Infra-Red Analysis of Aspirin, Advil and Tylenol
advertisement

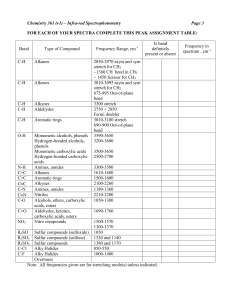
Infra-Red Analysis of Aspirin, Advil and Tylenol Introduction This laboratory experiment utilizes long wavelength electromagnetic energy called infra-red "light". The energy in the infra-red (IR) region of the "light" spectrum may be described as the energy contributing to the "heat" rising from the glowing red color of the wires one observes in a toaster. This overall energy, however, is "split" into each individual wavelength in an Infra-Red Spectrometer much like visible light is "split" into its component colors (red, orange, yellow, green, blue, indigo and violet) by a prism or by water vapor as in a rainbow. In IR spectroscopy, wavelengths are not used in studying compounds: wave numbers are. Wave numbers are reported in cm-1 (reciprocal centimeters). The importance of the wave numbers is that various functional groups behave differently when effected by IR energy of different wave numbers [and, hence, wavelengths] and the response in that wave number region is a "fingerprint" for THAT functional group. Charts from which comparisons may be made are typically reported in wave numbers, not in wavelengths -- to a small degree that has been changing, however, the older literature is the basis for many reference texts and are still reported in wave numbers. The CRC Handbook of Chemistry and Physics is the classic introductory students' reference text and reports most IR values in wave numbers. With all of this talk of IR energy effecting the functional groups on organic molecules, how does this phenomenon occur? If you think of organic molecules as consisting of balls joined together by springs (Figure top left): it makes the explanation easy. Although organic molecules are never really "still", these molecules may be forced to move in directions not done under standard conditions. IR energy "tweaks" the organic molecules and causes some to functional groups to "stretch" (much like the spring between 2 balls), Figures above right, 1 and causes other functional groups to "bend" (much like two balls held together by a spring, again), Figure immediately left. This is of incredible value to an organic chemist for these molecules, by its/their functional groups' responses, are now easy to identify. Each functional group leaves its own "fingerprint" or "peaks" at specific wave numbers OR over a wave number range. All one has to do is to obtain an IR spectrum of a compound and compare it to a book of spectra for identification of the molecule; OR write down the location of the major peaks, identify them and put the molecule back together. A few of the more important wave numbers and the phenomena associated with each region of energy are listed below in the table: Wave Number Phenomenon Functional Group 3600-3200 O-H stretch Alcohols, water 3550-3000 N-H stretch Primary amines 3000-2850 C-H stretch Alkyl groups 1800-1680 C=O stretch Carbonyl groups 1670-1615 C=C stretch Alkenes 1200-1070 C-O stretch Ethers 2 Mechanics of IR Spectroscopy When obtaining an IR spectrum of a compound, there is a certain set of information you need for the spectrum to be valid: 1. There must be some way to ascertain that the peaks are really where you say they are. Therefore, you must run a standard with each sample for which you are obtaining an IR spectrum. 2. The standard must be accepted unanimously within the scientific community. 3. There must be enough sample for the instrument to provide you with a spectrum, but not so much that it is difficult to interpret the spectrum. 4. Your results must be reproducible by someone other than yourself. The first and second points are easily met: polystyrene is the standard of choice and is accepted unanimously around the world. The IR spectrum for polystyrene is shown right: 3 Notice that it is fairly complex. How in the world is it possible to use this compound as a standard? That has also been unanimously agreed upon: one specific peak is used as the standard peak. That peak is at 1601 cm-1. Wherever the "1601 peak" falls on the chart paper is EXACTLY where 1601 cm-1 IS. It matters not of the peak falls 500 wave numbers away from where it is "supposed to be"! The presence of that peak IS where 1601 cm-1 falls (Figure below): The next three figures show IR spectra of several simple organic compounds. Note that the structures of the compounds are drawn with the scan as well as that the peaks of importance are identified for you. The compounds are isopropyl alcohol (rubbing alcohol), acetone (finger nail polish remover) and benzaldehyde (artificial oil of almond extract). Each compound has been selected especially for this experiment: Isopropyl alcohol, so you would observe the O-H stretch; acetone, so you would observe the carbonyl (ketone) peak at 1700 wave numbers; benzaldehyde, so you would be able to observe the aromatic (benzene ring) C-H interactions. Each one of these peaks will be of significance in the IR spectral analysis of aspirin, Tylenol and Advil. Listed with each IR spectrum of each compound is a table of functional groups that correspond by number to those in the jpeg. 4 5 RED number on jpeg 1 2 3 4 5 6 Functional group CH stretch; iso-propyl Iso-propyl Iso-propyl Secondary alcohol Iso-propyl; secondary alcohol Iso-propyl 7 C-H rock RED number on jpeg 1 2 3 4 5 6 7 8 Functional group C-H stretch C-H stretch CH stretch; "iso-propyl" "iso-propyl"; C-H bend "iso-propyl"; C-H bend Aliphatic ketone; C-O stretch C-O stretch; C-C stretch C-C stretch; CH rock RED number on jpeg Functional group 1 Aromatic 2 Aromatic aldehyde 3 Aromatic aldehyde 4 Carbonyl 5 Aromatic fingerprint region Although there are three methods of preparing samples for IR analysis, one will be discussed as the other two are discussed in the Operations section. Solid IR spectroscopy is fairly easy to perform if you have two pieces of steel, a piece of firm paper (a note card works) with a 0.75-1 cm hole punched in it, anhydrous KBr, aluminum foil and a mechanical press that is capable of attaining high pressures. A small amount of the sample is mixed with the KBr and placed on top of the aluminum foil wrapped around the first steel piece along with the paper with the hole. The sample needs to fir in the hole. The other piece of steel, also wrapped in aluminum foil, is placed on top of all of this and the stack of materials is placed on the high-pressure press. The stack is subjected to high pressures, which causes high temperatures within the chemical mixture and a "thermopressurized" thin "plastic" film is created. This is then analyzed by IR. Operating Steps of The Infra-Red Spectrometer 1. 2. 3. 4. 5. Turn on the instrument -- the switch is on the right side of the instrument near the back. Let warm up for 30 minutes. Put a pen in the pen holder on the chart recorder. Do NOT press it in hard, simply screw it in. Press 0.5 for "Chart Expansion". Press "3" for scan time. 6 6. Insert the polystyrene standard in the light beam path. Make certain you are starting on the LEFT side of the scan sheet, i.e., 4000 cm-1. 7. Press "Scan" and lift the wire that supports the pen with your fingernail -- not too high, now. 8. Watch the wave number panel (LED). 9. When the wave number panel reads "1630", let go of the wire that you have been holding so the pen makes contact with the paper. 10. When the wave number panel reads "1590", pick up the wire, again, lifting the pen from the paper. The deep peak left behind is your "1601 cm-1" standard for location on your chart paper. 11. Press "Chart", then press the "¯ " key on the "Parameter Adjust" panel to back your paper up to the original starting position of the paper (4000 cm-1, more or less). 12. Remove your polystyrene standard is it is very heat fragile. 13. Place the female end of the salt plate holder on a tissue in such a manner as to receive the salt plates and the male end. 14. Insert one salt plate. IMPORTANT: clean with methylene chloride (carcinogenic) or methanol -- NOT WATER!!!!! These plates are KBr, i.e., WATER SOLUBLE. Wipe with a Kim Wipe. Refrain from touching the optical surface with your fingers. The following steps are for NEAT samples. If you use a Nujol mull, skip down to step 1 after Step 20. 15. Place a drop of your "neat" ORGANIC sample on the salt plate, cover this with the other salt plate and screw in the male end of the plate holder snugly. 16. Change the pen to another color so that you can tell the difference between your standard and the sample. 17. Place the plate holder assembly in the light beam path and press "Scan". 18. Let Scan. 19. Remove the plate holder assembly in the light beam path. Tear off and label your spectrum. 20. Separate the assembly and clean the salt plates as described, below: a. b. c. d. e. Unscrew the assembly and remove salt plates. Separate the salt plates, holding them on their sides. Wipe with a Kim Wipe. Wipe with a Kim Wipe dampened with methanol or methylene chloride. Let air dry. 7 f. If you have completed your experiment, place the plates in their protective styrofoam cover and place in the plastic canisters in which they were set out. g. If you have not completed your experiment, return to Step 13 and repeat each step until you have completed your experiment. The following steps are for a Nujol mull. 1. Place a few crystals of your solid sample in the agate mortar. Add several drops (this takes practice) of Nujol or Fluorolube oil to your sample and grind/mix with the agate pestle to make the mull. 2. Place a drop of your mull on the salt plate, cover this with the other salt plate and screw in the male end of the plate holder snugly. 3. Change the pen to another color so that you can tell the difference between your standard and the sample. 4. Place the plate holder assembly in the light beam path and press "Scan". 5. Let Scan. 6. When the scan is complete, remove the plate holder assembly from the light beam path. Tear off and label your spectrum. 7. Separate the assembly and clean the salt plates as described in Step 20, above. LAST STEP: the last person to do their experiment is to turn OFF the instrument prior to leaving the laboratory. EXPERIMENTAL IR Spectrophotometer Agate & Ceramic pestle and mortar Nujol oil Fluorolube oil Plate holder assembly KBr salt plates Aspirin tablet Tylenol tablet Advil tablet Polystyrene standard Sample Holder IR Spectrophotometer Place one of the tablets in your ceramic mortar and pulverize it with your ceramic pestle. Place a few crystals of the sample in the agate mortar, add Nujol or Fluorolube oil, as above in the operating instructions, and grind together. Place a small sample onto one of the salt plates and cover with the other salt plate. Proceed as above in the operating instructions. 8 Label the major peaks of importance on all three spectra (you will find most of these in the Theory section if you look closely) and attach the labeled spectra to your lab write-up. Compare the IR of all three analgesics. Describe in the space, below, the similarities and the differences between the three spectra: Infra-Red Property Differentiation between Aspirin, Advil and Tylenol LINKS TO SCANS ARE BELOW: Aspirin IR Scan Similarities Ibuprofen IR Scan Aspirin Advil Differences 9 Tylenol IR Scan Tylenol Sources Carman, F.S. III: Organic Chemistry: An Introduction to Laboratory Methods for Tight Budgets, A One Semester Course. (Kinko's: Reno)© 1993. p. 25. This page reconstructed 11 August 2008, 1519 hours, PDT. 10