habitat. In contrast,under hard selection vores (Futuyma, 1983; Harvell, 1986; Ha- the contributionof a subpopulation to the vel, 1987; Levin, 1976). next generationdoes depend on the genoIn Figure 1 the reactions of only three typespresent,forinstanceon theirefficiency genotypesare plotted:a generalistand two in utilizingavailable resources. This may specialists.From Figure 1C it cannot be in- pertain to adult and female-reproductive ferredwhichgenotypewill win; thiswill de- traits(Holsinger and Pacala, 1990). Also, pend on the global fitnessof a genotype, under hard selection total population size averaged over the two habitats. Further- may change duringevolution, while under more,intermediategenotypesmay prove to softselectionitmayremainconstant(Chrishave an even higherfitnessthan a pure spe- tiansen, 1975; Via and Lande, 1985; Ancialist or generalist.In our approach the tonovics and Via, 1988), even thoughthe quantitativetraitis assumed to have a nor- population may be poorly adapted to one mal distributionof breedingvalues, but no or both habitats. a prioriconstraintsare imposed by limiting Dynamic equations for evolutionary the number of different genotypes,or pos- change will be derived, using standard sible phenotypes. Consequently, the pre- quantitative genetic techniques (Falconer, dicted outcome of selection is expected to 1981; Lande, 1979), withtheirassumptions depend primarilyon the selection regimes (see Via and Lande, 1985, 1987). One trait withinhabitats,and how theyare relatedto expressedin two habitatscan be viewed as selection at the population level. The pur- two characterstatesthatare geneticallycorpose of this study was to see under what related(Falconer, 1.952).The genotypicvalconditions selection leads to specialists or ues of the two characterstates(i1, Z2) have generalists,how thisrelatesto costs of phe- an additive and a nonadditive component. notypic adaptation, the frequenciesof the The expressed characterstate, z1 or z2, is different habitats,and the mode of regula- the sum of the genotypicvalue and an ention of population density(softor hard se- vironmentaldeviation. W1 and W2 are the lection).Evolutionarydynamicsare further fitnessesin habitat one and two, respecstudied in a case where environmentalfre- tively.When one assumes coststo plasticity, quencies graduallychangein time;thismay the fitnessof a genotype(il, Z2) in habitat lead to gradual or to punctuatedchangesin one, Wl(2l, Z2), dependsnot onlyon the the population, dependingon the mode of expressedphenotypezl, but also on the two regulationof populationdensity.In the dis- genotypicvalues (il, Z2), whichtogetherdecussion the importance of the results for terminethepotentialreactionofa genotype, empiricalworkare examined,and methods and thereforeprovide the link to the interforstudyingpatternsof adaptation are sug- nal costs of the reaction.Genotypeswith a gested. low-cost reaction will be favored by selection withineach habitat (Fig. 1C); whether THE MODEL such specialistsare also selectedat the level The model is a generalizationofthemod- of the global population requires further el that Via and Lande (1985) used to in- analysis. vestigateevolutionof a quantitativetraitin Soft Selection a coarse-grainedenvironment,in the absence of costs. Two modes of regulationof Lande (1979) showed, that Ai\ = gijo = gijVjln(14) gives the expected populationsize are also considered,i.e., soft ln(RW)/o9j and hard selection.Under softselection,the changein the mean value of traiti (Ai2) due contributionof each subpopulation to the to selection on traitj, where the gradient 1320 PETER H. VAN TIENDEREN operator(V1)standsfortakingthederivative withrespectto traitj, and gijis the additive geneticcovariancebetweentraiti and]. Under soft selection in two habitats, the expected selection response forthe character stateof thetraitin thefirsthabitatbecomes: AZ = qgl IVI(ln W1) + qg92V2(ln W1) + (1 - q)gIVI(ln W2) + (1 - q)g12V2(ln W2) is the same as the one obtained by Via and Lande (1 98 5). However, in theirderivation theyassumed thattherewas no selectionon traitsnot expressedin an environment[i.e., = 0]; the a ln(Wl)/022= 0, a ln(W2)/021 presentresultshows thatthisassumptionis easily relaxed. Hard Selection Under hard selection the frequenciesq withA21Ithe changein population mean for and (1 - q) referto the relativepopulation thecharacterstatein habitatone, and q and size in the two habitatsat colonization and (1 - q) the frequenciesof habitat one and before selection,and the weightingfactors two, respectively.No explicit assumptions qWllV/Wand(1 - q)JW2/Whave to be apare made regarding thegeneticvariancesand plied to,link selectionwithinhabitatsto secovariances, so that when the genetic co- lection in the entirepopulation, with W= variance between the characterstates (g12) qJWI+ (1 - q)W2. This leads to: is nonzero, directselectionon the traitexpressed in habitat two can resultin a corq(w /W)V1(ln W1) relatedresponsein thetraitexpressedin the firsthabitat.The fourcomponentsof selec- /= + (1 - q)(W22/W)V1(ln W2) G tion can easily be identified,with the first W1) q(WV /J'J)V2(ln term the result of direct selection on the + (1 q)(J'V2/ W)V2(ln W2) expressedtraitin habitatone, the second a correlatedresponse due to selectionon the G 'n1 [qqW+ (1 - q)] (2) unexpressedcharacterstate in thathabitat, term three direct selection on the unexpressed characterstate in habitat two, and The joint mean fitnessunder hard selecfinally,term four the correlated response tionequals thearithmeticmean ofthemean due to selection on the expressed trait in fitnessesper habitat, the same functionas habitat two. Under softselection,the first fora multiallelicautosomal locus. Again it two termsare weightedby the frequencyof does not depend on the assumption that the firsthabitat,the last two termsby the selection only acts on the expressed trait frequencyof the second habitat. (Via and Lande, 1985). For both traitsand usingmatrixnotation The dynamicequations forboth softand this becomes: hard selectioncan thusbe writtenin a form thatinvolves a singlefunctionforthe mean with reiv G qVI(ln W1) + (1 - q)VI(ln W2)\ population fitness,differentiated qqV2(ln W1) + (1 - q)V2(ln W2)J spect to both traits. This guarantees that when selectionis weak thepopulationmean fitness,given by these functions,increases = (1) monotonicallyduringselectionuntila local ln[W1qW21-] maximumis reached(Lande, 1979; Via and with A2 the vector of changes in the mean Lande, 1985). forboth characterstates,and G theadditive Costs ofAdaptation geneticcovariancematrixofcharacterstates. Thus the joint mean fitnessunder soft It will be assumed that the fitnessof a selectionequals the geometricmean of the genotype, W(Zl, Z2) has two components. mean fitnessesper habitat; this is identical The firstcomponentdescribes selectionon to the formuladerived fora singlemultial- theexpressedcharacterstate,and itdepends lelic autosomal locus (Cannings,1971). This on the differencebetween the expressed functiondescribesan adaptive landscape for phenotypeof an individual and an optimal softselection in a coarse-grainedenviron- phenotypicvalue forthecurrenthabitat.Asmentwithtwo habitattypes.This equation suming a gaussian fitness function, this EVOLUTION component becomes exp[- /2(zi -Z*)2/S,2] forthe traitexpressedin habitati, wherezi* and z2* are the optimal phenotypeswithin habitat one and two, respectively,and s1 and s2 (thewidthsof thegaussian functions) are inverselyrelated to the strengthof stabilizing selection on the expressed phenotypesin these habitats. The cost of a reaction to the environmentis assumed to be betweenhabassociated withthe difference itatsin average response. Consequentlythe second fitnesscomponent is a functionof the differencein the mean genotypicreaction between habitats and a reaction with the lowest (internal)costs. For a gaussian fitnessfunctionthis becomes exp[- '/2(Z2 Z2- *)2/ri2],withz2- * the cost-freerelaction and riinverselyrelatedto thestrength of selectionagainstdeviatingfromthecostfreereactionin habitati. Both a moreplastic and a less plastic reaction,relativeto Z2-1*, resultin extracosts. The cost parametersr, and r2are not necessarilyequal, forinstance in a harsh environmentthe fitnessconsequences of a specificreactionmay be more dramatic than under relatively favorable conditions. The mean fitness within habitats is given by Wi = v'[gi I I(1)i+ Pi)-I I] * exp[- /2( _- 0i)T(1i + Pi)1 *( - 0)] (3) with T denotingmatrixtransposition,01 = (Z1*, Z1I + 02 = Z2-1*), (Z2* - Z2-1*, Z2)T, P1, P2 the phenotypiccovariance matrices and 1)1, 1Q2 the selectionmatricesin habitat one and two, respectively(see Appendix). The selective forces on the two character statescan be evaluated fromthe inverseof the selectionmatrices = g (rl2 Vllrl2 + 1/s12 -l/r12) -1 _ l1r22 122-1 -\ -1 r2 2 llrl2J -1/r22 121/ r22 2 1321 OF GENERALISTS AND SPECIALISTS those associated with selection on the expressedcharacterstatedisappear. It can also be seen thatthereis correlatedselectionon z withinhabitats,since the off-diagonalelementsof the selectionmatrixare nonzero. Now thatwe have themean fitnesseswithin each habitat(Eq. 3) the next step to get dynamical results is to combine selection withinhabitats to selection at the population level, and performthe gradientoperation on the logarithmof the joint mean fitnesses(Eqs. 1 and 2), separatelyfor the softand hard selectioncase. RESULTS AdaptiveLandscape underSoft Selection Under softselection,the dynamic equations (1) are linear fora bivariate gaussian fitnessfunctionper habitat and a normal frequencydistributionof phenotypes.Consequently,the adaptive landscape has only a single peak that can be found by setting oln[WIqW2l-q])/o9i equal to zero fori = 1, 2. Figure2 gives an example oftheresulting adaptive landscape. Withoutcosts, it is the familiar bell-shaped function (Via and Lande, 1985); in this case the fitnesscontours are concentriccircles (Fig. 2A), because the frequenciesof the habitats and selectionregimeswithinhabitats are equal (q = 1 - q = 0.5, s, = S2). The second component, the cost attached to the phenotypicreaction,resultsin a ridgewiththe diagonal beingthetop oftheridge(Fig. 2B). Both fitnesscomponents togetherresultin an adaptive landscape with a single peak (Fig. 2C). The location of the peak depends on the selection parameters and the phenotypic (co)variance matrices.The solutionis quite lengthyand cannot be interpretedeasily. However, a very simple resultis obtained when the cost functionis the same in both habitats, r, = r2 = r, and the phenotypic variance in the population is relative low, i.e., P1 + 1ll - l, P2 + 2 -12: I I S22 Comparing the diagonal elements reveals thatselectionon the expressedtrait(1/r2 + 1/S,2) is moreseverethanselectionon the nonexpressedtrait(1/r2). If the cost componentis absent,ri- o?, all elementsexcept z2*Z2 -Ii zl* Z z*1 -_2 Z2- * 1 (1 -q)sI2 q(l -q)r2 + (I - q)s 12 + qS22 (4a) PETER H. VAN TIENDEREN 1322 ~~~~~~~~~70 70 60 60 50 50 50 40 40 400 30 30 30 20 20 20 10 10 10 -g, c 70 60 0 0 106 20 30 40 5'0 60 7a 0~~~~~~~~~~ 0 10 20 30 40 50 60 70 0i 0 10 20 30 40 50 60 70 trait in habitat 1 FIG. 2. Exampleof a topography of theadaptivelandscapeundersoftselection.Fitnesscontoursat 0.1 intervals,the outercontourbeing0.1. Threeadaptivelandscapesare plotted:(A) in theabsenceofcosts(with zj* = 20, z2*= 50, s1 = S2 = 10, r, = r2 ?c, q = 0.5, P1 = P2 with elementsPII = P22= 1, P12 = 0.6); (B) in theabsenceof selectionon theexpressedphenotype, of fitness i.e., involving onlythecostcomponent (as in ofbothselectionwithinhabitatsas in A and (A) exceptz2-I*= 0, s, = S2 ??, r, = r2 = 16); (C) combinatiQn costsas in B. In (C), theadaptivepeakconstitutes a compromise, locatedin betweenoptimalresponse(20, 50) and theclosestcost-free reaction(35, 35). freereaction,givenvalues forr, and r2,and the equilibriumpopulation will be close to Z2 Zi Z 2-1 optimal withinboth habitats. q22 When the elementsof the phenotypiccovariance matrixPi are largerrelativeto Q + + (4b) q(l -q)r2 (1 qS22 q)s12 thereis a wider distributionof phenotypes withii theequilibriummeanvalue forthe and the adaptive surfacebecomes smoothtraitexpressedin habitati. The lefthand er,because the population mean fitnessfolsidesof theseequationsgivethedeviation lows fromthe integrationof fitnessvalues over a widerrangeof phenotypespresentin fromtheoptimumforeachhabitat,ii -Z; betweenthereac- the population. The location of the joint relativeto thedifference tionthatcouldlead to thehighestfitnesses optimum only changes slightly,however, z2* - z1*, andthecost- and varyingthe parametervalues resultsin withinbothhabitats, freereaction,Z2-1*. The righthand side is similar changes in location (Table 1). Difa value betweenzero and one (Eq. 4a) or ferentialcostsbetweenhabitats(r, =#r2)also on affectthelocation ofthejoint optimum(Taminusone and zero(Eq. 4b), depending andthefrequenciesble 1), with the optimum closer to the optheselection parameters ofthetwohabitats.Itcaneasilybe seenthat timumforthehabitatin whichthecosts are thepopulationmeanis locatedcloseto the relativelylow (i.e., r relativelyhigh). The trajectoriesforthe change in popuoptimumin bothhabitats,(z,*, z2*), if the righthandsidesareclosetozero,i.e.,rvery lation mean duringselectiondepend on the ofthecost adaptive landscape as well as on thegenetic function largeso thatthefitness componentis veryflatand costsare low. (co)variances. However,thepopulationwill of,say,habitatone is evolve towards the optimum, unless there Also,ifthefrequency veryhigh,(1 - q) close to zero,or if the is no geneticvariance or the geneticcorreselectionin habitatone is moresevere,s1 lation between characterstates is equal to smallrelativeto s2, thepopulationmeanis one or minus-one(all leading to singularity close to the optimumin thathabitat,and of the G matrix). z*- zi - z2l farfromtheoptimumintheother relatively AdaptiveLandscapeunderHard habitat(seealso Table 1).WhenthedenomSelection inatorpartsofthelefthandsideoftheequaboth equations under hard sewithin The dynamic tionsare small,the optimum habitatscan be realizedwitha smallerand lection are nonlinear.Unfortunately,solvtherefore lesscostlydeviationfromthecost- ing forthe equilibriummean populationby EVOLUTION 70 70 Ci 60 50 C: 70 60 -60 10 20 30 40 50 60 70 0 10 304050650 40 40 0 - 20 40 1 30 30 30 20 20 20 10 10 10 0 1323 OF GENERALISTS AND SPECIALISTS 1-0 20 30 40 50 60 70 0( ~10 20 30 40 50 60 70 0 10 20 30 40 510 60 70 trait in habitat 1 FIG. 3. Exampleof a topography of theadaptivelandscapeunderhardselection.Fitnesscontoursat 0.1 intervals, theoutercontourbeing0.1. Threeadaptivelandscapesareplotted:(A) in theabsenceofcosts(with zj* = 20, z2* = 50, s, = s2 = 10, r, = r2 ?c, q = 0.5, P1 = P2 wi.thelementsPI IP22 = 1, P12 = 0.6); (B) in theabsenceof selectionon theexpressedphenotype, of fitness i.e., involving onlythecostcomponent [as in ofbothselectionwithinhabitatsas in A and (A) exceptz2-I* = 0, sI = S2 oo, ri = r2= 16];(C) combination costsas in B. In (C) threeadaptivepeaksare present, in betweentheoptimalresponse thefirst a "generalist" (20, 50) and theclosestcost-free reaction(35, 35),and two"specialists," eachcloseto beingoptimalforone of thehabitatsbutveryfarfromtheoptimumin theotherhabitat. takingthe derivativesof the mean popu- elementof P at row i, columnj, see Aplationfitness(Eqs. 2 and 3) does not lead pendix).Figure4A showsthelocationand to explicitsolutionsforpossible optima. numberofpeaksin theadaptivelandscape, Changesin theadaptivelandscapewiththe plottedagainston theabscissathestrength of themodelwerestudiednu- ofselection parameters againstdeviations fromthecostresults merically.Qualitativelydifferent werefoundcomparedto thesoftselection withan TABLE 1. The effectoftheamountofphenotypicvaricase, whichfirstwillbe illustrated example(Fig. 3). Withoutcosts,theadap- ance (P) in characterstateson thelocation oftheadaptivelandscapehas a peak at thejoint op- tive peak undersoftselection.All variables are scaled outin fourdi- in the standarddeviation unitsof the selectionwithin timum,witharmsstretching one (si). Deviationsare givenasD1 I(i1 -Z rections(Fig. 3A; Via and Lande, 1985). habitat -z [Z2* - zI* fromtheoptimum 1*11: thedeviation The costattachedto thereactionagainre- withinhabitats relative to the differencebetween the sultsin a ridge(Fig.3B). Lookingat Figures optimumand the reactionwiththe lowestcost (cf. Eq. 3A and 3B it becomesapparentthatwhen 4). D-values rangefrom0 and 1, fromoptimal forthe botharecombinedtheresultis an adaptive focal to optimal forthe alternativehabitat,respectivelandscapewiththreepeaks(Fig. 3C). With ly. No phenotypiccorrelation(P12 = 0)multiplepeaks in the adaptivelandscape, P- 0 P=1 thecourseofevolutiondependson theiniS2 D2 D2 SI ri r2 DI DI in thepopuof genotypes tial distribution 1 1 1 1 0.40 0.40 0.43 0.43 lation.Each peak has a certaindomainof q= 0.5 1 1 1 2 0.36 0.36 0.33 0.42 on theadaptivelandattraction, depending 1 1 2 1 0.36 0.36 0.42 0.33 scape, but also on the geneticcovariance 1 1 2 2 0.25 0.25 0.30 0.30 matrix. 1 2 1 1 0.18 0.73 0.23 0.69 of For further investigation oftheeffects 1 2 1 2 0.17 0.69 0.19 0.67 1 2 2 1 0.17 0.69 0.24 0.62 thedifferent on thenumberand parameters 1 2 2 2 0.14 0.57 0.19 0.56 locationofpeaksin theadaptivelandscape 1 1 1 1 0.21 0.63 0.21 0.69 0.75 q= a reference statewas takenwithbothhab1 1 1 2 0.20 0.61 0.16 0.68 itatsequallyfrequent (q = 0.5), stabilizing 1 1 2 1 0.18 0.53 0.20 0.57 selectionequal in bothhabitats(s, = S2 = 1 1 2 2 0.14 0.43 0.14 0.54 s), equal phenotypic variancematrices(P1 1 2 1 1 0.07 0.87 0.09 0.86 = P2 = P) and littlephenotypic 1 2 1 2 0.07 0.86 0.07 0.85 variation 1 2 2 1 0.07 0.82 0.09 0.80 relativeto the curvatureof the adaptive 1 2 2 2 0.06 0.75 0.07 0.76 landscape(PII= P22 = S2/8,P12 = 0, pij the a 1324 PETER H. VAN TIENDEREN freereaction(l/r),on theordinatethedif- population may evolve towards this ridge ferencebetweenthe optima withineach in the adaptive landscape, but its position on top oftheridgemay be affectedpredomhabitatand thecost-freereaction( I Z2 -z1 - Z2 1 1)- Onlyonepeakis present inregion inantlyby drift;thus experimentalistsmay betweentheoptima observe populations thatrangefromeither [G],whenthedifference reaction specialistto thegeneralistwithoutbeingable inthetwohabitatsandthecost-free in seis small.For Iz2* - z*-z2*I <2s this to explain the patternby differences of lection regimes.When costs are high,howofthestrength appearsto be irrespective adaptiveland- ever, the two specialistpeaks are separated selectionl/r.The resulting scape is similarto the softselectioncase, by deep valleys of maladaptation. Now theeffects ofvaryingtheparameters to and againtheadaptivepeakcorresponds populationthatis a compro- in this referencemodel will be dealt with. a "generalist" mise betweenthe optimawithinhabitats Figure 4B shows the adaptive peaks when and thecost-free reaction;forthesymmet- habitat one is more common (q = 0.75). state(q = 0.5, s1 = S2) and Three adaptive peaks are found under a ricalreference weakselection(P1 + Q1 ,- Q1 P2 + Q2 1- smaller range of parameter values when referencestate Q2), thepeakis locatedat thesameposition compared to the symmetric as undersoftselection.Therearetwopeaks (region[G + S1 + S2], Fig. 4B). Again only in theadaptivelandscapewhencostsofsi- one peak is found when the cost-freereacarehighandthecost- tion is close to the optimal reaction(I Z2adaptation multaneous fromthe Z1 - Z2 1* small).However,thispeaknow considerably freereactiondiffers betweentheoptimawithinhab- graduallyshiftswithincreasingIZ2 - Z1difference itats(Fig. 4A, region[S1 + S2]). At these Z2- 1 I and 1/rfroma generalist(region[G]) peaks the population is predominantly to a specialistforthe most common habitat adaptedto eitherone habitat,and can be ([S1]). There are two separate regions in referred to as "specialist"populations,in which two adaptive peaks are found,either populationof withtwo specialistpeaks (region[S 1 + S2]), contrastwiththe generalist region[G]. Finally,threepeaks are found or a combination of the generalistand the in a thirdregion[G + S1 + S2], thetwo specialistto the most common habitat (reThis occurs gion [G + S 1]). specialistsand the generalist. Figure4C shows thattherangeof paramwhenthe cost of deviationfromthecostfreereactionis low relativeto selection eter values in which three adaptive peaks withinhabitats(at least 1/r< 1/s),but at occur (region [G + Si + S2]) is again relthe same timea relatively largedifferenceativelysmall when selectionwithinhabitat betweentheoptimainbothhabitatsandthe one is more severe than in habitat two (1/ reaction(approx. IZ2*-z, - z2cost-free sI > 1/s2). In the regionwithtwo peaks, the > 3s). firstpeak is always the specialistto thehab- In somecaseswithtwoor threepeaksin itat in which selectionis less severe (in this theadaptivelandscapethesepeaksare sep- case habitat two), whereas the location of aratedbysaddlepointsthatareonlyslightly thesecondpeak is variable:a generalistwhen in fact,at the costs are low (leftpart of the region,[G + lowerin joint mean fitness; bordersoftheregions[G] and [S1 + S2] as S2], Figure4C), it graduallybecomes more wellas [G + Si + S2] and [Si + S2], the and morea specialist,thuslosingadaptation generalist peak changesfroman optimum to habitattwo, when costs are higher(right to a saddlepoint,and thenthereis a range part of the region[S1 + S2]). Again, in the of populationmeans withapproximatelyregionwithonlyone adaptive peak, a gradfrom ual shiftin location is foundfromgeneralist jointmeanfitnesses, equal population theone specialistto theother(cf.Fig. 3C). ([G]) to the specialistforhabitatone ([S1]). This is probablymoreimportant biologi- Figure 4D shows that a similar situationis observation that foundwhen deviatingfromthecost-freerecallythanthemathematical thereareexactlytwoorthreepeaks,because action is more costlyin habitatone (1l/r1> evolutionwillproceedveryslowlyin a di- l/r2).Whenever two peaks are found,one rectioninwhichtheincreaseinmeanfitness peak is the specialist to the environment is verysmall.Undersuchcircumstances a withthelowestcosts(in thiscase two),while 1325 EVOLUTION OF GENERALISTS AND SPECIALISTS A 6 5 S * N\ N 1 1+2 5 1+S2 X B 6 s1+ S1+S2 5~~~~~~~~~~2 44 GS 33 N * X 2 6 2 \ | 2 Si G+ G *N cost 0 1/8 ofdevitingfomfre 1/2 114 1 G reacton,1 2 1/8 6 '- 1/4 1/2 1 2 6 0 0 5 Si1+S2 1+S2 5 14 G Si+S2 Si1+S2 4 G+S2 E ~~~~~~~~~3 3 I~~~~~~~~~~G4-S21 4_j 0 G 1/8 1/4 cost ~ ~ 1/2 ~ 1 ~ 2 ~ 1 1/8 G 1/4 1/2 1 2 of deviating from free reaction, 1I/r FIG.4. The numberofpeaksin theadaptivelandscapeunderhardselection, plottedagainston theabscissa thestrength of selectionagainstdeviatingfromthecost-free reaction,l/r,and on theordinatethedifference betweentheoptimaperhabitatand thecost-free z *. Bothaxesexpressed z2 *- zreaction, relativeto the meanselectionwithinhabitats, s = (s, + s2)/2.Capitalletters refer to locallystableoptimain theseregions:G forthegeneralist, S forthe specialistto habitatone, S2 forthespecialistto habitattwo.(A) Symmetrical referencestate: q = 1 - q = 0.5, s1 = S2 = s, r, = r2 = r, P1 = P2. with elementsPI1 = P22 = s/8, P12 = 0. (B) Habitatone morecommon:as (A), exceptq = 0.75. (C) Stabilizing in habitatonemoresevere:as (A), selection exceptl/s1= 2 x 1/s2.(D) Costofdeviating fromthecost-free slopehigherin habitatone: as (A), exceptllr = 2 x I/r2,with r = (r1 + r2)/2.For meaningof parameterssee text. the otheris variable. In the leftpart of this + S2]). In the regionwitha singleadaptive region,the otherpeak is a generalist([G + peak, its location again varies froma genS2]); however, the location of this peak eralistto the specialist to the environment graduallychanges to a specialist with in- withthe highestcosts. creasingcosts (rightpart of the region,[SI In conclusion, generalistand specialist 1326 PETER H. VAN TIENDEREN strategiesmay occur as a single adaptive peak, in any combination of two peaks, or all threepeaks together,dependingon actual parametervalues. In general,one generalist peak is found when being adapted to both habitatsis relativelyeasy,and two specialist In betweenthese peaks whenthisis difficult. extremes,intermediatestates and combinations of state are found. diminishedpeak, does the population rapidlyevolve towardsthe new peak (Fig. 5C). Eventuallythepopulationbecomes adapted to the by then predominanthabitat. Phenotypicvariation between characterstates in the two habitats is presentonly during therelativelyshortperiod of change.Under these conditions evolution may thus be characterizedbyprolongedperiodsofstasis, interspersedwithperiods of a rapid change, Changeunder high phenotypicvariation, and low popuTrackingofEnvironmental Hard and SoftSelection lation size. This patternof evolutionarychange reSuppose thatthe frequencyof one of the two habitats changes slowly from one to sembles the episodic nature of evolution a successionalprocess,or proposed in the theoryof punctuatedequizero, representing a climatic change, and that these habitats libria (Eldredge and Gould, 1972; Gould, optimal phenotypes.Under 1980; cf. Charlesworthet al., 1982). In the have different softselection,this resultsin a gradual shift presentmodels theprocessis drivenentirely of the peak in the adaptive landscape and by selective forcesin a changingenvironthe population mean will follow this shift ment, and developmental/genetic con(Fig. 5A), provided thatgeneticvariationis straintsare notinvolved.Changestakeplace not constrainingthe evolutionaryresponse graduallywhena populationtracksa "movgenetic ing" adaptivelandscape.However,whenthe (i.e., G not singularand sufficient variationto keep up withthe change in lo- adaptive landscape is characterizedby mulcation of the adaptive peak). A difference tiple adaptive peaks that rise and fall, are betweencharacterstatesin the two habitats born or cease to be, the population may (reflectinga generalist,plastic population) remainunchangedforlong periods of time, becomes apparentat a low frequencyof the and then leap from one peak to another, second habitat, and persistsuntil the first e.g., initiatedby crossinga thresholdin the habitathas almost completelydisappeared. frequencyof habitats. The mean fitnessin the population firstdeDISCUSSION creases, remains relativelyconstant for a These models describe evolution of an longperiodoftime,and thenincreasesagain (Fig. 5B). However, rememberthat under arbitraryquantitativetrait,assuming ransoftselectionthe total population size need dom dispersal of individuals betweenhabitats,while stillindividuals spend theirlife not be affected(Christiansen,1975). Under hard selection,thepopulationalso in one habitattype.This situationmay apstartsas specialiststo theinitiallyprevailing ply to animals that live in an environment habitat (Fig. 5C), similar to softselection. with differentkinds of patches (carrion, The correspondingadaptive peak decreases dung), or to plants and othersessile organin heightas the frequencyof this habitat isms, although limited dispersal between graduallyfalls. However, neitherlocation habitats may violate the assumptions nor local stabilityof the peak is affected, (Bradshaw, 1972). The resultsshow thatuntherefore thepopulationmean remainscon- der softselection a population is expected stant and does not reflectthe ongoing en- to evolve towards a compromise between vironmentalchange.Only mean fitness(Fig. the reaction that would be optimal within 5D) and thus population size may show a each ofthehabitatsand a cost-freereaction. decline. Meanwhile, a second peak in the For instance, inducible defense reactions adaptive landscape startsto rise.Depending may reflectsuch a compromise, in which on the actual selection parameters(cf. Fig. the phenotype,e.g., levels of phenolic com4), this second peak may eitherbe the gen- pounds, is suboptimalboth in the presence eralistor the specialistpeak forthe habitat and absence of herbivores.Optimal levels increasingin frequency.But only afterthis withineach habitatare not reachedbecause second peak has absorbed the first,by then the benefitsare outweighedby highercosts, hard selection soft selection -Z z 1l Z2 Z A 40 C 4 0/ 30 30 20 L 20 ~~~~~~~~~~~~~~10 10 B 1.0 CO 2 50 50 >' 1327 OF GENERALISTS AND SPECIALISTS EVOLUTION 0.8 1.0 - 0.8 0.6 0.4 - 0.4 E 0.2 - 0.2 0 1 2 3 4 5 / 0.6 - C 0.0 o.. 6 generation 7 8 9 Thusands) 10 0.0 J 0 1 2 3 4 5 6 generation 7 8 9 10 (Thousands) in whichthefrequency of habitatone declines of changeduring10,000generations FIG. 5. Trajectories meanfitness undersoftselection. and (B) in (geometric) from1 to 0. (A) Changein meanphenotype linearly underhardselection. Undersoftselection and (D) in (arithmetic) meanfitness (C) Changein meanphenotype in time,underhardselectionpopulationsize followsthemeanfitness curve. populationsize remainsconstant Parameters forbothcasesare z, = 10,z2*= 40, z21l = 0, s12= S22 = 80, r12= r22= 400,p11= P22 = 10, P2 = 4,gll = 922 = 4, g12= 0. e.g., of making more precursorsor having underutilizedhabitat (Futuyma and Morehighersyntheticcapacity. The continuous, no, 1988). Under hard selection a similar gaussian cost functionimplies that fitness outcome is obtained under conditionsthat decreases withthe deviation fromthe cost- make it relativelyeasy to have a phenotype freereaction,and thereforethe outcome is that is close to the optimum forboth haba compromise. Other cost functionscould itats (low costs, and/or optimal and costbe analyzed in a similar fashion. For in- freereactionrelativelysimilar). When this multiplelocallystastance,some inducibledefensemechanisms becomes moredifficult, may perhaps cause a certain,fixed fitness ble equilibria exist. The change fromgenreduction,irrespectiveof the exact strength eralistto eitherspecialistunder hard selecoftheresponse;a (static)specialistmay then tionagreeswithresultsfromsimilarmodels evolve when costs are high,or a generalist of adaptation in a heterogeneousenvironwitha fullyoptimal phenotypein each hab- ment (e.g., MacArthurand Connell, 1966; itat when costs are low, but a compromise Lloyd, 1984). However,a possible existence generalistseems unlikelywith such a cost of threelocally stable solutions,the generalist and two specialists,was not foundprefunction. Optimization of the geometricmean fit- viously; furthermore,under some condiness under softselectionimplies frequency tions two locally stable equilibria can be dependent selection, so that a specialized present,in which one adaptive peak corpopulation always can be invaded by ge- respondsto a specialistpopulation and the notypesthatare betteradapted to theother, otherto a generalistpopulation. In between 1328 PETER H. VAN TIENDEREN the stable peaks are locally unstable equi- whatis optimal withinhabitats;it mightbe libria, eithervalleys or saddle points. The a compromisedue to costs.Historicalevents population is expected to evolve towards such as foundereffectsmay have driventhe one of the locally stable peaks (Felsenstein, population towards a local equilibrium. 1979; Kirkpatrick,1982), but the rate of Perturbationsmay cause a switchto a difevolution near valleys or saddle points can ferent equilibrium. Reciprocal-transplant be low. Valleys are sometimesonly slightly experiments, introduction ofspecialistsfrom lower than the adjacent adaptive peaks, es- neighboringpopulations, (temporary)perpeciallywhen thereare threepeaks, so that turbationsof frequenciesof habitats,or redriftmay cause a population to fluctuate productiveisolationofthedifferent habitats between the generalistand any of the spe- (to see whether specialization starts to cialists. Moreover, such peaks may easily evolve) could all give informationon the coalesce due to a temporaryincreasein phe- state of the population. The measurement notypicvariation(Kirkpatrick,1982). Evo- of contemporaryselection regimes in the lutionarydynamicsunderhard and softse- fieldmay also help to reveal therole ofcosts lection can differ substantially when in adaptations to a heterogeneousenvironfrequenciesof the habitats or selection re- ment. A firstapproach is to examine the gimeswithinhabitatschangein time.Under selection differentials in a natural populasoftselectionthe population may be able to tion (Falconer, 1981; Lande and Arnold, trackthe adaptive peak. Under hard selec- 1983; Arnold and Wade, 1984). A presence tion, however,the adaptive peak mightbe ofa compromiseequilibrium,i.e., a balance thepeak mightmove, or itmight between the optimallyadaptive phenotype unaffected, completelydisappear so thatthepopulation withinhabitatsand costs of the phenotypic will startto evolve towardsan entirelydif- reactionsinvolved, could be detectedin the ferentstate. followingway. Regressionsof fitnesswithin These resultshave some consequencesfor each of the habitats on the traitof interest the interpretationof fielddata: one cannot in that site may show that (i) thereis phealways assume that selective forcesdeter- notypicselection on the expressed traitin mine whethera generalistor specialiststrat- both habitatsifphenotypesare suboptimal, egy evolves. Instead, the outcome of selec- and (ii) the apparent selection differentials tion now may depend on initialconditions, (covariance between relative fitness and for instance, the mean traitvalue and ge- trait)in the subpopulations have opposite netic variation of migrantsthat startedthe signs (cf. Eq. 4). However, there are three population. Suppose that migrants were possible explanationsforsuch results.First, specialists and predominantlyadapted to the population is in a transientstate (i.e., habitatone. Under hardselectionthismight phenotypicselection present,selection rebe a locallystableequilibrium,and thepop- sponse present).Second, observed selection ulation would not change, despite the fact differentials do not resultin a change in the thatperhapsa generaliststrategy would lead population because thereis no appropriate to a much higherfitness.Under softselec- geneticvariation (i.e., phenotypicselection tion this could never happen, as the single present,no selectionresponse). Finally,the adaptive peak can be reachedfromall initial apparent selective forces are counterbalconditions,provided, of course, that there anced by selection for lower costs of the is sufficientgenetic variation (i.e., in the genotypicreaction (i.e., no phenotypicsepresentmodels nonsingularity ofthegenetic lection presentat the population level and covariance matrix). consequentlyno selection response); selection forhighervalues forthe characterstate Measuring Selection in the Field to in a certainhabitat are then accompanied Evaluate Costs by selectionforlower values in otherhabEvolution in a coarse-grainedenviron- itats. mentis complex. A populationcan be comThe regressionmethod formeasuringseposed of specialists, generalists,or inter- lection using fielddata on a natural popumediates, or it can be in a transientstate. lation,however,does not allow a directasThe equilibrium mightbe determinedby sessmentof selectionon costs and therefore EVOLUTION OF GENERALISTS AND SPECIALISTS cannot discriminateamong these possibilities. Data on clonallyreplicatedgenotypes or genotypeswithknown familialrelations such as sib-familiesare needed forthispurpose; this usually necessitates an experimental setup. Regressionof the overall fitness in the population (measured directly, or estimatedfromthe fitnesseswithinhabitats, the frequencies of habitats and the mode of population regulation)can thenbe used to test whetheror not the selective forceswithinhabitatscancel out at thepopulation level, suggestingequilibrium. Ideally, the data consist of measurementsof the expressedphenotypeand fitnessof each individual replicatein both habitats,as well as measuresof the mean phenotypeand the mean fitnessesofreplicatedgenotypes.This allows regressionof the fitnesswithin,say, habitat one (W1), on both the expressed phenotypes(zl) and the average reactionof 1329 ic variation is present.However, detecting selective forces mightbe more likely in a study of a population in a heterogeneous habitat than in a homogeneous habitat. In a compromise equilibrium state, selective forces are operatingevery generationand the equilibriumis maintainedby opposing selective forcesbetween habitats,while in a population in a homogeneous environment selection close to an adaptive peak may be weak and hard to detect. Comparison of observed patternswith predictions from models such as presented here also requiresinformationon the underlyinggenetic basis of traits. Fortunately,when experimentswith replicated genotypes or sib-families are employed, as sketched above, both selective forces and genetic (co)variances can be inferredat the same time, which will make such an effortmore promising.It seems thatsuch experiments, genotypes (2 - Z1). If thereis a generalist, togetherwith experimentalmanipulations compromiseequilibrium,theregressionsof such as perturbationsor introductionof fitnesswithinhabitatsare expectedto show othergenotypes,may be veryusefulto undirectionalselection on the expressedphe- ravel the complex patternsof adaptation, notypetowards the optimum forthe focal and the mechanismsinvolved. environment,and directional selection on thegenotypicreactiontowardsthecost-free ACKNOWLEDGMENTS reaction.The neteffectof both components I would like to thank J. Antonovics, B. of selection within each habitat would be directionalselectionforthe expressedchar- Bradshaw,G. de Jong,R. Lande, and S. Via acter state towards its optimal value (zl*), forstimulatingdiscussions and/orvaluable and directional selection of the character comments on a previous version of the stateexpressedin theotherhabitat(z2*)away manuscript.This work was fundedby the fromits optimum,the latterbecause thisis NetherlandsOrganizationforScientificReassociated withdecreasingcosts in thefocal search (NWO). habitat. The effectsare expected to cancel out in the regressionsof the overall fitness LITERATURE CITED over both habitats (or a constructfor the ANTONOVICS, J., AND S. VIA. 1988. Geneticinfluoverall fitness)on the genotypicmeans. If ences on the distributionand abundance of plants. In A. J. Davy, M. J. Hutchings,and A. R. Wata population is specialized to a specifichabkinson(eds.), Plant Population Ecology. Blackwell, itat, one would expect stabilizingselection Oxford,UK. on the expressed traitwithin this habitat, ARNOLD, S. J.,ANDM. J. WADE. 1984. On themeaand both stabilizingand directionalselecsurementof-naturaland sexual selection: Theory. tion on the genotypicreaction.In the habEvolution 38:709-719. itatto whichthe population is not adapted, BRADSHAw,A. D. 1965. Evolutionarysignificanceof phenotypicplasticityin plants.Adv. Genet. 13:115directionalselection on the expressed trait 155. towards its optimum is expected, but this 1972. Some of the evolutionary conseselective forceis counterbalancedby selecquences of being a plant. Evol. Biol. 5:25-47. C. 1971. Natural selectionat a mutiallelic tion forthe reactionwiththe lowest cost in CANNINGS, autosomal locus withmultipleniches.J.Genet. 60: the otherhabitat. 255-259. The measurementof these selectiveforc- CHARLESWORTH, B., R. LANDE, AND M. SLATKIN. es probablyrequireslargefieldexperiments, 1982. A neo-Darwinian commentaryon macroin whichsufficient evolution. Evolution 36:474-498. genotypicand phenotyp- 1330 PETER H. VAN TIENDEREN CHRISTIANSEN, F. B. 1975. Hard and softselectionin MAcARTHuR, R. H., AND J.H. CONNELL. 1966. The a subdivided population. Am. Nat. 109:11-16. biologyof populations.JohnWileyand Sons, N.Y. COOPER, W. S., AND R. H. KAPLAN. 1982. Adaptive MAYNARD SMITH,J.,R. BRURAN,S. KAUFMAN, P. AL"coin-flipping":A decision-theoreticexamination BERCH, J. CAMPBELL, B. GOODWIN, R. LANDE, D. of natural selection for random individual variaRAUP, AND L. WOLPERT. 1985. Developmental tion. J. Theor. Biol. 94:135-151. constraintsand evolution. Quart. Rev. Biol. 60: ELDREDGE, N., AND S. J. GOULD. 1972. Punctuated 265-287. equilibria: An alternativeto phyleticgradualism. VAN ALSTYNE,K. L. 1988. Herbivore grazing inIn T. J. M. Schopf (ed.), Models in Paleobiology. brown creasespolyphenolicdefensesin theintertidal Freeman and Cooper, San Francisco, CA. alga Fucus distichus.Ecology 69:655-663. FALCONER, D. S. 1981. Introductionto quantitative VAN TIENDEREN, P. H. 1990. Morphological variagenetics.Longman, London, UK. tion in Plantago lanceolata: Limits of plasticity. 1952. The problem of environmentand seEvol. Trends in Plants 4:35-43. lection. Am. Nat. 86:293-298. VIA, S. 1987. Genetic constraintson the evolution FELSENSTEIN, J. 1979. Excursions alongtheinterface ofphenotypicplasticity.In V. Loeschcke (ed.), Gebetween disruptiveand stabilizingselection. Genetic Constraintson Adaptive Evolution. Springer netics 93:773-795. Verlag,Berlin. FuTuYMA, D. J. 1983. Evolutionary interactions VIA,S., ANDR. LANDE. 1985. Genotype-environment among herbivorousinsectsand plants.In D. J. Fuinteractionand the evolution of phenotypicplastuymaand M. Slatkin(eds.), Coevolution. Sinauer, ticity.Evolution 39:505-522. Sunderland,MA. 1987. Evolution of genetic variabilityin a FuTuYMA,D. J., AND G. MORENO. 1988. The evospatiallyheterogeneousenvironment:Effectsofgelutionofecologicalspecialization.Annu. Rev. Ecol. interaction.Genet. Res. 49: notype-environment Syst. 19:207-234. 147-156. GOULD, S. J. 1980. Is a new and general theoryof evolution emerging?Paleobiology 6:119-130. CorrespondingEditor: M. G. Bulmer 1989. A developmentalconstraintin Cerion, withcommentson thedefinitionand interpretation of constraintin evolution. Evolution 43:516-539. APPENDIX HARVELL, C. D. 1986. The ecologyand evolution of inducibledefensesin a marinebryozoan:Cues, costs, The expressedcharacter statein habitati, z,,ofan and consequences. Am. Nat. 128:810-823. individualis thesumofa genotypic value 2,,and an HAVEL, J. E. 1987. Predator-induceddefenses:a re- environmental deviation(z, - 2,) witha univariate view. In W. C. Kerfootand A. Sih (eds.), Predation: normaldistribution t(z,- 2,)withzeromeanandvariDirect and indirectimpacts on aquatic communi- ance e,,.The meanfitness in habitati can be derived ties. UniversityPress of New England, Hanover, bytaking theaverageoverphenotypes, values breeding NH. In ourcasethelatteris mostconvenient, orgenotypes. inhetHEDRICK, P. W. 1986. Genetic polymorphism erogeneous environments:A decade later. Annu. = y(1, 2)W,(I, Z2) d2i d22 (Al) Rev. Ecol. Syst. 17:535-566. withy(i1,Z2) thedistribution ofgenotypic valuesbefore AND A. J. DOLMAN. HOEKSTRA, R. F., R. BuiLSMA, (underrandomdispersalbeingequalin both 1985. Polymorphismfromenvironmentalhetero- selection and W,(i1,Z2) thefitness ofgenotype geneity:Models are onlyrobustifthe heterozygote habitats) (i1, Z2) ofgenotypic valuesofthe is close in fitnessto the favoured homozygotein in habitati. Thedistribution character statesin thetwohabitatsis assumedto be each environment.Genet. Res. 45:299-314. 1990. Multi- bivariate normal, y(z-)= exp[- ?2( - 2)T(G + D)-1(i HOLSINGER, K. E., AND S. W. PACALA. matrices of ple-nichepolymorphismin plantpopulations.Am. - 2)], withG and D the2 x 2 covariance additiveand nonadditive forcharacter geneticeffects Nat. 135:301-309. and 2 and z thevectorsofpopuKIRKPATRICK, M. 1982. Quantumevolutionand states,respectively, values,respectively. punctuatedequilibria in continuous geneticchar- lationmeansand genotypic The averagecomponent offitness due to stabilizing acters.Am. Nat. 119:833-848. on theexpressed in habitati has phenotype LANDE, R. 1979. Quantitative genetic analysis of selection overall possibleenvironmental values multivariateevolution,applied to brain: body size tobe integrated thatthegenotype can attain, allometry.Evolution 33:402-416. LANDE, R., AND S. J. ARNOLD. 1983. The measurement of selectionon correlatedcharacters.Evolut(z,- 2,)exp[-1/2 (z, - z*)2/S2] dz, tion 37:1210-1226. + e)-'] _\[S,2(s,2 LEVIN, D. A. 1976. The chemical defensesof plants *exp[(A2) /2(2, - Z *)2/(S,2 + e)] to pathogensand herbivores.Annu. Rev. Ecol. Syst. 7: 121-159. The secondcomponent due to stabilizing on selection LEVINS,R. 1968. Evolution in changing environ- the reactionwiththe lowestinternalcost becomes ments.PrincetonUniv. Press, Princeton,NJ. and is assumedto be exp[- 1/2(22- -z2 *)2/r12], LLoYD, D. G. 1984. Variation strategiesof plants in genotype specific. heterogeneousenvironments.Biol. J.Linn. Soc. 21: Combining bothcomponents givestheaveragefit357-385. nessesofa genotype (i1, Z2) as w f ff EVOLUTION WI'(l, i2) f - exp[-Y/2(92 - = OF GENERALISTS AND SPECIALISTS *)21r,2] - z *)2/S2] t(z - z,) exp[- V12(z, dz (A3) Rewriting (A3)ina bivariate gaussianform, using(A2), yields W(Z) = + \[S,2(s,2 e')-l] - 0,)Tqf,-1(2 - 6)] *exp[-1/2( withT denoting matrixtransposition, = 62 = * I T, (21, X2) (Z2* 5 - 12+ ell 12 + ell 22+ S'2 Z2- 22+ 1 = (Zl*, *,Z2*)T, S12 Z* + Z2- and + ell SI + r,2 + el,J 2 r22 + e22 S22 + e22 e22 s 2 + e2 ) 1*),T (A4) 1331 thematrices describing selection at thegenotypic level in habitatoneand two,respectively. The meanfitness overall genotypes (Al) thusbecomes w, V[IQI (0Q+ P,)-II] exp[-V/2(f (0, + Pj)-1(2 - ,)], O,)T (A5) equation3 in thetext,withP, = G + D + E,, E, a 2 x 2 matrixofenvironmental deviationswithell elementsequal to e,,,and Q, = ', - E,. The off-diagonal elements ofthephenotypic covariancematrices P, are not undefined (cf.Via and Lande, 1985) despitethe factthatthephenotypic correlation betweencharacter statescannotbe measuredon thesameindividual.