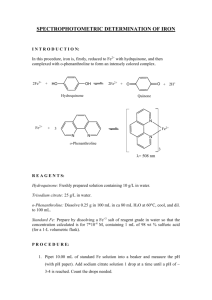
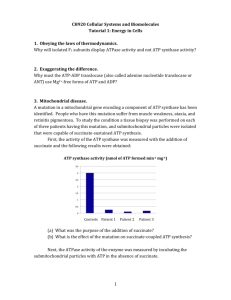
1. Outline the steps required to get one molecule of acetyl CoA from
advertisement

Chem*3560 1. Question set 7 Feb 23-27, 2004 Outline the steps required to get one molecule of acetyl CoA from inside the mitochondrion, where pyruvate dehydrogenase is located, to the cytoplasm. Acetyl-CoA is made in the mitochondrion by pyruvate dehydrogenase, by β-oxidation and by amino acid oxidation. However there is no transporter for acetyl-CoA in the mitochondrial membrane, so acetyl CoA has to be transported indirectly. (M) = in the mitochondrion; (C) = in the cytoplasm citrate synthase (mitochondrial) Acetyl-CoA(M) + oxaloacetate (M) → citrate (M) + HSCoA(M) Translocation across membrane: citrate translocator (mitochondrial membrane) citrate (M) + malate (C) → citrate (C) + malate (M) citrate lyase (cytoplasmic) Citrate (C) + HSCoA(C) + ATP(C) → acetyl-CoA(C) + oxaloacetate (C) + ADP(C) + Pi(C) malate dehydrogenase (cytoplasmic) Oxaloacetate (C) + NADH(C) + H+ → malate (C) + NAD +(C) For malate translocation, see above malate dehydrogenase (mitochondrial) malate (M) + NAD +(M) → oxaloacetate (M) + NADH(M) + H+ NADH is also not transported directly, so this process helps use up cytoplasmic NADH, while producing more mitochondrial NADH for use in oxidative phosphorylation. 2. Write down and sum the equations for citrate synthase (Intro Biochem, TCA cycle) and citrate lyase. citrate synthase Acetyl-CoA + oxaloacetate → citrate + HSCoA citrate lyase Citrate + HSCoA + ATP → acetyl-CoA + oxaloacetate + ADP + Pi ATP → ADP + Pi What prevents the combination of citrate synthase and citrate lyase from being a futile cycle? A futile cycle may occur when two opposed reactions (product of one is reactant of the other) differ in that ATP hydrolysis in one direction is not balanced out by ATP synthesis in the other direction. The net sum of the two reactions has ATP hydrolysis as the only outcome, e.g. Fructose-6-phosphate + ATP → Fructose-1,6-bisphosphate + ADP Fructose-1,6-bisphosphate + H2 O → Fructose-6-phosphate + Pi ATP → ADP + Pi Chem*3560 Question set 7 Feb 23-27, 2004 The two different reactions are necessary to ensure that each progresses in the designated direction, whereas a true reversal of the phosphofructokinase reaction is disallowed by the free energy change under cellular conditions (∆G = –26 kJ/mol). The difference with citrate synthase and citrate lyase reaction is that the acetyl-CoA product of the citrate lyase reaction is not the reactant of citrate synthase, because acetyl CoA substrate reacts in the mitchondrion, but the acetyl-CoA product is generated in the cytoplasm. Oxaloacetate eventually returns to the mitochondrion (see question 1), so the true sum to the two equations is: Acetyl-CoA(mito) + oxaloacetate → citrate + HSCoA Citrate + HSCoA + ATP → acetyl-CoA(cyto) + oxaloacetate + ADP + Pi Acetyl-CoA(mito) ATP → acetyl-CoA(cyto) + ADP + Pi The ATP hydrolysis in this process is not futile, but contributes chemical driving force to the export of acetyl CoA from the mitochondrion. 3. If fatty acid biosynthesis is the reverse of β-oxidation, a four step cycle, why is fatty acid biosynthesis a six step cycle? In most organisms, fatty acid synthase is a closely associated complex of seven catalytic centres surrounding acyl carrier protein (ACP) which carries a long pantetheine arm terminating in the HS- group that bonds to the substrate. In bacteria, the complex consists of independent polypeptides held together by non covalent forces, one for each catalytic component plus ACP. In animals, the fatty acid synthase components are strung out as multiple domains on a single polypeptide chain. The functional enzyme is a dimer of two identical chains. In fatty acid biosynthesis, the growing acyl chain becomes covalently bonded to the enzyme's ACP and its pantetheine chain. This increases fatty acid synthase's efficiency, since the substrate remains in close range of the six catalytic centres instead of disappearing into the surroundings, and having to return to the enzyme by random diffusion. The first four reactions of the fatty acid cycle backtrack through the four β-oxidation steps. 1) 2) 3) 4) Ketoacyl-ACP synthase reverses the thiolase reaction Ketoacyl-ACP reductase reverses the hydroacyl-CoA dehydrogenase step Hydroxyacyl-ACP dehydratase reverses enoyl-CoA hydratase Enoyl-ACP reductase reverses acyl-CoA dehydrogenase and this gives: CH3 -CH2 -CH2 -CO-S-ACP, which will be elongated by reaction with malonyl-S-CoA ↑ The malonyl radical reacts here, not at the CH3 - end, which is chemically inert. Chem*3560 Question set 7 Feb 23-27, 2004 If CH3 -CH2 -CH2 -CO-S-ACP reacted directly with malonyl-S-CoA, the product would be the ketoacyl-S-CoA, leaving HS-ACP vacant. The ketoacyl-S-CoA could then be lost from fatty acid synthase and would have to diffuse back to the enzyme, making the process less efficient. The extra two steps of acyl transferase and malonyl transferase overcome the problem: acyl transferase CH3 -CH2 -CH2 -CO-S-ACP + HS-Enz → CH3 -CH2 -CH2 -CO-S-Enz + HS-ACP where HS-Enz is a specific Cys sidechain in the ketoacyl-ACP synthase catalytic site. This keeps the growing chain covalently bonded to the fatty acid synthase, right in the catalytic site where it will next react, in ketoacyl-ACP synthase. malonyl transferase malonyl-S-CoA + HS-ACP → malonyl-S-ACP + HSCoA (free to leave) This covalently bonds the incoming malonyl to the fatty acid synthase ACP. Finally, ketoacyl-ACP synthase catalyzes the joining of the two substrates: ketoacyl-S-ACP synthase CH3 -CH2 -CH2 -CO-S-Enz + malonyl-S-ACP → CH3 -CH2 -CH2 -CO-CH2 -CO-S-ACP + HS-Enz + CO2 This leaves product on the ACP, ready to continue the next reaction cycle. 4. If cytoplasmic malic enzyme converts malate to pyruvate in order to generate NADPH, how does the TCA cycle in the mitochondrion get more oxaloacetate to sustain the TCA cycle? When there is sufficient glucose in the cell to supply NADPH by the pentose phosphate cycle, oxaloacetate returns to the mitochondrion by the following processes: (M) mitochondrial (C) cytoplasmic Citrate (C) + HSCoA(C) Oxaloacetate (C) citrate (M) citrate lyase (cytoplasmic) + ATP(C) → acetyl-CoA(C) + oxaloacetate (C) + ADP(C) + Pi(C) malate dehydrogenase (cytoplasmic) + NADH(C) + H+ → malate (C) + NAD +(C) citrate translocator (mitochondrial membrane) + malate (C) → citrate (C) + malate (M) malate dehydrogenase (mitochondrial) malate (M) + NAD +(M) → oxaloacetate (M) + NADH(M) + H+ regenerates citrate (M) Chem*3560 Question set 7 Feb 23-27, 2004 If acetyl-CoA is bing produced from amino acids instead of from glucose, there may be no substrate to make NADPH by the pentose phosphate pathways. In this case, malate can provide NADPH: malate (C) malate dehydrogenase (decarboxylating) + NADP+(C) → pyruvate (C) + CO2 + NADPH(C) + H+ but this poses the question of what supplies oxaloacetate to keep the TCA cycle going. Pyruvate enters the mitochondrion through a specific transporter, and is converted to oxaloacetate by pyruvate carboxylase: pyruvate (M) pyruvate carboxylase + CO2 + ATP(M) → oxaloacetate (M) + ADP(M) + Pi(M) This pathway costs an extra ATP to make NADPH in place of the the NADH produced in the pathway that uses malate transport. citrate ↓ ATP used oxaloacetate + acetyl-CoA ↓ NADH used malate ↓ transport malate ↓ NADH made oxaloacetate citrate ↓ ATP used oxaloacetate + acetyl-CoA ↓ NADH used malate ↓ NADPH made pyruvate + CO2 ↓ transport pyruvate ↓ ATP used oxaloacetate NADPH is not a substrate for oxidative phosphorylation, so deriving its energy equivalence in units of ATP is not straightforward. However the process shown here is one example of several that suggests that in terms of energy equivalence, NADPH = NADH + ATP. The extra energy content of NADPH is the consequence of the high ratio [NADPH] / [NADP +]. 5. What effects other than fatty acid biosynthesis may be expected from the presence of citrate in the cytoplasm? Cytoplasmic citrate is a negative allosteric regulator of phosphofructokinase 1 and a positive regulator of fructose-1,6-bishosphatase, so would normally shut down glycolysis and activate gluconeogenesis. Insulin can stimulate phosphofructokinase 2 in liver, and the fructose-2,6-bisphosphate produced would override the effect of citrate. This allows glycolysis to continue feeding substrate to fatty acid biosynthesis in the liver.