Name: Chem 232 Analytical Chemistry Test 2
advertisement
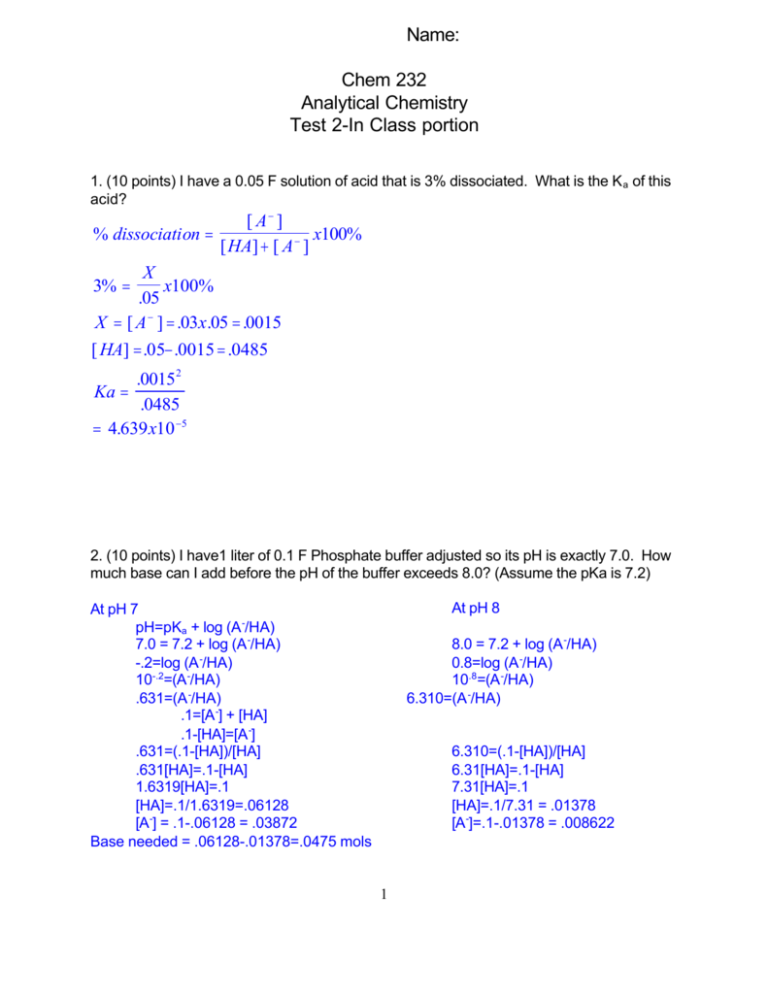
Name: Chem 232 Analytical Chemistry Test 2-In Class portion 1. (10 points) I have a 0.05 F solution of acid that is 3% dissociated. What is the K a of this acid? [ A− ] % dissociation = x100% [ HA] + [ A − ] X 3% = x100% .05 X = [ A − ] = .03x.05 = .0015 [ HA] = .05− .0015 = .0485 .00152 Ka = .0485 = 4.639 x10 −5 2. (10 points) I have1 liter of 0.1 F Phosphate buffer adjusted so its pH is exactly 7.0. How much base can I add before the pH of the buffer exceeds 8.0? (Assume the pKa is 7.2) At pH 8 At pH 7 pH=pKa + log (A -/HA) 7.0 = 7.2 + log (A -/HA) -.2=log (A -/HA) 10-.2=(A -/HA) .631=(A -/HA) .1=[A -] + [HA] .1-[HA]=[A -] .631=(.1-[HA])/[HA] .631[HA]=.1-[HA] 1.6319[HA]=.1 [HA]=.1/1.6319=.06128 [A-] = .1-.06128 = .03872 Base needed = .06128-.01378=.0475 mols 8.0 = 7.2 + log (A -/HA) 0.8=log (A -/HA) 10.8=(A -/HA) 6.310=(A -/HA) 6.310=(.1-[HA])/[HA] 6.31[HA]=.1-[HA] 7.31[HA]=.1 [HA]=.1/7.31 = .01378 [A-]=.1-.01378 = .008622 1 3. (10 points) If we were titrating one of the amino acids whose equivalence point was at pH 9.5, what would be the most appropriate indicator? Indicator Transition Range Methyl Red 4.8-6.0 Bromothymol Blue 6.0-7.6 Phenolphthalein 8.0-9.6 Thynolphthalein 8.6-10.5 Midpoint of Methyl red transition is (6+4.8) /2 = 5.4 Midpoint of Bromothymol Blue transition is (6+7.6) /2 = 6.8 Midpoint of Thynolphthalein transition is (8.6+10.5) /2 = 9.55 You want the midpoint of the transition (the pKa of the indicator) to match with the pH of the equivalence point as closely as possible, hence Thynolphthalein is the best choice 4. (10 points) I am titrating 25 mls of .0258M HClO4 with .0398M KOH. A What is the initial pH of the acid solution? Strong acid = [HClO4] = [H+] pH = -log (.0258) = 1.59 B. What is the volume of base added at the equivalence point? M1V2=M2V2; 25(.0258)=X(.0398) X=(25x.0258)/. 0398 =16.21 ml C. What is the pH at the equivalence point? Strong acid-Strong base titration, pH at equivalence point = 7.0 D. What is the pH of the solution after you have added 25 ml of base? This is after the equivalence point so... [OH-] = conc of base X dilution factor = .0398 X (25-16.21)/50 = 6.997x10-3 pOH = -log OH- = 2.16; pH=14-pOH = 11.84 2 5. (10 points) I am titrating 25 mls of .01345M NH3 with .005047M HCl. (Ammonia had a K a of 5.7x10-10) A What is the initial pH of the ammonia solution? Use the weak base equation K b = X 2/.01345-X KB = 1x10-14/5.7x10-10 = 1.75x10-5 = X 2/.01345-X Successive approximation 1: X 2 = 1.75x10-5 (.01345) X=4.85x10-4 2: X 2 = 1.75x10-5 (.01345-4.85x10-4 ) X=4.7610-4 3: X 2 = 1.75x10-5 (.01345-4.76x10-4 ) X=4.7610-4 X=[OH-] = 4.76x10-4 ; pOH = 3.32; pH=14-3.32 = 10.68 B. What is the volume of acid added at the equivalence point? V1B1=V 2B2 25(.01345)=X (.005047) X=25(.01345)/(.005047) X = 66.62 ml C. What is the pH at the equivalence point? At this point the base has been converted to the acid form use X=[H+]=[A -] Ka =X 2/F-X F = original conc X dilution facotor F= .01345 X [25/(25+66.62)] = 3.67x10-3 Successive approximation 1: X 2 = 5.7x10-10(3.67x10-3 ) X=1.45x10-6 2: X 2 =5.7x10-10(3.67x10-3 -1.45x10-6 ) X=1.45x10-6 X=[H+] = 1.45x10-6 ; pH = 5.84 3 6. (10 points) Aminobenzoic acid has the following structure in it fully protonated form: NH3+ COOH This molecule has 2 pKA’s 2.08 and 4.96. A. What groups on the molecule are associated with each pKa? NH3+ 4.96 COOH 2.08 B. What is the charge on the H2A molecule +1 HA molecule 0 or +1/-1 A molecule -1 C. Roughly sketch the titration curve of this compound when it is dissolved in the H2A form and titrated with base. (Sketch and explain, do no calculations) Too hard to do with computer graphics - Sorry Mostly I am looking for 2 buffer regions at roughly 2 and 5, and 2 equivalence points D. Using the terms H2A, HA and A, what are the principal species at pH 2, 4, 6, 8, and 10? 50:50 H2A and HA 2 4 HA A6 8 AA10 4 Name: Chem 232 Analytical Chemistry Test 2-Take home portion Open book - use any resource you want except other people (but you can ask Dr. Z. if the question is unclear) SHOW ALL WORK ON SEPARATE PAGES FOR PARTIAL CREDIT 7. (10 points) Calculate the titration curve for 25 mls of .25M Bromoacetic acid being titrated with .26M NaOH. Include the following points in your titration curve: 0, 5, 10, 20, 25, and 30 ml of base added. 8. (20 points) Calculate the titration curve for 25 mls of .25M Histidine in its fully protonated form being titrated with .25M NaOH. Include the following points in your titration curve: 0, 10, 25, 30, 50, 70, 75, and 80 mls of base added 9. (10 points) Use the successive approximation method to calculate the pH of .1M Piperidine(base). 5 7. (10 points) Calculate the titration curve for 25 mls of .25M Bromoacetic acid being titrated with .26M NaOH. Include the following points in your titration curve: 0, 5, 10, 20, 25, and 30 ml of base added. 1st find equivalence point 25(.25)=X(.26); X=25(.25)/.26 = 24.04 ml 0 pH of .25M Bromoacetic Acid X=[H+] = [A -] Ka = X 2/.25-X Ka = 1.25x10-3 = X 2/.25-X 1st approximation: X 2 = 1.25x10-3 (.25) X=1.77x10-2 2nd X2 = 1.25x10-3 (.25-1.77x10-2 ) X=1.70x10-2 rd 2 -3 -2 3 X = 1.25x10 (.25-1.70x10 ) X=1.71x10-2 4th X2 = 1.25x10-3 (.25-1.71x10-2 ) X=1.71x10-2 + -2 [H ] = 1.71x10 , pH=1.77 5, 10 and 20 are in the buffer region - Use Henderson-Hasselbalch 5 RXN table HA OH A6.25mMole 1.3mMol -1.3 -1.3 +1.3 4.95 0 1.3 pH = 2.90 + log (1.3/4.95) =2.32 10 HA OH 6.25mMole 2.6mMol -2.6 -2.6 3.65 0 pH = 2.90 + log (2.6/3.65) A+2.6 2.6 =2.75 20 HA OH 6.25mMole 5.2mMol -5.2 -5.2 1.05 0 pH = 2.90 + log (5.2/1.05) A+5.2 5.2 =3.59 25 is .96 ml past the equivalence point [OH] = .26(.96/50) = 4.99x10-3 pOH = 2.30, pH = 11.70 30 ml is 5.96 ml past the equivalence point [OH] = .26(5.96/55) = 2.82x10-2 pOH = 1.55, pH = 12.45 6 8. (20 points) Calculate the titration curve for 25 mls of .25M Histidine in its fully protonated form being titrated with .25M NaOH. Include the following points in your titration curve: 0, 10, 25, 30, 50, 70, 75, and 80 mls of base added Histidine is a triprotic amino acid so will call it H3A it has pKa’s of 1.7, 6.02, and 9.08 The first equivalence point is at: M1V1 = M2V2 25(.25) = X (.25) so we have equivalence points at 25, 50 and 75 mls! 0 ml - pH 0f .25M His with a pKa of 1.7 or a K a of 10-1.7 = 2.0x10-2 [H+] = [A -] = X 2.0x10-2 = X 2/(.25-X) X=.071 1st approx X2= 2.0x10-2 X .25 nd 2 -2 2 X = 2.0x10 X (.25-.071) X=.0599 X2= 2.0x10-2 X (.25-.0599) X=.0617 3rd th 2 -2 4 X = 2.0x10 X (.25-.0617) X=.0614 X2= 2.0x10-2 X (.25-.0614) X=.0614 5th [H+] = .0614, pH = 1.21 10 ml use rxn table moles H3A = .25(25) = 6.25mmol moles OH- = .25(10 = 2.5 mmol rxn Net H3A 6.25 -2.5 3.75 + OH- 6 2.5 -2.5 0 H2A + H2O 0 +2.5 2.5 pH = 1.7 + log (2.5/3.75) pH = 1.52 25 ml - 1st equivalence point The quick and dirty answer pH = (pKa1 + pKa2)/2 = 3.86 Since K is fairly large you might suspect that the quick and dirty answer could be wrong. The CORRECT ANSWER uses the long equation: 7 [HA] = .25(25/50) = .125 [H+ ] = K1 K 2 [ HL] + K1 KW K1 + [ HL] 2 x10 − 2 (9.55x10− 7 ).125 + 2 x10− 2 (1x10 −14 ) = 2 x10− 2 + .125 = 128 . x10− 4 pH=3.89 30 mL use rxn table moles H3A = .25(25) = 6.25mmol moles OH- = .25(30) = 7.5 mmol rxn Net rxn2 Net H3A 6.25 -6.25 0 + OH- 6 7.5 -6.25 1.25 H2A + H2O 0 +6.25 6.25 H2A+ OH- 6HA + H2O 6.25 1.25 0 -1.25 -1.25 +1.25 5 0 1.25 pH = 6.02 + log (1.25/5) pH = 5.42 50 ml 2nd equivalence point The quick and dirty answer pH = (pKa2 + pKa3)/2 = 7.55 At this point the K is small enough that you don’t need to use the long equation 8 70 ml use rxn table moles H3A = .25(25) = 6.25mmol moles OH- = .25(70) = 17.5 mmol rxn Net rxn2 Net H3A 6.25 -6.25 0 + OH- 6 17.5 -6.25 11.25 H2A + H2O 0 +6.25 6.25 H2A+ OH- 6HA + H2O 6.25 11.25 0 -6.25 -6.25 +6.25 5.0 6.25 HA 6.25 -5 1.25 rxn3 net +OH- 6A 5 -5 +5 0 5 + H2O pH = 9.08 + log (5/1.25) pH = 9.68 75 ml Final equivalence point Treat as strong base [A] = .25(25/100) = .0625 KB = 1x10-14/8.38x10-10 = 1.19x10-5 [HA]=[OH-] = X 1.19x10-5 = X 2/(.0625-x) 1st approx X2 = 1.19x10-5 (.0625) X=8.62x10-4 nd 2 -5 -4 2 approx X = 1.19x10 (.0625- 8.62x10 ) X=8.56x10-4 3rd X2 = 1.19x10-5 (.0625- 8.56x10-4 ) X=8.56x10-4 [OH-] = 8.56x10-4 pOH = 3.07, pH =14-pOH = 10.93 80 ml 5 ml past last equivalence point treat as excess base [OH-] = .25(5/105) = 1.19x10-2 pOH = 1.92 pH = 14-pOH = 12.08 9 9. (10 points) Use the successive approximation method to calculate the pH of .1M Piperidine(base). KB = K w/KA = 1x10-14/7.53x10-12 = 1.328x10-3 KB=X 2/(.1-X) 1.328x10-3 = X 2/(.1-X) 1st 2nd 3rd 4th X2 = 1.328x10-3 (.1) X2 = 1.328x10-3 (.1- 1.15x10-2 ) X2 = 1.328x10-3 (.1- 1.08x10-2 ) X2 = 1.328x10-3 (.1- 1.09x10-2 ) X=1.15x10-2 X=1.08x10-2 X=1.09x10-2 X=1.09x10-2 [OH-] =1.09x10-2 ; pOH = 1.96 pH = 12.04 10