Imject ® Maleimide Activated SuperCarrier® System
advertisement

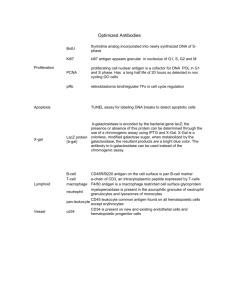
INSTRUCTIONS Imject Maleimide Activated ® SuperCarrier System ® 3747 N. Meridian Road P.O. Box 117 Rockford, IL 61105 77156 0140w Product Description Number Description 77156 Imject® Maleimide Activated SuperCarrier® System* Contents: 1. Primary carrier for injection: 5 x 2 mg of maleimide activated cBSA. 2. Non-relevant carrier for ELISAs: 5 x 2 mg of maleimide activated KLH. 3. 10 x 5 ml gel filtration columns, contains 0.02% sodium azide as a preservative. 4. Conjugation Buffer: 1 x 30 ml of 0.083 M sodium phosphate, 0.9 M NaCl, 0.1 M EDTA, 0.02% Sodium Azide, pH 7.2, plus stabilizer. 5. Purification Buffer: 5 x 4.96 gm dry powder to prepare 5 x 60 ml of buffer forgel filtration separations. 6. Adjuvant: 1 x 50 ml of Imject® Alum. *US Patent 5,142,027 The Imject® Maleimide Activated SuperCarrier® Immune Modulator is based on the cationized BSA (cBSA), which will carry an antigen that is covalently coupled to it into the Antigen Presenting Cell, resulting in more efficient processing and presentation of that antigen and enhanced immune response. Antigen presentation is a three-step process. First the antigen must be internalized by the appropriate cell type, either a macrophage or B cell. The B cell is more efficient at internalization because it has an antigen specific receptor, a membrane attached form of the antibody molecule. The macrophage does not bind antigen but takes up molecules by nonspecific pinocytosis. Therefore, uptake is dependent on antigen and macrophage concentration. Secondly, the antigen must be processed or partially degraded. The immunogenic peptide/MHC complex is transported to the cell surface of the APC for the last step, presentation to the T cell. The antigen stimulated Th then begins to proliferate and produce a variety of lymphokines. During B cell, activation, the same peptide/MHC structure on the B cell is recognized by the T cell providing help for the antibody response. It serves as the antigen-specific recognition structure. Small molecules or haptens are able to interact with the products of an immune response, but they cannot stimulate that response. Haptens are incomplete antigens, but they can be made immunogenic by coupling to a suitable carrier molecule. A carrier is a large molecule capable of stimulating an immune response. The most commonly used carriers are highly immunogenic proteins that are capable of imparting immunogenicity to covalently coupled haptens. To enhance the immune response, the antigen is usually mixed with an adjuvant prior to injection. The adjuvant, such as Freund's (Complete or Incomplete) or alum, is believed to non-specifically enhance the response by localizing the antigen for an extended time and attracting the appropriate cells (T, B and APC cells) to interact with it and each other. cBSA, because of its positive charge, has greater affinity for the negatively charged cell surface membrane of the APC. It can bind to the membrane and be internalized by receptor-mediated endocytosis rather than by pinocytosis. This results in more efficient uptake and processing of the antigen into a more immunogenic form with preferential stimulation of Th cell help rather than Ts. Telephone: 800-8-PIERCE (800-874-3723) or 815-968-0747 • Fax: 815-968-7316 or 800-842-5007 Internet: http://www.piercenet.com 1 SuperCarrier® Immune Modulator is effective for enhancing the antibody response to proteins with low pI, and it is useful with haptens. Native charge seems to be more important in determining the potential for enhancement using SuperCarrier® Immune Modulator. There may be additional factors that also influence the effectiveness of the conjugate. SuperCarrier® Immune Modulator is based on the fact that cBSA will carry the antigen that is covalently coupled to it, regardless of size, into the APC, resulting in more efficient processing and presentation of that antigen and yielding an enhanced immune response. The cBSA has been modified via its amine groups through the NHS-ester end of sulfo-SMCC, yielding a maleimide activated carrier. This preactivated cBSA is supplied lyophilized and ready to couple via free sulfhydryls on your hapten. After the hapten has been added to the Maleimide Activated SuperCarrier® Immune Modulator, an incubation of only 2 hours is all that is necessary for preparation of the immunogen. Also included with the Imject® Maleimide Activated SuperCarrier® System is a preactivated non-relevant carrier (Keyhole Limpet Hemocyanin) for use in ELISAs. Standard Protocol Note: If you intend to quantitate the conjugate, refer to the Note indicated in Step 2 and to the Quantitation of Conjugation section. Note: Complete and efficient conjugation is ensured by using a molar excess of hapten (peptide) over the carrier protein’s maleimide groups (15-25 moles maleimides per mole cBSA). For example, if the hapten’s molecular weight is 2000 daltons, then 2 mg (1 µmole) of hapten should be added to 2 mg of carrier protein (~0.65 µmoles of maleimide groups). Alternatively, if a molar excess of hapten is not possible, then after the hapten’s conjugation, a sulfhydryl containing compound, such as cystine, can be added to quench any remaining active maleimide groups. 1. Dissolve one bottle of the purification buffer salts in 60 ml of distilled, deionized water. 2. Reconstitute up to 2 mg of the sulfhydryl-containing hapten or peptide to be conjugated in 200 µl to 500 µl (smaller volume recommended) of the Conjugation Buffer. If an excess of the peptide solution is made at this time, an estimate of the degree of conjugation may be determined later. Note: The peptide may be added directly to the maleimide activated carrier solution if it is freely soluble and can be weighed out in the appropriate quantity (up to 2 mg peptide/2 mg of activated carrier). Note: If the peptide to be coupled is already in solution, it may be used directly if it is in a buffer containing no additional sulfhydryls and is at a pH between 7.0 and 7.5. Note: To quantitate the conjugation, perform an Ellman’s assay (see Quantitation of Conjugation) on at least 10 µl of the peptide solution from step 2. For accurate results, this initial assay of the non-conjugated peptide must be done as soon as possible after reconstitution since disulfide bonds can form during even short term storage of the peptide. 3. Reconstitute one 2 mg vial of maleimide activated carrier protein with 200 µl of distilled, deionized water. 4. Immediately mix the peptide solution with the activated carrier solution and allow them to react for 2 hours at room temperature. Purification of the Conjugate for removal of EDTA and free hapten 1. Remove top and bottom caps from one gel filtration column, respectively. 2. Allow storage solution to drain, and wash column with 5 ml of the purification buffer. 3. Apply the hapten-carrier conjugate mixture directly to the top of the disc, taking care to avoid the sides of the column. 4. Collect the eluate. 5. Add 0.5 ml aliquots of purification buffer to the column. 6. Collect each fraction in a separate test tube, such as Product No. 15082. 7. Pool all conjugate fractions. The hapten-carrier conjugate may be used for injection purposes without further purification. Telephone: 800-8-PIERCE (800-874-3723) or 815-968-0747 • Fax: 815-968-7316 or 800-842-5007 Internet: http://www.piercenet.com 2 8. If the immunogen is to be stored for several days, sterile filter the conjugate fractions and store them in a sterile container at 4°C or keep them frozen at -20°C. NOTE: For extended storage, the conjugate can be lyophilized and kept at 4°C. Quantitation of Conjugation The degree of conjugation may be estimated by using the following microwell plate protocol. Ellman’s Reagent (Product No. 22582, 5 g), 5,5’-dithiobis-(2-nitrobenzoic acid), will react with sulfhydryl groups to produce a chromophore with maximum absorbance at 412 nm. E412 = 1.44 x 104 cm-1M-1 Using the protocol below, make a standard curve with known quantities of cysteine. Add 10 µl of varying concentrations of cysteine in place of the peptide to wells containing 200 µl of conjugation buffer. This assay will estimate the total numbers of peptide sulfhydryls present before and after conjugation. The presence of EDTA in the buffer salts prevents redox interferences in this assay caused by the carrier protein. The cysteine will produce a similar response to a peptide containing one free sulfhydryl group. A comparison of the absorbance of your samples after modification with Ellman’s Reagent will give you an estimate of the number of free sulfhydryls present on your peptide before and after conjugation. Note: For correct determination of conjugation, the dilution of the peptide solution by addition of the carrier protein must be taken into account. Note: For accurate results, the initial assay of the non-conjugated peptide must be done as soon as possible after reconstitution since disulfide bonds can form during even short term storage of the peptide. 1. Add 200 µl of conjugation buffer to each appropriate well of a microwell plate. Add 210 µl of this buffer to one well for use as a blank. 2. To each of two wells containing buffer, add 10 µl of hapten solution. 3. Add 10 µl of the conjugate to each of two buffer-containing wells. 4. Add 20 µl (1 mg/ml reagent in buffer) Ellman’s Reagent to each well with conjugate, peptide and blank. 5. Incubate microwell plate for 15 minutes at room temperature. 6. Determine absorbance of the wells at 412 nm using a microwell plate reader. Note: Efficiencies vary greatly in this protocol due to differences in the sizes and structure of haptens. The protocol is designed to yield effective immunogens for the widest variety of applications, but is not necessarily optimal for use with all haptens. It may be possible to use less hapten and still obtain good results. If the hapten is precious, be sure to recover uncoupled hapten from the gel filtration column. Example Immunization Protocols Note: Before attempting to immunize animals, consult with someone experienced in these techniques. Mixing the Immunogen with Adjuvant The concentration of the immunogen before mixing with adjuvant will determine the amount of conjugate that will be administered per injection. The conjugate peak coming off the gel filtration column will be obtained in 2-3 fractions. When diluted with an equal volume of adjuvant, the mixture will be at a carrier concentration of about 330-500 µg/ml or 33-50 µg/100 µl injected. This concentration range is suitable for stimulating an immune response in small animals such as mice, rats and rabbits. You may prefer to use higher quantities for larger animals, although it may not be necessary. Preparation of Antigen for Injection 1. Add Imject® Alum dropwise with stirring to the conjugate solution. The ratio of Imject® Alum to conjugate should be in the range of 1:4 to 1:2 for a final Aluminum Hydroxide concentration of 1.12 to 2.25 mg/100 µl. Imject® Alum contains aluminum hydroxide at a concentration of 45 mg/ml. 2. Continue stirring suspension for 30 minutes. Telephone: 800-8-PIERCE (800-874-3723) or 815-968-0747 • Fax: 815-968-7316 or 800-842-5007 Internet: http://www.piercenet.com 3 3. After the suspension is thoroughly mixed, sample with a syringe while continuing to stir. 4. Inject the mixture into the appropriate sites on the animal Note: To avoid anaphylaxis, do not use adjuvants for intravenous injection. Injection and Bleeding The following protocols represent suggested procedures that have proven successful for routine immunizations. The schedules can be customized for convenience or when the condition of the animals warrants such consideration. In any case, injections should be discontinued whenever a severe reaction is observed in the animals—either locally or systemically. Individuals unfamiliar with these techniques should consult an experienced investigator before attempting to immunize. 1. Immunization in Mice: Day 0: Inject 50-100 µg of immunogen (equal to 100-200 µl of antigen/adjuvant mix) per mouse. Typical routes of injection include intraperitoneal or subcutaneous when working with mice. One or two injections may be made per animal. Day 14: Boost with an equivalent amount of immunogen in adjuvant. Day 21: Test bleed to measure antibody response by ELISA. Day 28: Boost again if necessary. Continue with a similar schedule of alternating boosts and test bleeds until a satisfactory response is observed. For monoclonal antibody production, inject either i.p. or i.v. 4-5 days before fusion with the immunogen dissolved in saline (no adjuvant). 2. Immunization in Rabbits: Day 0: Collect pre-immune serum from the rabbit to use as a blank when doing ELISAs after immunization. Store frozen. Inject 100 µg of immunogen (equal to about 200 µl of the antigen/adjuvant mix) into each of 8-10 subcutaneous sites on the back of the rabbit. Day 14: Boost with an equivalent amount of antigen. Day 21: Test bleed to measure antibody response by ELISA. Day 28: Boost again if necessary. Alternate boosts and test bleeds until a satisfactory response is observed. References 1. 2. 3. 4. 5. 6. 7. 8. Germain, R.N. (1986). The ins and outs of antigen processing and presentation. Nature (London) 322, 687-689. Harlow, E. and Lane, D. (1988). Antibodies: a laboratory manual; Cold Springs Harbor Laboratory, Cold Spring Harbor, N.Y. pp. 56-100. Male, D., Champion, B. and Cooke, A. (1987). Advanced Immunology J. B. Lippincott, Gower Medical Publishing, London, Section 8.1-8.8. Sell, S. (1987). Immunology, Immunopathology and Immunity, Elsevier, New York, pp. 69-78. Muckerheide, A., Apple, R.J., Pesce, A.J. and Michael, J.G. (1987). Cationization of protein antigens. I. Alteration of immunogenic properties. J. Immunol. 138, 833-837. Muckerheide, A., Domen, P.L. and Michael, J.G. (1987). Cationization of protein antigens. II. Alteration of regulatory properties. J. Immunol. 138, 2800-2804. Domen, P.L., Muckerheide, A. and Michael, J.G. (1987). Cationization of protein antigens. III. Abrogation of Oral Tolerance. J. Immunol. 139, 31953198. Apple, R.J., Domen, P.L., Muckerheide, A. and Michael, J.G. (1988). Cationization of protein antigens. IV. Increased antigen uptake by antigenpresenting cells. J. Immunol. 140, 3290-3295. © Copyright Pierce Chemical Company, 2/1999 Printed in U.S.A. Telephone: 800-8-PIERCE (800-874-3723) or 815-968-0747 • Fax: 815-968-7316 or 800-842-5007 Internet: http://www.piercenet.com 4 Telephone: 800-8-PIERCE (800-874-3723) or 815-968-0747 • Fax: 815-968-7316 or 800-842-5007 Internet: http://www.piercenet.com 5