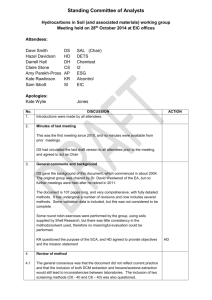
How to Grow Crystals:
advertisement

How to Grow Crystals: Step 1: Considerations about the charge of your molecule. Small Scale Tests 1. If you expect your molecule is negative you may need to add a cation to your solution. Potential cations include - Tetrabutylammonium Halides (TBABr, TBACl, TBAI) - Tetraethylammonium Halides (TEABr, TEACl, TEAI) - Tetramethylammonium Halides (TMABr, TMACl, TMAI) - Tetramethyphosphonium halides (TMPBr, TMPCl) - Tetrabuytlphosphonium halides (TBPBr, TBPCl) - Triphenylmethyl halide (TPMBr, TPMCl) 2. If you expect your molecule is positive you many need to add an anion to your solution. Potential anions include: - Ammonium hexafluorophosphate (NH4PF6) - Ammonium borohydride (NH4BH4) - Alkali Earth Metal /Amomonium nitrate (ANO3/NH4 NO3) - Alkali Earth Metal /Ammonium Perchlorates (AClO4/NH4ClO4) 3. If you expect your molecule is neutral and you believe you will have trouble getting crystals here are some molecules which can help yield better crystals. - Triphenylphosphine - Triphenylphosphine oxide (TTPO) Step 2: Different methods of crystallization 1. Undisturbed solution: leaving solution in a location where it will be undisturbed by vibrations or movement. 2. Slow evaporation: allowing the concentration of you solution to slowly increase (leading to saturation) by solvent evaporation. 3. Slow cooling: allowing your solution to cool from a higher temperature to room temperature over a long time period (anywhere from several hours to multiple days.) 4. Vapor diffusion: allowing a solvent of high volatility to slowly diffuse into a sample of lower volatility. (Figures from: http://www.chemistryviews.org/details/education/2538941/Tips_and_ Tricks_for_the_Lab_Growing_Crystals_Part_3.html) Air Stable: Air-free 5. Layering (Solvent Diffusion): Uses the advantage of density for two different solvents. The solution (dc) is either layered under (if density of layering solvent is lighter dc > dl) or on top of (if density of layering solvent is higher dc < dl) the layering solvent (lc). 6. Sublimation: heat the sample solution under reduced pressure until it vaporizes and allow it to undergo deposition on a cool area of the surface. Step 3: Is your molecule soluble in polar or non-polar solvents? 1. List of Polar Solvents A. Aprotic Polar Solvents -Acetonitrile (MeCN) Layer with ether, ether/hexane (1:1), acetone, acetone/hexane (1:1), dichloromethane (DCM aka methylene chloride), chloroform, toluene, THF Diffusion with ether, E/H, Me2CO, DCM -Dimethylsulfoxide (DMSO) Layer with ether, acetone, ether/acetone Diffusion with E/H, Ether/Acetone, Acetone -Dimethylformamide (DMF) Layer with ether, acetone, ether/acetone, hexane, ether/hexane Diffusion with E/H, Hexane, Ether/Acetone, Acetone -Acetone (Me2CO) B. Polar Protic Solvents -Ethanol (EtOH) Layer with acetone, ether, ether/acetone, water, acetonitrile Diffuse with acetone, ether, ether/acetone -Methanol (MeOH) Layer with acetone, ether, ether/acetone, water, acetonitrile Diffuse with acetone, ether, ether/acetone -tertButanol (tBuOH) Layer with acetone, ether, ether/acetone, water, acetonitrile Diffuse with acetone, ether, ether/acetone -Water (H2O) Layer with acetone, alcohols Diffuse with alcohol, methanol -nPropanol (nPrOH) Layer with acetone, ether, ether/acetone, water, acetonitrile Diffuse with acetone, ether, ether/acetone C. Borderline Aprotic Solvents -Tetrahydrofuran (THF) Layer with alcohols, acetonitrile, DCM, nitromethane, chloroform Diffuse with alcohols, DCM -Ethylacetate (Et2OAc) -Dichloromethane (DCM) Layer with ether, ether/hexane, hexane, acetonitrile D. Nonpolar Solvents -Pentane (P) Layer with ether, acetone, ether/acetone, acetonitrile Diffusion with ether, acetone -Hexane (H) Layer with ether, acetone, ether/acetone, acetonitrile Diffusion with ether, acetone -Cyclohexane (CH) Layer with ether, acetone, ether/acetone, acetonitrile Diffusion with ether, acetone -Benzene (Bz) Layer with ether, acetone, ether/acetone, acetonitrile Diffusion with ether, acetone -Toluene (Tol) Layer with acetonitrile, hexanes, pentane, ether, DCM Diffusion with hexane, ether, DCM -Chloroform (CHCl3) Layer with ether, hexane, ether/hexane, pentane, ether/pentane, acetonitrile Diffuse with ether, hexane -Ethyl Ether (Et2O) Layer with acetone, acetonitrile, ethyl acetate, hexane **If these solvents do not work you can also layer any solvent with another solvent it is miscible with (see chart below) Step 4: What if crystals are not growing? 1. Try adding one of the neutral molecules to act as a cocrystallizaer. 2. Place reaction in fridge, sometimes a change in temperature (or cooler temperatures) can induct crystalliations. 3. Use a seed crystal or slightly scratch the glass to create a nucleation site. Step 5: I have crystals but they’re not suitable for data collection. What do I do? 1. Recrystallization – dissolve your crystals in another solvent (test for solubility by using a small amount of crystals and scanning different solvents) and layer back with the solvent they originally crystallize from or one from the miscibility chart. 2. Place vial in the fridge to slow the process of crystallization and hopefully yield better crystals. 3. Decrease the concentration of your solution so the crystals have more room to form single crystals. 4. Try using a similar solvent system (i.e. chloroform for DCM, nitromethane for acetonitrile, ethanol for methanol) or a mixture of solvents (pick two solvents which are miscible).