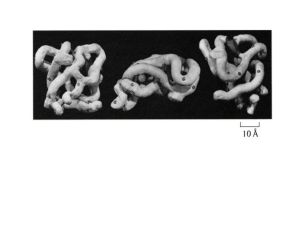
Angstrom – The man and the unit
advertisement

Angstrom – The man and the unit
Brief description:
The angstrom or {ångstrom (Å)} is an internationally recognized unit of length equal to
1 × 10-10 meters (m) or 0.1 nanometer (nm). The unit is widely used in physics, chemistry and
biology to express the sizes of atoms, molecules, and the electromagnetic wavelengths. Also,
the unit is used to measure the integrated circuits components in electronic engineering.
However, the angstrom unit is not recognized as part of the International System of Units (SI).
Nevertheless, the angstrom is often used in the natural sciences and technology to express the
sizes of atoms, molecules, and microscopic biological structures, the lengths of chemical bonds,
the arrangement of atoms in crystals, the wavelengths of electromagnetic radiation, and the
dimensions of integrated circuit parts.
The unit was named after the Swedish physicist Anders Jonas Ångström ([
ŋstrøm]; 1814–
1874). The symbol is always written with a ring diacritic, as in the Swedish letter.
The man behind the unit:
Anders Jonas Angstrom was born into a wealthy, upper class family in
Logodo, Sweden in 1814 during a solar eclipse. He went to the University
of Uppsala where he studied physics and astronomy and eventually
became the private docent of physics. He also got his doctorate at the
University of Uppsala in 1839. Anders had a better chance at an education
and a good life because he was born into the clergy, which is what the upper class was called in
Europe. Back then your social class made all the difference so him being in the clergy ()כמורה
which is the highest and has connections with the church gives him the advantages and
opportunities.
Also in 1839 he became a professor at Uppsala University and got his own observatory. Since
he was first to discover hydrogen in our atmosphere he got to study the aurora borealis
( )הזוהר הצפוניalso known as the northern lights, which was a bright line in the yellow green
region, at his observatory. He then was able to disprove the hypothesis about the Northern
Lights that said that they were caused by sunlight being reflected through airborne ice crystals
and proved that it was excited oxygen atoms. The oxygen atoms emitted the light because they
were "super charged" by then sun in a way which made them produce color (emit photons at
certain wavelengths as we studied). He also invented an instrument that became standard in
1905 for measuring solar radiation. With this knowledge he also helped invent spectroscopy
which is the study of spectral lights.
His most known work was on Optiska Undersökningar. Optsika was one of his optical studies. It
dealt mainly with electrical sparks and the two spectral lines that it produced. In this he also
determined that gas emits light at the same wavelength that it absorbs. He also worked in
thermal studies. He invented a way to measure the temperature considering its electrical
conductivity. He also studied geomagnetism which is also known as terrestrial magnetism
()דיסציפלינה העוסקת בשדה המגנטי של כדור הארץ. Most of his work dealt with spectrums which he
was first to discover such as solar, and atomic spectrums. Despite the importance of his
work he was never honored even in his own country. He was honored more at the university
than in the scientific community. A reward at the University of Uppsala was, for example,
receiving the chair of physics. Though he did get rewarded Secretary of the Royal Society of
Science in Uppsala and other organizations.
Anders Jonas Angstrom contributed many things to the scientific community and left a legacy in
his son and grandson. The pyrheliometer that he invented that measures solar radiation is still
used today. Also he invented the micrometer which was named the angstrom when he invented
it. The micrometer is still a standard SI unit today when measuring the small lines in spectra's.
Anders Jonas Angstrom made many contributions to the scientific field involved with
spectrometry. He was the first to examine the Northern Lights and figure out why they actually
show up as colors. Anders Jonas Angstrom was also the start of a 3 generation long chain of
scientists that contribute to the area of spectrometry. His son and grandson both contributed
much to the scientific field of spectrometry too. His son, Knut Angstrom, was known for the
research that he did while at the University of Uppsala. Also, Knut was known for making his
father's invention, the pyrheliometer, electronic. Knut's son, Dr. Anders Angstrom, named after
his grandfather, used his father's and grandfather's inventions and tools to do field work. All
three of them received many awards, though mainly just in Sweden but none the less, they were
recognized for their major contributions in the field of spectra's. Even though Anders Jonas
Angstrom died in 1874 his work, in a way, carried on for almost another century through his son
and grandson.
Honors:
The Angstrom unit (1 Å = 10−10 m) with which the lengths on a scale of the wavelength of light
or interatomic spacings in condensed matter is measured are named for him.[2] The unit is used
in crystallography as well as spectroscopy.
The crater Angstrom on the Moon is named in his honor.
One of the main building complexes of Uppsala University, the Angstrom Laboratory, is named
in his honor.
The Pyrheliometer
The northern lights
Using the Angstrom unit:
Atomic radius
The concept is difficult to define because the electrons do not have definite orbits, or sharply defined
ranges Rather, their positions must be described as probability distributions that taper off gradually as
one moves away from the nucleus, without a sharp cutoff. Moreover, in con
condensed
densed matter and molecules,
the electron clouds of the atoms usually overlap to some extent, and some of the electrons may roam
over a large region encompassing two or more atoms.
Despite these conceptual difficulties, under most definitions the radii of isolated neutral atoms range
between 0.3 and 3 angstroms. Therefore, the radius of an atom is in many cases approximated to 1 Å.
A pyrheliometer is an instrument for direct measurement of solar irradiance.. Sunlight enters
the instrument through a window and is directed onto a thermopile which converts heat to an
electrical signal that can be recorded. The signal voltage is converted via a formula to measure
watts per square meter. It is used with a solar tracking system to keep the instrument aimed at
the sun. A pyrheliometer is often use
used in the same setup with a pyranometer.
But the main usage of the angstrom unit is measuring the wave length of electromagnetic
waves.
Angstrom exponent is the name of the exponent in the formula that is usually used to
describe the dependency of the aerosol optical thickness, or aerosol extinction
coefficient on wavelength.
Depending on particle size distribution, the spectral dependence of the aerosol optical thickness
is given approximately by
The Angstrom exponent is inversely related to the average size of the particles in the aerosol:
the smaller the particles, the larger the exponent. Thus, Angstrom exponent is a useful quantity
to assess the particle size of atmospheric aerosols or clouds, and the wavelength dependence of
the aerosol/cloud optical properties.
A spectrophotometer is a photometer (a device for measuring light intensity) that can
measure intensity as a function of the light source
wavelength. Important features of spectrophotometers are
spectral bandwidth and linear range of absorption or
reflectance measurement.
easurement. A spectrophotometer is
commonly used for the measurement of transmittance or
reflectance of solutions, transparent or opaque solids, such
as polished glass, or gases. However they can also be
designed to measure the diffusivity on any of the listed
light ranges that usually cover around 200
2000 Å - 25000 Å
using different controls and calibrations
calibrations. Within these ranges of light, calibrations are needed on
the machine using standards that vary in type depending on the wavelength of the photometric
determination.
Convertions:
Astronomical Units - Angstroms Converter
http://www.thecalculatorsite.com/conversions/length/astronomical-units-to-angstroms.php
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
1 angstrom = 100 picometers
1 Å = 100 pm
1 angstrom = 0.1 nanometer
1 Å = 0.1 nm
angstrom = 0.0001 micron
1 Å = 0.0001 µm
1angstrom = 0.0001 micrometer
1 Å = 0.0001 µm
1angstrom = 1.0 × 10-7 millimeter
1 Å = 1.0 × 10-7 mm
1angstrom = 1.0 × 10-8 centimeter
1 Å = 1.0 × 10-8 cm
1angstrom = 1.0 × 10-9 decimeter
1 Å = 1.0 × 10-9 dm
1angstrom = 1.0 × 10-10 meter
1 Å = 1.0 × 10-10 m
1angstrom = 1.0 × 10-11 dekameter
1 Å = 1.0 × 10-11 dam
1angstrom = 1.0 × 10-13 kilometer
1 Å = 1.0 × 10-13 km
1angstrom = 6.68458712267 × 10-22 astronomical unit
1 Å = 6.68458712267 × 10-22 au
1angstrom = 1.05702341103 × 10-26 light year
1 Å = 1.05702341103 × 10-26 ly
1angstrom = 3.24077649 × 10-27 parsec
1 Å = 3.24077649 × 10-27 pc
1 angstrom = 0.003937007874 microinch
1 Å = 0.003937007874 µin
1 angstrom = 3.937007874 × 10-6 mil
1 Å = 3.937007874 × 10-6 mil
1 angstrom = 3.937007874 × 10-9 inch
1 Å = 3.937007874 × 10-9 in
1 angstrom = 3.28083989501 × 10-10 foot
1 Å = 3.28083989501 × 10-10 ft
1 angstrom = 6.21371192 × 10-14 mile
1 Å = 6.21371192 × 10-14 mi
1 angstrom = 1.09361329834 × 10-10 yard
1 Å = 1.09361329834 × 10-10 yd
1 angstrom = 5.39956803456 × 10-14 nautical mile
1 Å = 5.39956803456 × 10-14 M