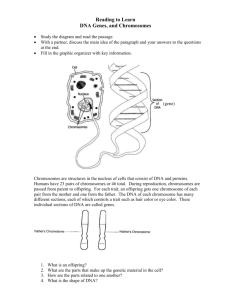
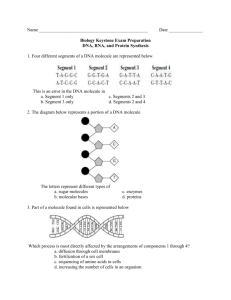
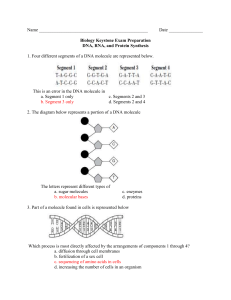
NOTES FOR BIOLOGY 101: Dr. Charles Masarsky, Instructor
advertisement

NOTES FOR BIOLOGY 101: Dr. Charles Masarsky, Instructor (Chapter, Table & Figure numbers refer to the Campbell, et al text.) LAB #1: Scientific Method: PP 3-7 in the lab manual, with modifications TBA. LECTURE #1: Chapters 1&2 What Is Science? Scientists seek to enlarge their knowledge of nature in a self-correcting way. Someone cannot simply announce that his or her latest hunch is “new knowledge” and be considered a scientist. A scientist is required to describe the process by which they gathered observations that led them to this “new knowledge”. Other scientists can then duplicate the same process or develop additional processes for gathering observations – also known as data – relevant to the alleged “new knowledge”. If repeated future observations fit the “new knowledge”, it gains scientific support. If some observations do not fit, the “new knowledge” must be revised to explain all existing observations. Any “new knowledge” in science must be tested with repeated, independent observations. It is this self-correcting aspect of science that makes it different from most other methods of gaining knowledge. One method of gathering data is descriptive research. For example, the Spanish scientist Santiago Ramon y Cajal looked at nerve cells in brain tissue and spinal cord tissue under his microscope in the late nineteenth century. Everything he had read about this tissue told him that the nerve cells are all directly connected to each other. However, when he looked in his microscope, he saw gaps between the nerve cells in this tissue. He carefully described his technique for preparing and examining his tissue samples. After a number of years, many other scientists reported that they independently observed the gaps first described by Cajal. Eventually, these gaps (later named “synapses”) were considered critical to our understanding of the nervous system, and Cajal received the Nobel Prize. Another method of gathering data is experimental research. First, a scientist develops an educated guess that will form the groundwork for an investigation into some aspect of nature. Such an educated guess is called a hypothesis, from a Greek root meaning “groundwork” (the word “hypothetical” comes from the same root). Often, a scientist’s hypothesis will grow out of the observations typical of descriptive research. This is followed by a type of reasoning that goes from the specific to the general. Such reasoning is called inductive reasoning or induction. For example, in 1964, Aklilu Lemma, an Ethiopian scientist, described a large number of dead snails close to where a group of women were washing their clothing in a stream. He found out that the women were using a local berry for soap. He hypothesized that the reason these specific snails were dying was because the berry was poisonous to this variety of snail in general. Lemma’s hypothesis was significant, because this particular type of snail carries a disease that afflicts some 200 million people worldwide (schistosomiasis). 1 The next stage of experimental research is to develop a means of testing the hypothesis. This usually requires a deduction – a form of reasoning that goes from the general to the specific. For example, if the local berry is poisonous to the disease-carrying snails in general, then an extract of the berry should kill live snails in the laboratory. This type of formal test of a hypothesis that comes from deductive reasoning is an experiment. Lemma did, in fact, perform this experiment. Dr. Makhubu continued this line of research on the berry at the University of Swaziland, and the berry is now being used in the fight against schistosomiasis. Biology as a Science Biology is the science of life. Living things and living processes can be studied with both descriptive and experimental methods at many levels of organization. Some fields of study within biology concern themselves with the largest level of biological organization – the biosphere – the life-bearing layer of the planet Earth. Other fields of study within biology study smaller-scale levels of organization. The smallest level of biological organization is the molecule – the domain of biochemistry and molecular biology. Before biologically significant molecules can be understood, even smaller levels of organization have to be discussed. These levels form the domain of basic chemistry. Basic Chemistry Matter takes up space and has mass. An element is a form of matter that cannot be broken down into another substance by chemical reactions. The smallest bit of an element you can have without turning it into some other form of matter is an atom. Atoms are composed of subatomic particles. The subatomic particles we will discuss are protons, neutrons, and electrons. Most of the mass of an atom is in the protons and neutrons, with the mass of the electrons being almost negligible. The unit used to represent the mass of subatomic particles is the Dalton or the atomic mass unit (AMU). A proton’s mass is approximately one AMU; same for a neutron’s mass. An atom’s protons and neutrons are located in the center of the atom – the nucleus. The electrons orbit about the atom. The number of protons in the nucleus of an atom of a particular element is its atomic number. Every atom of an element has the same atomic number. The atomic number is written as a subscript to the left of the symbol for that element. For example, the four most common elements in living organisms are carbon, hydrogen, nitrogen and oxygen. Their chemical symbols, with their atomic numbers are 6C, 1H, 8O, and 7N. Other biologically significant elements include (but are not limited to) phosphorus (15P), sulfur (16S), calcium (20Ca), potassium (19K), and sodium (11Na). Although the atomic number of every atom of an element is the same, the atomic mass can be different. Atoms of the same element but of differing atomic mass are called 2 isotope of that element. The atomic mass is usually noted as a superscript just above the atomic number. Write the notation for the most common isotope of carbon – carbon twelve:_____________. Some isotopes are unstable, and the nucleus will shed particles and energy from time to time. These are radioactive isotopes. An example that is important in certain lines of research is carbon fourteen. LAB 2: Scientific Method, pages 7-9 in lab manual with modifications TBA LECTURE 2: Chemical Bonding, Chapter 2, continued The electrons orbiting the nucleus of an atom occupy various energy levels or electron shells. The electrons in an electron shell have a natural tendency to pair up. Each electron shell can only accommodate so many pairs of electrons. The number of electrons orbiting an atom is normally the same as the number of protons in the nucleus. A diagram of how the electrons are arrayed in their electron shells around a particular atom is called an electron configuration. The first electron shell can accommodate no more than one pair of electrons. So, the electron configuration of 1H would be: The electron configuration of helium (2He) would be: Notice that the one electron shell of a helium atom is completely filled. An atom in which the outer electron shell is completely filled is very stable – it does not have any tendency to enter chemical reactions with other atoms. The chemical behavior of an atom depends on the number of electrons in the outermost electron shell. This shell is called the valence shell, and the electrons in that shell are called the valence electrons. Atoms with completed valence shells, such as helium, do not readily enter chemical reactions with other atoms. The next two electron shells can accommodate a maximum of 4 electron pairs each. An electron shell fills with unpaired electrons first; then the electrons begin to pair up. So, the electron configuration of 6C would be: The electron configuration of 7N would be: The electron configuration of 8O would be: The presence of unpaired electrons in the valence shell gives an atom a tendency to chemically bond with other atoms. Chemical bonds tend to complete the valence shell in one or both of the atoms involved with the bond. The periodic chart arranges atoms with similar chemical properties in the same row. For example, He, Ne and Ar are all in 3 the same row, and they all have a completed outer valence shell – no unpaired electrons. Therefore, these elements do not readily form chemical bonds. H, on the other hand, has one unpaired electron in its valence shell. Therefore, each H atom can form a single bond with another atom. For example, one H bond can bond with another. By sharing their electrons, the H atoms complete each other’s valence shells. This molecule of hydrogen would be represented in this way: H-H O has two unpaired electrons in its valence shell. Therefore, it can form two single bonds, such as in water (H2O): O / \ H H O can also form double bonds, as in molecular oxygen: O=O C has four. N has three. These are also the number of bonds an atom of each of those elements can form. The number of unpaired electrons in an atom’s valence shell also represents the number of chemical bonds it can form, and is called the atom’s valence. The sorts of chemical bonds in which the electrons are shared between atoms are called covalent bonds. Sometimes the electrons in a covalent bond are shared more or less equally between atoms. Such a covalent bond is called a nonpolar covalent bond. The covalent bonds in molecular hydrogen and molecular oxygen are examples. The amount of attraction an atom has for the electrons in a chemical bond is called electronegativity. If one atom in a chemical bond is more electronegative than another, it tends to hold the electron more closely. Such a bond is called a polar covalent bond. For example, oxygen is more electronegative than hydrogen. Therefore, the water molecule has a slightly negative pole at the oxygen side and a slightly positive pole at the side with the two hydrogen atoms. Water is a polar molecule. Extremely electronegative atoms can completely strip away an electron from another atom. For example chlorine (17Cl) has one unpaired electron in its valence shell. So does 11Na. However, if chlorine completes its pair, it also completely fills its valence shell. This makes chlorine very electronegative. When it bonds with sodium, it completely strips away sodium’s one valence electron. In effect, sodium is left with a complete valence shell (the second electron shell), because the one unaccompanied electron has been stripped away from the third electron shell. Chorine also has a complete valence shell (the third electron shell). This type of bond is called an ionic bond. The formation of chemical bonds between atoms of different elements creates a substance different than the original elements. For example, hydrogen and oxygen are 4 both colorless, odorless gasses at room temperature. Yet, when one oxygen atom and two hydrogen atoms are combined with covalent bonds, they form water – a liquid at room temperature. To take another example, sodium is a metal, and chlorine is a toxic gas. Neither substance is edible. Yet, when you combine an atom of each with an ionic bond, you have table salt, which is often used to flavor food. (In general, ionic bonds form salts). These substances such as water and table salt formed by fixed ratios of elements are called compounds. They have properties completely different from the properties of the individual elements composing them. These different properties that appear as you combine simple components (such as atoms of elements) into entities with more complexity (such as molecules of compounds) are called emergent properties. Another example of emergent properties is the combination of one atom of carbon – usually a black solid at room temperature – with four atoms of the colorless, odorless gas hydrogen. The result is a combustible gas called methane. The molecular formula (a “recipe” indicating the ratio of the elements composing a compound) for this gas is CH4. The structural formula (a diagram indicating the single and double bonds) is: H | H-C-H | H Another example: hydrogen and nitrogen are both odorless gasses at room temperature. However, combine them into ammonia, and you have a gas with a powerful odor. Nitrogen, with a valence of three can combine with three hydrogen atoms. The molecular formula for ammonia is NH3. The structural formula is: The making and breaking of chemical bonds are called chemical reactions. The starting materials are reactants, and the end results are products. If a reaction converts all of the reactants into products, it is an irreversible reaction. However, most reactions are reversible. For example, when hydrogen and nitrogen form ammonia, some of the ammonia reverts to the original reactants. The chemical equation for this reaction looks like this: 3H2 + N2 __________ 2NH3 LAB 3: Selected Properties of Water In your experience, does water tend to flow uphill or downhill? _____________ A stalk of celery contains a great deal of water. Develop a hypothesis about what would happen to the water in a stalk of celery placed in an upright position; would the water move up or down in the stalk?__________________________________________ 5 Experiment #1: Place 100 ml of water in a graduated cylinder, and add enough food coloring to give it a definite color. Place a stalk of celery in the cylinder so that the bottom of the stalk reaches all the way down to the bottom of the beaker. Put this experimental preparation aside while you go on to the rest of the lab. When you remove the celery stalk from the graduated cylinder at the end of the lab, do the results support or refute your hypothesis?______________________________________________________________ _______________________________________________________________________ Do these results suggest that water molecules are neutral, or that water molecules have the ability to cling to the sides of a small tube, allowing a column of water to rise up a plant?___________________________________________________________________ ________________________________________________________________________ * * * A water molecule is composed of two hydrogen atoms and one oxygen atom. Using the periodic table in the back of your lab manual, calculate the mass of the typical water molecule in A.M.U. (round off to the nearest A.M.U.). __________________________ Staples are made of steel. Steel is composed mostly of the element iron, also known as ferrium, the chemical symbol for which is Fe. Using the periodic table, what is the atomic mass of an iron atom? _________ Based on this information, plus your own personal experience, which would you expect to be heavier – water or steel? ________ Based on this understanding, develop a hypothesis about whether or not a steel staple will float or sink in water.___________________________________________________________ Experiment #2: Hold a steel staple a few inches above the surface of a beaker of water. Drop the staple. Do the results support or refute your hypothesis?__________________ Experiment #3: Place a steel staple on a piece of tissue paper. Using the tissue paper like a stretcher or a hammock, gently lay the paper on the surface of the water. Observe what happens to the staple. Do the results support or refute your hypothesis?______________________________________________ Does the behavior of the steel staple suggest that water molecules are neutral, or does this behavior suggest that water molecules are polar entities that can cling to each other strongly enough to create a sort of “skin” on the surface of a quiet body of water?____________________________________________________________ At a naturally-occurring quiet body of water such as a pond, can you observe something that behaves in a manner similar to the staple in Experiment 3? If so, what?___________________________________________________________ * * * In your experience, can two objects occupy the same space at the same time? __________ Based on your answer, if you took an object whose volume was 10 ml and 6 another object whose volume was also 10 ml, how much space would you hypothesize both objects together would occupy:________________________________________. Experiment #4: Place 10 ml of water in a graduate cylinder. In another graduated cylinder measure out 10 ml of table salt. You now have two 10 ml objects. Now, combine them in one cylinder. How much space is occupied by these two objects together?____________________ Do these results support or refute your hypothesis?___________________________________ Table salt is primarily NaCl. Molecules of NaCl are held together with ionic bonds, with the Na side being positively charged and the Cl side being negatively charged. What property would a substance need to be an excellent solvent of NaCl?__________________________________________________________________ _______________________________________________________________________ * * * Based on your experience, is sweating a common reaction of the human body to hot weather? _____________ If the evaporation of water only took a small amount of heat, would the human body’s reaction to hot weather be a good cooling strategy?____________ How about if the evaporation of water took a large amount of heat?______________________________________________ LECTURE 3: Chemistry of Water, Chapter 3 Because oxygen is more electronegative than hydrogen, and because the water molecule is asymmetrical the molecule ends up having a positive pole and a negative pole, like a battery or a magnet. The existence of poles makes water a polar molecule. Just as the positive pole of a magnet will attract the negative pole of another magnet, the positively charged hydrogen side of one water molecule will attract the negatively charged oxygen side of another. This attraction between water molecules is the basis for hydrogen bonding. See Figure 3.2. One of water’s emergent properties due to hydrogen bonding is the fact that water molecules will attract each other. This property is called cohesion. A closely related emergent property is the attraction of water for other substances. This property is called adhesion. These two properties together explain the transport of water in plants such as a tree (See Figure 3.3) or a stalk of celery (as in your lab experiment). Adhesion allows water to stick to the walls of the water-conducting cells within the plant. Cohesion preserves the integrity of this rising column of water, preventing the column from breaking up. A property directly resulting from cohesion is the formation of an ordered arrangement of molecules that creates a sort of “skin” at the interface between a quiet 7 body of water and air. This property is called surface tension. Surface tension explains the behavior of the paper clip in your lab experiment, and the water strider in Figure 3.4. The energy of motion of any body is called kinetic energy. The total amount of kinetic energy of the molecules composing any particular object is its heat. The average kinetic energy of the molecules at a particular location in a particular object is that location’s temperature. For example, a swimmer’s body may have a higher temperature than the ocean they are swimming in, but the total heat of the ocean will be immensely greater than the total heat in the swimmer’s body. The amount of heat required to raise the temperature of one gram of a substance one degree Centigrade is that substance’s specific heat. The specific heat of water is 1 cal/g/oC. This is greater than the specific heat of most substances, because the kinetic energy of the heat must overcome the hydrogen bonds between water molecules before the molecules will move faster. This makes water a good heat buffer, keeping most places on earth within a range sustainable for life. (See Figure 3.5.) Compared to most liquids, more heat is required to evaporate water. This is also due to hydrogen bonding. One result of the heat absorbed and then carried away by water during evaporation is evaporative cooling. This mechanism is the reason that sweating and panting enable animals to cool themselves during hot weather. Hydrogen bonding also causes water molecules to organize themselves into a crystalline lattice when liquid water is frozen (Figure 3.6). This lattice structure locks the water molecules into a less dense configuration than the than they assume in the liquid form. Therefore, ice floats. Therefore, liquid water remains under a frozen pond, a frozen lake, or the Arctic ice, enabling marine and aquatic life to survive. The polar nature of the water molecule enables the oxygen end to pull at anything with a positive charge, and the hydrogen to pull at anything with a negative charge. For example, in table salt (NaCl), the oxygen ends of several water molecules will surround the Na+ ion, while the hydrogen ends of several water molecules will surround the Cl- ion (Figure 3.7). In other words, the polarity of the water molecule makes it a very versatile solvent. A substance dissolved in water, such as table salt, is called a solute. Any solution with water as the solvent is an aqueous solution. The vast majority of chemical reactions in living organisms take place in aqueous solution. If you take the molecular mass of a substance, but express it in grams, you have a mole of that substance. A mole of a substance always contains the same number of molecules (approximately 6.02 x 1023, which is called Avogadro’s number). If you dissolve a mole of solute into one liter of water, you have a one molar (1 M) solution. For example, table salt is NaCl. The atomic mass of Na is 23 AMU (rounded off to the nearest AMU or Dalton), and the atomic mass of Cl is 35 AMU. Therefore, the molecular mass of one molecule of table salt is 58 AMU. To make a 1 M solution, you would dissolve 58 grams of NaCl into 1 liter of water. Biological functions require specific molarities of certain substance to proceed normally. In pure water, a very small percentage of molecules will break up into ions: H+ and OH-. If there are more H+ than OH-, you no longer have pure water, but a solution known as an acid. If your have more OH- ions, you have a base. There is a scale to represent how acid or how basic a solution is; it is called the pH scale (Figure 3.9). Pure water has a pH of 7. The “ultimate” acid would have a pH of 0. The “ultimate” base 8 would have a pH of 14. Biological functions in any organism require a certain range of pH levels. Substances that resist changes in pH are called buffers. These substances can be critically important in maintaining pH within tolerable limits for a particular organism. 9 LAB 4: Microscope – pp 12- 21 LECTURE 4: Building Blocks of Carbon-Based Molecules, Chapter 4 Hydrogen has 1 unpaired electron in its outer shell, giving it a valence of 1. Oxygen has a valence of 2. Nitrogen has a valence of 3. Carbon is very chemically versatile, because it has a valence of 4, giving it the ability to bond with 4 other atoms. This makes it the backbone of a diversity of molecules. Figure 4.4 10 Molecules formed of carbon and hydrogen only are hydrocarbons. They are found in petroleum, and are major constituents of fats. Both petroleum and fats are formed with mostly non-polar bonds, making them difficult to mix with water = hydrophobic. Hydrocarbons can vary in length, they may include both single and double bonds, they can involve branching, and can sometimes form ring structures. Organic molecules with the same number of atoms of the same elements but different structures are called isomers. Figure 4.5 Figure 4.7 11 Although hydrocarbons are hydrophobic, they can be attached to certain other chemical groups with more polar bonds. The resulting organic molecule becomes more hydrophilic, and therefore soluble in water, and available for chemical reactions in aqueous solution. These important portions of organic molecules are called functional groups. Functional groups include: Hydroxyl group: When this group is the major functional group, the molecule is an alcohol, which usually ends in the suffix “ol”. -OH. Carbonyl group: When this is the major functional group, and it is located at the end of the carbon skeleton, it is an aldehyde. If it is within the carbon skeleton, it is called a ketone. \ C=O / Carboxyl group: When this is the major functional group, the molecule is an organic acid. The H of this group tends to dissociate, giving up an H+ ion. O // -C \\ OH Amino group: When this is the major function group the molecule is an amine. If it also has a carboxyl group, it is called an amino acid. H / -N \ H Sulfhydryl group: When this is the major functional group, the molecule is called a thiol. -SH Phosphate group: When this is the major functional group, the molecule is an organic phosphate. O || -O-P-O| O Methyl group: When this is the major functional group, molecule is a methylated Figure 4.10 compound. (-CH3) 12 LAB 5: Microscope, p. 21-end LECTURE 5: Macromolecules, Part I, Chapter 5 There are four main classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic acids. Some of these molecules are huge molecules called macromolecules. Macromolecules are polymers (poly = many; meris = part), which are made up of smaller units called monomers. The typical monomer has at least one hydrogen atom and at least one hydroxyl group at some site on the molecule. Building up polymers is generally done by bonding a hydrogen atom from one monomer with a hydroxyl group from another, leaving the original monomers bonded together and releasing a molecule of water. This process is dehydration synthesis. The reverse process – breaking a polymer down to smaller fragments, and eventually to its individual monomers – is done by adding water. This process is hydrolysis. You can almost think of this as a more complex form of the changes you see when water interacts with table salt. Adding water breaks the salt molecules down into smaller units (sodium and chlorine ions) – a process analogous to hydrolysis. Taking the water away (by evaporation, for instance) allows the sodium and chlorine to get back together again, to form the larger unit – molecules of table salt. See Figure 5.2 13 Carbohydrates Think, “hydrated carbon”. The molecular formula for any carbohydrate is some multiple of carbon plus a molecule of water – CH2O. The monomers of carbohydrates are simple sugars, called monosaccharides. Sugars, including monosaccharides, usually end in the suffix “–ose”. Simple sugars are classified in two basic ways. One form of classification is by the number of carbons in the molecule. For example, any 3-carbon sugar is a triose; any 5-carbon sugar is a pentose; any 6-carbon sugar is a hexose. Glucose is one of the more important 6-carbon sugars in biology. Any hexose, including glucose has the molecular formula C6H12O6. The other form of monosaccharide classification is by the location of the carbonyl group, which all simple sugars have. If the carbonyl group appears at one end of the carbon skeleton, it is an aldehyde, which makes the sugar an aldose. Glucose is an example of an aldose. On the other hand, if the carbonyl group appears within the carbon skeleton, it is a ketone, which makes the sugar a ketose. For example, fructose, like glucose, is a hexose, but it differs from glucose in being a ketose. See Figure 5.3 The carbon atoms in a monosaccharide are numbered, with the carbon at the end closest to the carbonyl group being carbon #1. In aqueous solution, monosaccharides exist in both linear and ring forms. See Figure 5.4 When monosaccharides are joined by dehydration synthesis, the resulting bond between monosaccharides is called a glycosidic linkage. See Figure 5.5 Very large carbohydrates include energy storage molecules, such as starch in plants and glycogen in animals. Some large carbohydrates are not for energy storage, but are used for structural purposes, such as cellulose and chitin. See Figures 5.6, 5.8, and 5.10 14 Lipids Lipids are mostly non-polar, and therefore hydrophobic. The most characteristic lipids are fats. One building block of fat is the three-carbon alcohol known as glycerol: H | H-C-OH | H-C-OH | H-C-OH | H The other building block of fat is a long hydrocarbon chain with a carboxyl group at one end – a fatty acid: O H HH \\ | | | C-C-C-C…………….etc. / | | | OH H H H In a dehydration synthesis reaction, the carboxyl group of the fatty acid gives up its OH group, while an OH group of glycerol gives up a hydrogen atom. The result is the creation of a water molecule and a bond between the carbon of the fatty acid’s carboxyl and the oxygen of glycerol’s OH – an ester linkage. One fatty acid forms an ester linkage to each OH site on the glycerol molecule. This combination of glycerol and 3 fatty acids is a fat, aka a triglyceride. If all of the bonds in the carbon skeletons of the fatty acids composing a fat are single bonds, then the molecule is completely saturated with hydrogen – a saturated fat. Saturated fats are solid at room temperature, and are often found in animal fats (the fats in meat or in butter, for example). If some of the bonds in the carbon skeletons of the fatty acids composing a fat are double bonds, then the molecule is not completely saturated with hydrogen – an unsaturated fat. Unsaturated fats are liquid at room temperature, and are often found in vegetable and fish oils. Certain isomers of unsaturated fats – the trans isomers – are reputed to be even more of a risk factor for cardiovascular disease than saturated fats. For this reason, there has been a lot of negative press about trans fats. See figures 5.11 and 5.12 15 In a phospholipid, there are two fatty acids attached to glycerol instead of three. The third position on the glycerol is occupied by a phosphate group, which carries a slight negative charge. This creates a long fatty acid “tail” which is non-polar, and therefore hydrophobic, with a polar phosphate “head” which is hydrophilic. This feature comes into play in the formation of the plasma membrane of a cell. The major building block of such a membrane is a double layer of phospholipids, with the hydrophobic tails inside and the hydrophilic heads in contact with the water outside. Additional small molecules can be attached to the phosphate, creating a variety of phospholipids. See Figures 5.13 and 5.14 16 Cholesterol is a lipid with composed of four hydrocarbon rings, with a hydroxyl group at one end. Cholesterol and all molecules built on cholesterol are called steroids. Steroids are components of certain cell membranes and certain hormones. See Figure 5.15 17 Nucleic Acids In the 1980s, a series of satellite probes rendezvoused with Halley’s comet, and analyzed the gasses in its tail. The basic elements necessary for life – carbon, hydrogen, oxygen and nitrogen – are all present in the comet’s tail. Also, the % of each element is approximately the same as that in a typical living organism. Yet, the tail of a comet is not a living organism. You need more than just the right elements to make something alive. A living organism is not just matter; it’s matter organized by biological information. At the molecular level, this information is stored and handled by a class of compounds called nucleic acids. The monomer of a nucleic acid is called a nucleotide, which consists of a pentose, a nitrogenous base, and a phosphate group. The pentose is either ribose or deoxyribose. There is a numbering system for each of the five carbons in the pentose; the carbon that sticks up from the ring is the #5 carbon, usually written as “5’carbon” (“five prime carbon”). A phosphate group is attached to the 5’ carbon. A nitrogenous base is attached to the 1’ carbon (the carbon at the end closest to the carbonyl group, in the sugar’s linear form). A nucleic acid built up from ribose monomers is ribose nucleic acid, or RNA. A nucleic acid built up from deoxyribose monomers is deoxyribose nucleic acid or DNA. DNA uses four nitrogenous bases: adenine, thymine, guanine and cytosine. In RNA, adenine, guanine and cytosine are also used, but uracil replaces thymine. Joining nucleotides together to form a polymer is accomplished by two pentose sugars being linked by a phosphate group. This creates a backbone of repeating sugarphosphate units, with nitrogenous bases extending from the backbone. One free end of the polymer is attached to the 5’ carbon, and is called the “5’ end”, while the opposite end is attached to the 3’ carbon, the “3’ end”. The sequence of bases is the code for the biological information that will organize matter into a living organism. See Figure 5.27 18 DNA consists of two polynucleotide chains spiraled around each other, with each base on one chain linked by hydrogen bonds to a base on the other chain. The result is a molecule that looks like a spiral staircase, or a twisted ladder. This structure was first described by James Watson and Francis Crick in 1953, and is called a double helix. (Watson and Crick didn’t figure out the double helix all by themselves. Their work was made possible by many years of research by other scientists, including Gordon Avery, Maurice Wilkins, and Rosalind Franklin, among many others.) The bases do not connect randomly. An adenine on one strand can only link to a thymine on the opposite strand. A guanine on one strand can only connect to a cytosine on the other. Also, moving from one end of the DNA molecule to the other, you are moving from the 5’ end to the 3’ end of one strand, and the 3’ end to the 5’ strand of the other, sort of like a divided highway. A modified nucleic acid – adenosine triphosphate – usually abbreviate ATP, is essential in any reactions involving the expenditure of energy. See Figure 5.28 19 LAB 6: Chemical Aspects of Life, p. 26-31 LECTURE 6: Macromolecules, Part II, Chapter 5, continued Proteins Remove water from a typical living organism, and approximately 50% of the dry weight will consist of proteins. The monomers from which proteins are built are amino acids. Each amino acid consists of a carbon attached to a carboxyl group, a hydrogen atom, an amino group, and a side-chain or “R group”: H R O \ | // N-C-C / | \ H H OH Each amino acid is basically the same, except for the R group. The R group can be as simple as a hydrogen atom; this would be the amino acid glycine. It can be as complex as a nine-carbon side-chain with a double ring structure; this would be the amino acid tryptophan. The R group determines the special chemical properties of the amino acid. The R group can make the amino acid polar, non-polar, acidic or basic. See Figure 5.17 Chains of amino acids, called polypeptides, are formed by dehydration synthesis. A hydrogen atom from the amino group of one amino acid is combined with the hydroxyl from the carboxyl group of another amino acid. A water molecule is released, and the two amino acids are linked by a carbon-nitrogen bond, called a peptide bond. A chain formed in this manner – a polypeptide – will have a free amino group at one end and a free carboxyl group at the other. See Figure 5.18 20 Long polypeptides with a complex three-dimensional structure are called proteins. There are four levels of protein structure. One level is simply the sequence of amino acids from the amino end to the carboxyl end of the polypeptide chain. This is called the primary structure. The polypeptide chain usually forms some sort of coil or pleated sheet. This shape is held together by hydrogen bonds, and is called the secondary structure. This coil or pleated sheet is folded into a more complex structure when in aqueous solution. This is formed when water drives hydrophobic areas to the inside of the protein molecule, with hydrophilic areas on the outside, in contact with the water. Ionic interactions and bonds between sulfur atoms, called disulfide bridges, further maintain this complex shape. This complex shape is the tertiary structure. The final level of protein structure is created by more than one polypeptide chain linked together. For example, a blood protein called hemoglobin has four chains wrapped around each other. The structural protein collagen has three chains. There are approximately 1.2 proteins of known primary structure. We only know the full structure of approximately 8,500 of them. The three-dimensional structure of protein determines its function. There are all sorts of proteins with a variety of functions. A protein’s three-dimensional structure is often a delicate thing. It often depends on the existence of a specific range of temperatures, a specific range of pH, a polar or non-polar environment, and a number of other factors. When these factors are altered, the three-dimensional structure of the protein can be disrupted or destroyed. This disruption of the protein’s structure is called denaturation. A familiar example of irreversible denaturation takes place in the proteins of an egg when you boil it. See Figures 5.21, 5.23 and Table 5.1 21 LAB 7: Enzymes, Part I, pp 34-38 LECTURE 7: Examination I (Note: I will announce any changes in the planned exam schedule. This will only happen if the overall course schedule is disrupted due to inclement weather or other unforeseen circumstances.) LAB 8: Enzymes, Part II, pp 38-40 LECTURE 8: Introduction to the Cell, Chapter 6 It is quite difficult to see objects smaller than 0.3 millimeter in diameter, and most cells are smaller than 100 micrometers (0.1 millimeter) in diameter. Therefore the exploration of the cell requires the use of microscopes. Thanks to the development of the microscope shortly before 1600, many observations of the microscopic world were made during the 17th century. Hooke first described the cell in 1665, and van Leeuwenhoek first described microorganisms shortly thereafter. The light microscopes of today can achieve magnification of approximately 1,000x the object’s actual size, allowing clear visualization of 0.2-micrometer objects. This allows visualization of all animal and plant cells, much of the internal structure of these cells, and many bacteria. A micrometer is 1/1,000,000 (one millionth) of a meter. A nanometer is 1/1,000,000,000 (one billionth) of a meter. Visualization of the smallest bacteria, viruses, very small structures of animal and plant cells and individual molecules requires the use of the electron microscope. All cells are surrounded by a plasma membrane. The basic structure of any plasma membrane is a double layer (or bilayer) of phospholipids, with the hydrophilic phosphate heads on the outside and the hydrophobic fatty acid tails on the inside. Embedded in this basic structure are various proteins and carbohydrates that provide channels for certain substances to move into or out of the cell and receptor sites for the recognition of certain chemical signal, among other functions. The plasma membrane contains a fluid called cytosol. All cells also contain chromosomes, which carry most or all of the cell’s DNA, and ribosomes – small pieces of cellular machinery where proteins are synthesized. Small pieces of cellular machinery – including ribosomes – are called organelles. See Figure 6.7 Relatively small (typically1-10 micrometers) and primitive organisms such as bacteria and archaea (more about these in a later lecture on classification) are prokaryotes. Prokaryotic cells do not have any membrane-bound organelles. There is no true nucleus; the DNA is located in a “nucleoid region” of the cell. Outside the plasma membrane is a rigid cell wall. Outside the cell wall there is often a jelly-like capsule. See Figure 6.6 22 Animals, plants, fungi, and microorganisms called protists are eukaryotes. Eukaryotic cells are usually larger than prokaryotic cells, and have a much more complex internal structure. The most striking feature of the eukaryotic cell is the presence of membrane-bound organelles. The membranes of the organelles have the same phospholipid bilayer framework as the plasma membrane. Many of these organelles are connected, either by physical contact between their membranes, or by indirect contact through tiny sacs of membrane (vesicles). This connected series of membrane-bound organelles within the cell is referred to as the endomembrane system. One structure on the endomembrane system is the nucleus, which contains most of a eukaryotic cell’s DNA intertwined with specialized proteins. This complex of DNA and protein is called chromatin. The DNA-bearing chromatin within the nucleus is organized into a number of individual units called chromosomes. The nucleus also contains ribosomal RNA, which will be combined with certain proteins to form the building blocks of ribosomes. In addition, there is messenger RNA in the nucleus, which carries the genetic instructions coded in the DNA to the ribosomes located outside of the nucleus. See Figures 6.10 & 6.11 23 Connected to the nuclear membrane is an extensive network of membranes called the endoplasmic reticulum – the next structure of the endomembrane system. Some regions of endoplasmic reticulum have ribosomes attached – rough endoplasmic reticulum. These regions of the endoplasmic reticulum are devoted to the synthesis and transport of certain proteins. Portions of the endoplasmic reticulum devoid of ribosomes – smooth endoplasmic reticulum – are involved in a number of functions, including the synthesis and transport of lipids, the detoxification of certain poisons, and the storage of certain ions. The next portion of the endomembrane system – the Golgi apparatus, processes many of the substances produced and transported by the endoplasmic reticulum. The Golgi apparatus is a cellular version of a package delivery system such as UPS or FedEx. The packages delivered by the Golgi apparatus are vesicles. Larger vesicles are called vacuoles. See Figures 6.12, 6.13, & 6.14 24 One type of vesicle formed by the endoplasmic reticulum and then refined and released by the Golgi apparatus is the lysosome – a sac of digestive enzymes. These are found in animal cells only. Another characteristic of animal cells is a small organelle called a centrosome, which contains centrioles; these organelles become important in cell division. Plant cells also have unique characteristics. A large vacuole in a plant cell, called a central vacuole, serves as a storage compartment for nutrients and pigments, a place where waste products are broken down to harmless chemicals, and a place where macromolecules are hydrolyzed to their monomers. Just outside the plasma membrane, a plant cell has a cell wall; a structure composed mostly of the polysaccharide cellulose, which protects the cell and maintains its shape. There are small pores through the cell walls that allow one plant cell to connect to another one; these pores are called plasmodesmata. One of the most striking features of a plant cell is an organelle containing a green pigment called chlorophyll. Chlorophyll – located in organelles called chloroplasts - captures the energy of sunlight, and utilizes that energy to convert carbon dioxide and water into simple sugars. See Figures 6.9, 6.15, 6.14, & 6.18 25 Although most of a eukaryotic cell’s DNA is located in its nucleus, the chloroplast contains its own DNA. Another organelle containing its own DNA is the mitochondrion. The mitochondria are the energy-producing organelles of the cell. It is here that sugars and other nutrients react with oxygen in a process that results in carbon dioxide, water and ATP; this process is called cellular respiration. A cell may contain hundreds or even thousands of mitochondria. A membrane-enclosed compartment similar to a vesicle, but not formed by the Golgi apparatus, is the peroxisome. This organelle synthesizes hydrogen peroxide (H2O2), which is a caustic chemical. It is helpful in breaking down certain toxins, breaking down macromolecules into smaller components, and several other functions. When there is an excess of hydrogen peroxide, enzymes within the peroxisome break it down into water. See Figure 6.17 and 6.19 26 A network of protein tubules and filaments maintains the structure of a cell. This network is called the cytoskeleton. In addition to structural support, some types of filaments can become shorter or longer. The cell can use this property to move, to change its shape, or to move components around within the cytoplasm. Sometimes motile protein tubules (microtubules) project to the outside of the cell, to form flagella or cilia. See Table 6.1 and Figures 6.23, 6.25, & 6.27 27 LAB 9: Physical Aspects of Life: Diffusion, Osmosis, Plasmolysis LECTURE 9: Membranes, Chapter 7 In the current concept of what membranes are like, the phospholipids, which compose the framework of any biological membrane, are in constant lateral movement. Smaller molecules such as H2O, O2, and CO2 are able to move through the shuffling phospholipid molecules. Embedded in this moving (fluid) bilayer of phospholipids is a mosaic of various proteins. This concept is called the fluid mosaic model. Plant cells have unsaturated fatty acids in their phospholipids. These unsaturated fatty acids make plant cells resistant to freezing. Animal cells have saturated fatty acids in their phospholipids, making them somewhat vulnerable to freezing. However, animal membranes often include cholesterol molecules, which add a little bit of resistance to freezing. The embedded proteins serve a number of functions. Some of them transport various substances across the membrane. Others are enzymes. Some are involved in sending or receiving signals. A specialized group of proteins serve as identification tags. There are other functions as well. See Figures 7.5 and 7.9 28 Methods of transport across a membrane include diffusion. This is movement from high concentration to low concentration, and does not require the expenditure of cellular energy (does not require ATP). Therefore, it is a type of passive transport. When water moves across a membrane from high to low concentration, this is a form of passive transport called osmosis. Animal cells are vulnerable to changes in shape due to osmotic pressures. For example, if you placed an animal cell in a solution with a greater concentration of solute than the cytoplasm (a hypertonic solution), water would leave the cell, causing it to collapse. If you placed an animal cell in a solution with a lesser concentration of solute than the cytoplasm (a hypotonic solution), water would rush into the cell and cause it to burst (lyse). The shape of a plant cell is supported by its cell wall, so a hypertonic solution will not collapse the cell. However, the plasma membrane more-or-less caves in within the cell wall (plasmolysis). The plasma membrane of a plant cell will press against the cell wall in a hypotonic solution – such a cell is called turgid. Some substances can diffuse across a membrane, but only if they are assisted by specialized transport proteins. This form of passive transport is called facilitated diffusion. See Figures 7.11, 7.13 & 7.15 29 Forms of transport across a membrane that require ATP expenditure are forms of active transport. Some of the most important active transport mechanisms are those that help maintain a slight negative electrical charge on the inside of a cell and a slight positive charge on the outside. It is as if a membrane’s outside is a positive pole – like the positive pole of a battery or a magnet. The inside of the membrane forms a negative pole. This existence of positive and negative poles creates a voltage across the membrane, called a membrane potential. The existence of this membrane potential means that cations (positively charged ions) are drawn into the cell and anions (negatively charged ions) are driven out. In animals, the main active transport mechanism that creates the membrane potential is the sodium-potassium pump. This mechanism pumps Na+ ions out of the cell, and K+ ions into the cell, with more Na+ going out than K+ coming in. The end result is a slight positive charge on the inside of the membrane, positive charge outside. In plants, fungi and bacteria, the membrane potential is created mainly by a mechanism called the proton pump, which actively transports H+ ions out of the cell, leaving the inside relatively negative, and the outside relatively positive. In addition to the active transport mechanisms related to membrane potential, there are mechanisms for transporting “bulk” – large molecules or particles – into and out of the cell. These mechanisms involve the creation of membrane packages. Bulk transport into the cell is called endocytosis. Endocytosis of a solid is called phagocytosis, and the resulting package is called a food vacuole. Endocytosis of a liquid is called pinocytosis, and results in a vesicle called a pinocyte. Receptor-mediated endocytosis involves the engulfing of a receptor protein and the molecule it has recognized. The process opposite endocytosis – exocytosis – removes substances from the cell by joining a vesicle to the plasma membrane. This process is useful in secretion and excretion. See Figures 7.16, 7.18 & 7.20 30 LAB 10: Cell Structure: pp 47-53 LECTURE 10: Cell Division, Chapter 12 The phases of cell division in eukaryotic cells are collectively called the cell cycle. The cell cycle includes interphase, mitosis and cytokinesis. Interphase occupies the majority of the cell cycle. During this time, the cell is mostly growing and occupying itself with everything else it does besides dividing. The only event during interphase directly related to cell division is the duplication of the cell’s nuclear DNA – the creation of a copy of the cell’s genome. As a result of this DNA duplication, each chromosome is also duplicated. During most of interphase, each chromosome consists of two arms of DNA-protein complex (chromatids) joined together by a centromere. After duplication, each of these arms is linked to a sister chromatid, which carries a copy of the original chromatid’s genetic information. At this point, the chromosomes are not condensed into compact bodies visible under the light microscope; they are in a sort of wispy, uncoiled form. The first phase of mitosis is prophase. (Note: In your text, the phase called “prophase” in most texts is divided into “prophase and prometaphase”. For the purpose of this class, the events described in your text’s description of prophase and prometaphase will all be part of prophase. This will bring the description of mitosis in line with the description in your lab manual.) During this phase, the nuclear membrane dissolves, the chromosomes condense into their visible forms. The nuclear envelope (the nuclear membrane) fragments and disappears. The centrosomes move to opposite poles of the cell. A number of short microtubules extend from the centrosomes in animal cells, creating a star-like formation called asters. Longer microtubules form a structure called the mitotic spindle. Some of the microtubules reach the centromere of each chromosome, attaching to a protein structure called the kinetochore – these are called the kinetochore microtubules. Other microtubules interact with the microtubules from the opposite pole of the cell, to complete the spindle. The sister chromatids of the chromosomes are joined all along their length by specialized proteins. In metaphase, which is typically the longest phase of mitosis, the microtubules of the mitotic spindle manipulate the chromosomes towards the center of the cell. At this point, if the centrosomes look like the North and South poles of the earth, the chromosomes are arranged along the equator. This chromosome arrangement is called the metaphase plate. Each kinetochore is attached to kinetochore tubules from opposite poles. In anaphase, an enzyme breaks down the specialized protein holding the sister chromatids together. The sister chromatids separate, the centromere breaks apart, and two identical chromosomes move to opposite poles of the cell. In telophase, two nuclei form, and envelop the chromosomes. The chromosomes become less condensed, reverting to their wispy interphase structure. This is the final phase of mitosis. See Figure 12.6 31 In cytokinesis, the last stage of the cell cycle, the cytoplasm divides to form two new (daughter) cells. In animal cells, this division of the cytoplasm takes place at a structure called the cleavage furrow. In plant cells, vesicles filled with the materials for the construction of a cell wall are released by the Golgi apparatus and move to the middle of the cell, forming a cell plate. This cell plate divides the cell into two daughter cells, complete with new cell walls. Prokaryotes – including bacteria – have much simpler cellular structures, and therefore much simpler cell division. A bacterial cell simply replicates its single circular chromosome, while the cell as a whole elongates to twice its original length. Afterwards, the plasma membrane pinches inwards and creates two new bacterial cells. This process does not involve a mitotic spindle, and is called binary fission. See Figures 12.9, 12.10, and 12.11 32 In multicellular organisms, there are chemical signals that control the cell cycle. For example, if you remove cells from the tissues of a multicellular organism and grow the cells on a culture dish, the cells will usually divide until they form a single layer of cells, and then cell division stops. This phenomenon is controlled by biochemical signals given off by the cells when they reach a certain density, and is called density-dependent inhibition. In animal cells, division will usually not happen unless they are anchored to some surface, such as the inside of the culture dish or the extracellular matrix of the tissue to which they belong. When cell division depends in this way on anchorage to a surface, it is called anchorage dependence. Occasionally, a cell in a multicellular organism transforms to a state in which it divides without the normal controls such as anchorage dependence and density-dependent inhibition. A mass of such cells is called a tumor. If the tumor stays put, it is benign. For example, a wart is an example of a benign tumor. If the tumor extends crab-like appendages of itself to invade the surrounding tissue, such a tumor is no longer benign, but is cancerous, and is called a malignant tumor. When pieces of the malignant cancerous tumor break off and travel through the body, this process is called metastasis, and the cancer is called a metastatic cancer. See Figures 12.19 & 12.20 LAB 11: Cell Division: pp 53-60. LECTURE 11: Cellular Respiration, Chapter 9 When a fuel is burned, the fuel reacts with oxygen; this is what most of us mean when we say, “oxidation”. In chemistry, the term oxidation has a more general meaning – it may or may not involve actual oxygen at all. In this more general sense, the term oxidation means the loss of one or more electrons from a reactant. A specific example would be the reaction of hydrogen with oxygen. The hydrogen, being less electronegative, loses an electron to the oxygen. Therefore the hydrogen has been oxidized. The opposite situation, the acquisition of one or more electrons, is reduction. Therefore, in the aforementioned example, oxygen is reduced. Such reactions are often called redox (reduction-oxidation) reactions. Cellular respiration is a redox reaction in a food molecule such as glucose is oxidized to carbon dioxide, while oxygen is reduced to water. This does not happen all at once, as in a fire; it happens in a controlled, step-by-step manner, with each step controlled by an enzyme. Cellular respiration takes place mostly in the mitochondria of eukaryotic cells and the plasma membranes of many prokaryotic cells. The reaction produces energy, primarily in the form of ATP: 33 C6H12O6 + 6O2 Æ 6CO2 + 6 H2O + Energy (ATP) In the first phase of cellular respiration – glycolysis (“sugar splitting”) – the main oxidation agent is not oxygen itself, but a positively-charged organic molecule derived from the vitamin niacin – nicotinamide adenine dincucleotide (NAD+). In a series of reactions involving NAD+ and ten different enzymes, glucose is oxidized to two molecules of pyruvate (the ionized form of pyruvic acid). In the process, two molecules of water are formed, along with energy in the form of two molecules of ATP. Glycolysis takes place entirely in the cellular fluid – the cytosol. In the absence of oxygen, or in organisms that normally live in an oxygen-poor environment, glycolysis is followed by a reaction to renew the NAD+ for use in a new round of glycolysis. The end product of this reaction – fermentation – is lactate (an ionized form of lactic acid) or ethanol. If oxygen is present, cellular respiration proceeds to the next step, which takes place in the mitochondrion of eukaryotes or the cytosol of certain prokaryotes – the citric acid cycle (aka the Krebs cycle). Figures 9.8 , 9.9 and 9.18 The citric acid cycle consists of eight steps in which a pyruvate molecule is oxidized to carbon dioxide. In addition to NAD+, another oxidizing agent is also used in this process – a molecule derived from the vitamin riboflavin called flavin adenine dincucleotide (FAD). At the end of each round of the citric acid cycle, three molecules of CO2 and one molecule of ATP are produced. This happens twice for each original glucose molecule, because each glucose produces two molecules of pyruvate. Therefore, at the end of glycolysis and the citric acid cycle, the oxidation of glucose has produced four molecules of ATP. Glycolysis has also produced two molecules of reduced NAD+ - NADH – for every original glucose molecule. The two turns of the citric acid cycle produce an additional eight NADH molecules, along with two molecules of the reduced form of FAD – FADH2. NADH and FADH2 – are fed into the electron transport chain, the next phase of cellular respiration. See Figures 9.11 and 9.12 34 The electron transport chain utilizes a series of enzymes and electron carriers to oxidize NADH and FADH2. One of the key electron carriers in the electron transport chain is coenzyme Q (aka ubiquinone). The transfer of electrons is used to create a gradient of H+ ions within different compartments of the mitochondrion – a process called chemiosmosis. The pressure of this H+ gradient is used to activate an enzyme called ATP synthase. This process is very productive of ATP molecules – 32 to 34 ATP molecules are produced by the electron transport chain and chemiosmosis per glucose molecule. All told, 36-38 ATP molecules are produced by complete cellular respiration of one glucose molecule. See Figures 9.13, 9.14, 9.16, and 9.17 35 LAB 12: Recipe Analysis The following recipe for apple and banana fritters was modified from The Grand Diplome Cooking Course, Volume 2, Anne Willan (Editor), The Danbury Press, 1972. p. 58. I. Fritter Batter Ingredients: ½ cup flour Pinch of salt 2 egg yolks 1 tablespoon vegetable oil ½ cup milk 1 egg white Method: Sift flour with salt into a bowl, make a well in the center and add egg yolks and oil. Add milk gradually, mixing to form a smooth batter, and beat thoroughly. Store at cool room temperature (do not refrigerate) for 30 minutes. Just before use, whip the egg white until stiff, and then fold into batter. II. The Fritters Per Se Ingredients: 2-3 tart apples 2 bananas 2 teaspoons lemon juice fritter batter approx ½ inch frying oil in frying pan granulated sugar (for sprinkling) Method: Pare and core apples and cut into ½ inch slices. Peel bananas and cut diagonally into 3-4 pieces. Sprinkle the fruit slices with lemon juice. Heat the oil to 350-375 degrees F. Coat half of the fruit with batter. Remove from batter with a slotted spoon and place into the oil. Fry until golden brown. Drain fried fritters on paper towels, then arrange on a platter. Repeat with the remaining fruit. Sprinkle the finished fritters with powdered sugar before serving. 36 Questions for Recipe Lab H | H-C-OH | H-C-OH | H-C-OH | H 1. What is the name of this monomer?___________ What large biological molecules does this monomer contribute to?______________ What recipe ingredients are especially rich in this type of biological molecule?________________________ If you substituted butter for vegetable oil in the batter, what would it be harder or easier to beat, and why? _________________________________________ H R O \ | // N-C-C / | \ H H OH 2. What is the name of this monomer? _________ What large biological molecules are composed of this monomer?_____________ What recipe ingredients are especially rich in this type of biological molecule?______________________________ As the fritters are browned, what is the heat of frying doing to the structure of these biological molecules?_____________________ Can this process be reversed?_____________ What sorts of bonds are being affected by the heat in this process?______________ 3. Is the pH of these fritters high, low, or neutral, and why?_______________________________________________________ 4. In which ingredients would you find sugars?_____________________ Starches?____________________________________ Structural carbohydrates?______________________________________ 37 5. If you decided to serve these fritters with wine, what alcohol would be found in that beveredge?_______________________ Draw the structural formula for that alcohol: What stage and process of cellular respiration is responsible for that alcohol? ________________ Is that a high-yield or low-yield process in terms of ATP production?_____________________________ LECTURE 12: Meiosis, Chapter 13 The basic unit of hereditary information is the gene. The genes in eukaryotes are coded in the DNA located in the chromosomes. A gene’s specific place on a chromosome is called its locus. Some organisms can reproduce without a partner, producing genetically identical copies of themselves – clones. This is accomplished through mitosis or binary fission, and is called asexual reproduction. This form of reproduction does not produce very much variation from one generation to the other. What little variation does occur takes place entirely due to accidental changes in genes – mutations. There is another form of reproduction that requires two parents, and produces great variation from one generation to the other – sexual reproduction. This form of reproduction requires a type of cell division involving a process called meiosis, in which each original cell produces four cells with half the usual number of chromosomes. These cells are called gametes. Meiosis alternates with fertilization in the life cycle of a sexually reproducing organism. Cell not involved in gamete production are called somatic cells. In humans, each somatic cell has 46 chromosomes. 23 of these are from one’s biological mother, and 23 from one’s biological father. If images of chromosomes are arranged starting with the longest, the result is a karyotype. Most maternal chromosomes can be paired with a paternal chromosome of equal length; these paired chromosomes are called homologous chromosomes or homologs. The chromosomes determining a person’s gender are called the X and Y chromosomes; collectively they are the sex chromosomes. All other chromosomes are autosomes. The number of chromosomes in each set – maternal and paternal – is referred to as n. The number of chromosomes in the normal somatic cell (46 for humans) containing both sets is referred to as 2n. A cell containing 2n chromosomes is diploid cell, while a cell containing n chromosomes is a haploid cell. In meiosis, one diploid cell (a germ cell) produces four haploid cells (gametes). See Figures 13.2, 13.3, 13.4, 13.5, & 13.7 38 The phase of a germ cell’s life cycle prior to meiosis – interphase – is characterized by the replication of the chromosomes – just as in mitosis. The first phase of meiosis is prophase I. Just as in mitosis, the nucleus disintegrates, and a spindle forms. What is different is that homologous chromosomes pair up and align with each other – a process called synapsis. This pairing of a chromosome with its two sister chromatids and a homologous chromosome with its sister chromatids is called a tetrad. During this process, segments of homologous chromosomes may get stuck to each other; these stuck-together segments are called chiasmata. At these places, DNA from one chromosome may be exchanged with the DNA of its homolog – a process called crossing over. The DNA of these chromosomes is now a new combination of genes; such chromosomes are called recombinant chromosomes. The creation of recombinant chromosomes by crossing over during the synapsis of prophase I is one source of variability in sexual reproduction. In metaphase I, the pairs of homologous chromosomes – the tetrads – are lined up in a metaphase plate. Not all of the maternal chromosomes are on the same side of the plate; neither are all of the paternal chromosomes. In anaphase I, enzymes break down the proteins responsible for causing the homologs to stick to each other, and the homologs move towards opposite poles of the cell. Because of the arrangement noted in metaphase I, not all of the paternal chromosomes will end up on the same side; neither will all of the maternal chromosomes. This phenomenon is called independent assortment. In telophase I, the movement of the homologous chromosomes away from each other is complete. New nuclei may or may not form, depending on the species. Cytokinesis follows, just as in mitosis. Prophase II, metaphase II, anaphase II, and telophase II resemble the equivalent phases of mitosis, except that sister chromatid separation will take place with only the haploid number of chromosomes. Also, due to the crossing over that took place in prophase I, not all of the sister chromatids are identical any more. After cytokinesis, the original germ cell has yielded four gametes. Due to crossing over, any gamete you produce can have any one of 223 combinations of your maternal and paternal chromosomes. If one of your gametes meets someone else’s in the fertilization process, approximately 70 trillion combinations are possible. Added to this is the variation due to crossing over. When you consider all of these factors, it is understandable that sexual reproduction is a powerful generator of genetic variation. See Figures 13.8, 13.9, 13.11 &13.12 39 LAB 13: Review for Lab Midterm LECTURE 13: Exam 2 40 LAB 14: Midterm Test LECTURE 14: Mendelian Genetics, Chapter 14 Beginning around 1857, an Austrian monk by the name of Gregor Mendel began a series of experiments on heredity. He chose pea plants, because there are many varieties, and they are normally self-pollinating, so pea plants tend to be true-breeding: tall plants produce only tall plants, plants with green seeds only offspring with green seeds, and so forth. A pea plant can only be pollinated by a pea plant other than itself if the gardener carries out the pollination artificially; this enabled Mendel to perform carefully controlled crosses. Up until the time of Mendel’s work, it was assumed that hereditary material from both parents would simply blend. So, if you pollinated a tall pea plant with a short pea plant, you would get a pea plant intermediate in height. If you crossed a pea plant with green seeds with one with yellow seeds, you would get a plant bearing yellow-green seeds. This was called the blending hypothesis of inheritance. Much to Mendel’s surprise, when he crossed a pea plant with purple flowers with a plant with white flowers, all of the plants of the next generation produced exclusively purple flowers, not a pale purple. These results refuted the blending hypothesis. In an experiment like this, the original purple and white flowers are the parental generation, usually symbolized by the letter P. The next generation is the first filial generation, usually symbolized by the letter F with the subscript 1: F1. When Mendel crossed one F1 plant with another, the resulting F2 plants had either pure purple or pure white flowers. The ratio of purple to white flowers in this generation was 3:1. Mendel got similar results when performing crosses involving stem length, seed color, seed size, and a number of other characteristics. Mendel developed a new hypothesis to replace the blending hypothesis. First, he proposed that heritable factors (which in modern parlance, we call genes) come in two or more alternative forms, accounting for differences in inherited characteristics. Today, we refer to these alternative forms as alleles. Second, he proposed that each organism inherits two alleles, one from each parent. Third, if the alleles in an organism differ, one will dominate, and completely determine the observable expression of the inherited character in the organism. The allele that dominates is called the dominant allele, and the other is the recessive allele. Fourth, when an organism creates gametes, the two alleles for a heritable character separate, with any one gamete having a 50:50 chance of getting one allele or the other. This is called the law of segregation. The genetic makeup of a parental organism’s gametes and the resulting genetic makeup of possible offspring – genotype – and the observable expression of the trait inherited by the offspring – phenotype – can be diagrammed in a format called the Punnett square for clear analysis. When you do this, taking dominance and segregation into account, you have a very good explanation of Mendel’s experimental results. See Figures 14.1, 14.2, 14.3, Table 14.1, Figure 14.5 & 14.6 41 An organism with a pair of identical alleles for a trait is homozygous, while one with two different alleles is heterozygous. To find out whether a plant with the dominant phenotype (purple flowers, for instance) is homozygous or heterozygous, you can cross it with an organism of recessive phenotype. This is called a testcross. In the plant in question is homozygous, the testcross will produce offspring with all dominant phenotypes, just like the F1 generation in Mendel’s experiment with purple and white flowers. If the plant in question is heterozygous, ¼ of the offspring will have the recessive phenotype, just like the F2 generation in Mendel’s experiment. In later experiments, Mendel went on to follow two heritable characters in his crosses instead of one. He found that the two characters are not transmitted to the progeny as a package, but rather each pair of alleles segregates independently of each other pair of alleles during gamete formation. This is known as the law of independent assortment. See Figures 14.7, 14.8, & 14.9 42 The pea plant characteristics that Mendel was fortunate enough to choose were fairly simple to follow, because they exhibited complete dominance. There are some instances in other organisms that demonstrate incomplete dominance. For example, if you breed red snapdragons with white snapdragons, you get a pink flower. There are also instances where there are more than two alleles – multiple alleles. An example of great practical significance in health care is the ABO system of antigens (substances that can cause an immune response) on red blood cells. Allele “IA” causes a person’s red blood cells to produce the A antigen. Allele “IB” causes the red blood cells to have the B antigen. Allele “i” does not cause the production of any antigen in the ABO blood group. Any human receives one of these alleles from their biological mother and one from their biological father. The combinations of alleles represent the four blood groups. In Mendel’s pea plants, each gene seemed to be responsible for a single trait. There are some genes, however, which create more than one phenotypic effect. This phenomenon is called pleiotrophy. For example, there are plants in which one gene controls both flower color and seed color. Sometimes a gene at one locus affects the expression of a gene at a second locus epistasis. For example, black fur is dominant to brown in certain species of mice. The alleles are generally represented as B for black and b for brown. However, these alleles alone will not determine the mouse’s fur color. There is an epistatic gene for pigment, and only if the dominant allele is present – C – will there be color at all. If the mouse has inherited the recessive pigment allele from both parents – cc – then the mouse will be albino. Since you cannot carry out experimental breeding experiments on human beings, human genetics is studied examining matings that have already occurred. This process is called pedigree analysis. A number of human traits follow Mendelian inheritance patterns, and can easily be studied by pedigree analysis. See figures 14.10, 14.11, 14.12, 14.15, 14.16 & 14.17. 43 LAB 15: Mendelian Genetics, pp 66-82 – selected topics LECTURE 15: Chromosomal Basis of Inheritance, Chapter 15 In the late nineteenth century, cytologists discovered and observed chromosomes in dividing cells. Mendel’s Law of Segregation and Law of Independent assortment seemed to correspond with the behavior of these chromosomes. These observations led to the theory that chromosomes carried the genes described by Mendel. The chromosome theory of inheritance was strengthened by Morgan’s work with the species Drosophila melanogaster. This species of fruit fly has four easily-observed chromosomes – three autosomes and the sex chromosomes. Just as in humans, the male genotype is XY, and female genotype is XX. Morgan discovered a male fruit fly with a mutant phenotype – white eyes. The typical – or wild – phenotype is red eyes. When he crossed the mutant male with a normal female, all of the offspring had red eyes, suggesting that the wild-type allele is dominant. However, in the F2 generation, only male flies had the mutant trait. This suggested that the allele for this trait was located on the X chromosome, with no corresponding allele on the Y. This was the first evidence of a specific gene being carried by a specific chromosome. A gene located on either sex-linked chromosome is a sex-linked gene. In humans, sexlinked genes are responsible for a number of traits, including normal color vision, and its mutant allele, color blindness. Only one X chromosome is active in a cell. If the individual is female (in a species where the XX individual is female), one X becomes inacitivated. The inactivated X chromosome is visible as a compact object in the nucleus called a Barr body. Which X chromosome will become inactivated is random, so the individual becomes a mosaic of cells in which either the paternal X or the maternal X is active. See Figures 15.2, 15.3, 15.4, 15.5, 15.7 & 15.8. 44 Morgan found some traits that did not seem to obey the Law of Independent Assortment, but rather seemed to be inherited together. He concluded that the genes for these traits must be located on the same chromosome. Such genes are called linked genes. Sometimes, linked genes undergo recombination as a result crossing over, and wind up on different chromosomes. The further apart two genes are on a chromosome, the more likely this is to happen. The percentage of recombination of two linked genes is expressed as the number of map units apart they are on the chromosome. See Figures 15.9, 15.10, & 15.11 45 Sometimes, there are errors in the process of meiosis, such that homologous chromosomes are not separated during meiosis I, or sister chromatids fail to separate during meiosis II. This is called nondisjunction. The result is one gamete with an extra chromosome, and one gamete with a chromosome missing. In the resulting zygotes, one will be monosomic for that chromosome, and the other will be trisomic. For example, trisomy of chromosome 21 in humans results in Down syndrome, which causes mental retardation, heart defects, and a number of other health problems. Human males with an extra X chromosome (Klinefelter syndrome) and human females with a missing X chromosome (Turner syndrome) are sterile. There are traits not inherited through chromosomal DNA, but rather through the DNA of the organelles. Chloroplasts in plants and mitochondria in all eukaryotes contain their own DNA. See Figure 15.13, 15.16, 15.19. 46 LAB 16: Mendelian Genetics, pp 66-82 – selected topics LECTURE 16: Molecular Genetics, Chapter 16 The chromosome theory of inheritance implied that the genetic code was located on one of the chemical components of the chromosome, but which one? The code either had to be on DNA or on protein. In 1928, a British medical doctor (Frederick Griffith) mixed a harmless strain of the bacteria Streptococcus pneumonia with the cellular remains of heat-killed cells of a disease-causing (pathogenic) form of the bacteria. Some of the harmless bacteria turned into pathogenic bacteria. Griffith had no evidence that DNA from the pathogenic strain was the culprit, but today we recognize that cells can incorporate external DNA, thereby changing the cell’s genotype and phenotype. This process is called transformation. Following up on Griffith’s experiment, American bacteriologist Oswald Avery used treatments to inactivate the RNA, the DNA, or the protein of the heat-killed pathogenic bacteria. Only when he allowed the DNA to remain intact did transformation take place. By 1944, Avery and his colleagues were able to announce that DNA was the transforming agent – a major piece of evidence indicating that DNA is the molecule that contains the genetic code. In 1950, the biochemist Chargaff demonstrated that different species had different percentages of the four nitrogenous bases in their DNA – further evidence of the role of DNA as the molecule of inheritance. Chargaff also noted that the adenine was always present in the same ratio as thymine, while guanine was always present in the same ratio as cytosine. Any future model of DNA’s structure would have to take this into account. Further evidence implicating DNA as the carrier of the genetic code came from an experiment by Alfred Hershey and Martha Chase in 1952 involving a virus. There is a virus known as the T2 phage, which infects the bacterium Escherichia coli. When infection takes place, the virus forces the bacterial cell to make copies of the virus. It was known from previous research that the T2 phage consists almost entirely of protein and DNA, just like a chromosome. Hershey and Chase prepared batches of T2 phage tagged with a radioactive isotope of sulfur, and another batch with a radioactive isotope of phosphorus. DNA is rich in phosphorus, while protein is rich in sulfur; therefore, the isotopes would enable the scientists to track protein and DNA movements into the infected cells. They found that only the radioactive phosphorus entered the bacteria, indicating that the viruses injected DNA, not protein, into the cells to infect them. See Figures 16.2, 16.3, 16.4, and 16.5 47 After absorbing the results published by Griffith, Avery, Chargaff, Hershey and Chase, the scientific community was in substantial agreement that DNA was the substance of heredity. Now, the race was on to figure out the three-dimensional structure of this critically important molecule. One of the scientists working on the problem was Rosalind Franklin of King’s College, England. She was gathering data on DNA structure by passing x-rays through fibers of DNA, and photographing the resulting patterns. This technique is called x-ray diffraction. Another King’s College scientist, Maurice Wilkins, took one of Franklin’s xray diffraction photographs to James Watson at Cambridge. Based on Franklin’s data, Watson and his research partner Francis Crick quickly solved the DNA puzzle with their now-familiar double helix model in 1953. Their model positioned adenine in such a way that it could form hydrogen bonds only with thymine, and guanine could only hydrogen bond with cytosine. This explained Chargaff’s findings. This rule for base pairing also implied that one strand of DNA could form a template for the other. Based on this, Watson and Crick proposed a mechanism for DNA replication in 1954. In the years since the critical publications by these scientists, molecular biology has acquired much additional information about the DNA replication process. See Figures 16.6, 16.1, 16.7, 16.8, and 16.9 FYI, See Figures 16.12, 16.13, 16.14, 16.15, and 16.17. 48 The DNA replication process is not free of error. An error in sequence takes place in approximately one out of every 100,000 bases. There are systems of enzymes in all organisms studies so far that proofread and repair the base sequence in replicating DNA, to limit the number of errors that get passed down to the next generation of cells. One of the more important systems is called nucleotide excision repair. On the other hand, certain factors in the environment can increase the number of errors in DNA replication. These factors can be chemical (asbestos, certain components of cigarette smoke, etc.) or physical (x-rays, ultraviolet rays, etc.). In eukaryotes, the ends of DNA molecules are sometimes lost in the replication process. Fortunately, the segments of DNA at the ends of the molecule do not contain genes. These non-coding segments of DNA are called telomeres. With each successive mitotic division, a telomere tends to shorten. Once the telomere is gone, actual gene degeneration may take place. There are some scientists who hypothesize that telomere shortening may be one of the molecular components of the aging process. See Figures 16.18, 16.19 and 16.20 49 LAB 17: The DNA Molecule LECTURE 17: Protein Synthesis, Chapter 17 Bread mold can grow on a very minimally nutritious jelly or agar called minimal medium. In the late 1930s, George Beadle and Edward Tatum bombarded bread mold with x-rays, and discovered mutant bread mold that did not grow on minimal medium. By preparing a series of media with various amino acid combinations, they discovered that only one amino acid supplement was necessary for the mutant mold to grow. From this, they determined that the mutation must involve a single gene that produces a single enzyme involved in the synthesis of a single amino acid. This led them to the idea that each gene was responsible for the production of one enzyme – the one gene – one enzyme hypothesis. Today, we understand that genes may carry the code for all sorts of proteins and smaller polypeptides, not just enzymes. Also, genes can carry the code for specific RNA molecules that never get translated into proteins. The most frequent genes code for polypeptides, so one gene – one protein covers most situations (the more complete rule would be one gene – one polypeptide or RNA). In the eukaryotic nucleus, when it is time for a gene to become active, the DNA molecule will “unzip” to expose the base sequence of that gene. A strand of RNA will be synthesized according to a base-pairing rule similar to the one for replicating a new DNA molecule. The only difference is that adenine in DNA will form a hydrogen bond with uracil from RNA rather than thymine. The resulting strand of RNA carries the genetic code for the polypeptide out of the nucleus, and is called messenger RNA or mRNA. Because mRNA carries a transcript of the gene, the process of producing the mRNA strand is called transcription. After transcription, the information coded on the mRNA must be translated into the polypeptide – a process called translation. Translation takes place at the ribosome. See Figures 17.2 and 17.3 50 The “letters” of the DNA “language” are the bases are the bases adenine, thymine, guanine and cytosine. The “words” are each three bases long, each three-base sequence standing for an amino or a “start” or “stop” signal directing RNA where to begin translating the protein. These three-base sequences are called triplets. The mRNA language is the same language, just a slightly different “dialect”, in which uracil is substituted for thymine, as explained before. The three-base sequences in mRNA are called codons. At the ribosome, the mRNA will be exposed one codon at a time. Floating in the cytoplasm of the cell are short segments of RNA only three bases long. These short pieces of RNA are each attached to an amino acid. This RNA is called transfer RNA or tRNA. The three-base sequence on the tRNA is the complementary sequence to the codon representing that amino acid, and is called an anticodon. The anticodon will base-pair with the codon, placing the first amino acid in just the right place. For example, let’s say the first amino acid in a protein is phenylalanine. The DNA triplet for the amino acid phenylalanine is adenine-adenine-adenine, or AAA. The mRNA codon is UUU. At the ribosome, the tRNA anticodon AAA pairs with the mRNA codon UUU. The tRNA is attached to the amino acid phenylalanine. The first amino acid is now in place. After this, the ribosome will expose the next mRNA codon, which will be joined by a tRNA anticodon, placing the next amino acid in the right sequence. This is done again and again, until all of the amino acids have been placed in the proper sequence. The tRNA disengages from the amino acids, and the translation of the protein is complete. Naturally, a complex series of enzymes control the processes of transcription and translation. See Figures 17.4, 17.5, and 17.13. FYI, See Figures 17.7, 17.8,17.9, 17.10, 17.11, 17.12, and 17.14-17.21 51 LAB 18: Taxonomy, pp 86-89 (Note: Your lab manual uses a five-kingdom system of taxonomy. This is a bit different from the system in Chapter 26 of your textbook, which uses a system of three domains. I will explain this a bit during the lab with, hopefully, a minimum of confusion.) LECTURE 18: Exam 3 LAB 19: Taxonomy, pp 86-89 LECTURE 19: Darwinian Theory, Chapter 22 The ancient Greek philosopher, Aristotle, maintained that species were unchanging, and had been present since the world’s earliest times. Each species had a particular level of complexity, one species being higher or lower than another on the “scale of Nature”. The eighteenth Century Swedish physician and botanist, Linnaeus, also saw species as unchanging entities present since the beginning of Creation. He developed a system of grouping similar species together in genera (plural for genus). Since the time of Linnaeus, the scientific name for any organism is a two-part or binomial name. The genus name is capitalized, and the species name is not; both parts of the binomial name are underlined or italicized. For example, the scientific name for our species is Homo sapiens. During the late eighteenth and early nineteenth Centuries, the French scientist Georges Cuvier compared fossils from layers or strata of sedimentary rock laid down at different times. He noticed that the older the stratum, the more dissimilar its fossils were compared to current life forms. He also noted that from one stratum to the next, some new species appeared, while other species disappeared. These were important observations supporting the idea that species were not unchanging categories A new graduate of Cambridge University in divinity by the name of Charles Darwin was a naturalist by inclination. In 1831, he joined the survey ship HMS Beagle in a fiveyear voyage around the world. During this time, he collected a huge number of plant and animal specimens, both contemporary and fossilized. His observations of coral atolls led him to believe that they took approximately one million years to form – suggesting that the earth is much older than many of his contemporaries supposed. He found fossils of marine organisms high in the Andes, suggesting that these mountains were once under an ocean, yet another indication of the earth’s great age and changeability. Darwin also noted that the animals of the Galapagos Islands, while somewhat similar to mainland South American animals, were clearly unique species. Darwin hypothesized that South American animals had colonized the Galapagos, and that these animals had somehow adapted to their new habitat, thereby changing into new species. Back home in England, Darwin pondered his experiences. In 1844, he crystallized his ideas in an essay on what he called descent with modification. He further developed these ideas in the 1858 book, On the Origin of Species. (The term “evolution” was not used 52 until the book’s sixth edition.) The key idea behind descent with modification is the process of natural selection. In brief, Darwin maintained the following: 1. Members of a population of organisms often show a great deal of variation in heritable traits. 2. All species are capable of producing more offspring than the environment can support. Therefore, many of those offspring do not survive. (The book, Essay on the Principle of Population by Thomas Malthus influenced this idea. Malthus maintained that the human food supply could not keep up with human population growth in the long run. Darwin applied this idea to the broader natural world.) 3. Some individuals will have heritable traits better suited to survival in a given environment than other individuals. These individuals are more likely to pass on their traits to future generations. 4. Over time, these traits will become increasingly common in a particular natural habitat. The local population of a particular species becomes more and more of a match to the little bit of nature in which it finds itself. In essence nature itself selects certain heritable traits for survival – natural selection. See Figures 22.5, 22.6, 22.7, 22.10, 22.11, 22.12 53 Evidence for Darwin’s natural selection concept comes in many forms. John Endler at the University of California, Santa Barbara has studied a species of guppies with an enormous amount of variation in adult male color pattern. Endler’s field observations indicated that the more brightly colored the male, the more likely he is to attract a female to mate with, and thereby pass on his coloration to the next generation. On the other hand, the more predators of adult guppies there are, the more likely is the brightly colored adult male guppy to be eaten, and thereby fail to pass on his coloration to the next generation. When Endler followed up his field observations with a controlled experiment, he found that the choice of predator makes a significant difference in guppy color patterns in 22 months – 15 guppy generations. There are strains of the human immunodeficiency virus (HIV) that are resistant to the drug 3TC. In the absence of 3TC, this trait is not an advantage; this strain of HIV replicates more slowly than non-resistant viruses. However, studies indicate that within 3-8 weeks of beginning 3TC therapy, 100% of a patient’s HIV will be resistant. Modern-day paleontologists have confirmed Cuvier’s observations many times over; fossilized organisms differ from modern-day organisms, with many older species having become extinct. Also the older the rock strata, the more different the fossilized organisms are from modern life forms. Where it is possible to track a type of organism through successively more modern rock strata, evolutionary changes are seen over time. For example, a series of fossils unearthed recently show a gradual transition from life on land to ocean-dwelling life in ancestors of the modern whale. There are many modern organisms with body parts that have retained similar structures, even though they have evolved different functions – evidence of common ancestry. These structurally similar body parts are called homologous structures, and the phenomenon is called homology. For example, the forelimbs of the bat, the whale, the cat and the human share the same basic skeletal structure, even though each species uses the limb very differently. This is evidence of common ancestry among mammals. Some homologous structures are not apparent in the adult organism, but are readily seen in the embryo. All vertebrate embryos have a tail and a pair of gill slits. In fish, these structures develop into a true tail and a set of gills; in humans, they do not. Some homologous structures inherited from an ancestor species no longer serve a significant function in the modern species. For example, the land-living ancestor of the whale had a fully developed pelvis and hind limbs. Modern whales have small remainders of these bones, but they no longer form functional hind limbs. Such homologous body parts of marginal significance are called vestigial structures. Some homologous structures are only recognizable at the molecular level. Some genes found in bacteria are also found in humans, suggesting the existence of a distant common ancestor. In a sense, genetic code itself is the ultimate homology – it is the same in all species known to exist. See Figures 22.13, 22.14, 22.15, 22.16, 22.17, & 22.18 54 LAB 20: Looking at Homologous Structures, I (Not in your lab manual) LECTURE 20: Origin of Species, Chapter 24 It is easy to see how a population of a particular species can change over time through natural selection, but when does this change become sufficient to consider the population to be a new species? The development of new species is called speciation. External similarities or differences are easily observed, but they are not reliable guides to whether or not individuals belong to the same species. For example, there are birds that look strikingly similar, but are members of different species. On the other hand, all dogs are members of the same species, but a poodle does not look much like a boxer or a greyhound. Humans also show a great variety in external characteristics. In 1942, biologist Ernst Mayr proposed the biological species concept. This concept holds that a species is a group of populations whose members have the potential to interbreed in nature and produce viable, fertile offspring. While there are situations in which this concept does not readily apply, it is very useful in most situation involving multicellular, sexually reproducing organisms. If the biological species concept is valid, then speciation depends on the development of barriers to certain organisms interbreeding. The existence of such barriers is called reproductive isolation. There are a number of factors which can reproductively isolate a population, leading to the development of a new species. For example, a population of organisms can be geographically isolated. Over time, as differing forces of natural selection act the isolated populations, they may become sufficiently different so that they can no longer interbreed. This process is called allopatric speciation (Greek: allos = other; patra = homeland). This is the type of speciation Darwin hypothesized when he found unique species on the Galapagos Islands. Although unique, these species were more similar to those found in nearby South America than they were to anything found in far-away Europe, Africa, Asia, or Australia. Darwin reasoned that mainland South American organisms happened onto these islands, and developed generations of reproductive isolation into new species. Similar geographic isolation is provided by the Hawaiian Islands; these islands also are home to unique species. A further example of allopatric speciation can be seen in North America, where two distinct but related species of squirrels live on opposite sides of the Grand Canyon. Also, distinct but related species of frogs live on Madagascar and India; geological evidence suggests that these landmasses were once connected, then drifted apart millions of years ago. See Figures 24.2, 24.5, 24.6, 24.7, & 24.9 55 Speciation can also take place without geographic separation. This is called sympatric (Greek: syn = together; patra = homeland) speciation. For example, mutation or other genetic change may give rise to populations better adapted to various food sources within the same habitat. For example, there have historically been some 600 species of a type of fish known as cichlids in Lake Victoria, Africa. Different species of cichlids have adapted to different food sources within the same lake. Over time, cichlids with different diets have developed differing male breeding colors, creating a genuine reproductive barrier between species. Interestingly enough, this reproductive barrier may be breaking down. Pollution in Lake Victoria over the past 30 years has made the water murky. This has obscured the differences in male breeding color, leading to matings between species. Since the speciation was relatively recent, the offspring are often viable. This merging has contributed to the reduction in the number of species of Lake Victoria cichlids. Sympatric speciation can be brought on by a mutation in a single gene. There are two species of snail in Japan in which a difference in a single gene caused a difference in the direction of the shell’s spiral. The difference in shell direction causes a difference in genital positioning, creating a complete reproductive barrier. These snails are distinct species. In other organisms, speciation is related to multiple genes with complex interactions. See Figures 24.12, 24.26, & 24.19 56 LAB 21: Looking at Homologous Structures, II LECTURE 21: Bacteria and Archaea, Chapter 27 Prokaryotes are unicellular organisms without membrane-bound organelles. They typically have cell walls, which are biochemically different from the cell walls of eukaryotes like plants and fungi. Prokaryotic cell walls are composed of a combined carbohydrate-protein polymer called peptidoglycan. Organisms of one of the prokaryotic domains – bacteria – can be characterized by different abilities of their cell walls to take up stains. For example, Gram stain can be used to categorize bacteria into species that take up the stain – Gram-positive bacteria, and those that do not take up the stain – Gram-negative bacteria. For a number of biochemical reasons, Gram-negative bacteria are more likely to cause human disease than Gram-positive bacteria. Outside the cell wall, many prokaryotes have a sticky layer of polysaccharide or protein – a capsule. Capsules and extensions called fimbriae enable prokaryotes to adhere to surfaces or to each other. Some prokaryotes have the ability to move. Usually, a whip-like appendage called a flagellum makes this movement possible. Some species can cover 50 times their body length per second. That would be like a human being running at a speed of approximately 190 mph. A prokaryote’s genome is usually located in a single circular chromosome. Many prokaryotes also have smaller bits of DNA called plasmids. See Figures 27.3, 27.4, 27.5, 27.6, & 27.8 57 Prokaryotes reproduce quite rapidly through a cell division process quite a bit simpler than eukaryotic mitosis - binary fission. Some prokaryotic species can produce a new generation every 20 minutes. This rapid reproductive rate gives prokaryotes the ability to genetically change in a relatively short period of time. For example, a spontaneous mutation in a single gene of a typical species of intestinal bacteria – E. coli – will happen about one in every 10 million cell divisions. However, the rapid reproductive rate of this bacterium, some 20 billion new bacteria are produced every day in the average human being’s intestines, leading to some 2,000 mutations in that one gene alone. When one considers all 4,300 genes in the E. coli genome, the average human being has some 9 million mutant bacteria generated in their intestines every day. In addition to mutation, prokaryotes can also ingest DNA from other members of its species or closely related species. This is called transformation. Also, viruses that infect bacteria – bacteriophages - can carry bacterial genes from one cell to another. This is called transduction. In addition, genetic material can be transferred from one bacterial cell to another cell of the same or different species through a tube called a sex pilus. This is called conjugation. All of these processes – mutation, transformation, transduction and conjugation – combined with rapid rates of reproduction – give prokaryotes the ability to adapt very rapidly to environmental pressures. For example, if you attack disease-causing bacteria with an antibiotic, the likelihood of at least some mutant bacteria in your body carrying genetic resistance to the antibiotic is high. These survivors will then make copies of themselves through binary fission, and/or pass on their resistance to other bacteria through transformation, transduction or conjugation. You will soon have a very high population of bacteria resistant to that antibiotic. When faced with harsh environmental conditions, some bacteria form a tough wall around its chromosome called and endospore. An endospore can survive dry conditions, boiling water, and very high levels of salinity. When environmental conditions are more favorable, the bacterium reconstitutes a full, active cell. Bacteria have been brought back to active life after lying dormant as endospores in 118-year-old cans of meat, 166-year-old bottles of beer, 3,000,000-year-old layers of Siberian permafrost, and even 250,000,000-year-old salt deposits. (Sources for this information: 1.“A Case of Bacterial Immortality,” Nature, 10-19-2000, p. 844; 2. “Earth’s Hidden Life,” Economist, 12-21-1996, p. 111, 3. “Sleeping Beauty,” New Scientist, 10-21-2000, p. 12.). See Figures 27.10, 27.11, 27.12, and 27.9 58 Prokaryotes, and all other organisms for that matter, can be categorized according to their mode of nutrition. Organisms that obtain energy form light are phototrophs. Those that gain energy from chemicals are chemotrophs. Organisms that need only inorganic carbon sources such as carbon dioxide are autotrophs. Those that require at least one organic nutrient are heterotrophs. These categories combine to describe the four major modes of nutrition: photoautotrophs, chemoautotrophs, photoheterotrophs, and chemoheterotrophs. Prokaryotes are represented in all four groups. Mode of oxygen metabolism is another way of categorizing organisms. Among prokaryotes there are obligate aerobes (organisms which need oxygen for survival), obligate anaerobes (organisms for whom oxygen is poisonous), and facultative anaerobes (organisms that can use oxygen when present, but can live without it). One of the most important types of prokaryotes – in fact, one of the most important types of organisms altogether – are the cyanobacteria (in some older texts, these are referred to as blue-green algae). These are capable of photosynthesis. Much of the atmosphere’s free oxygen supply is the result of these prokaryotes. Cyanobacteria are abundant wherever there is fresh or salt water. The ancestors of modern-day cyanobacteria are the most likely generators of the first free oxygen in earth’s atmosphere; this event is recorded in reddish bands of iron oxide in rock strata dated at 2.7 billion years old. Eukaryotic photosynthetic organisms, including green plants, may very well owe their chloroplasts to these older cyanobacteria. A chloroplast contains a single circular chromosome, is sensitive to certain antibiotics, and has bacteria-like ribosomes and nucleotide sequences – all characteristics of cyanobacteria. It has been hypothesized that early large cells ingested ancient cyanobacteria, and the two organisms cooperated with each other. This hypothetical model is called endosymbiosis. Some cyanobacteria can also take atmospheric nitrogen and use it to synthesize amino acids – a process called nitrogen fixation. Nitrogen-fixing cyanobacteria are often found in close association with the roots of eukaryotic plants, which cannot fix their own nitrogen. In some species of cyanobacteria, one cell cannot carry out photosynthesis and nitrogen fixation at the same time. Therefore, colonies form in which some cells are photosynthetic and some cells are nitrogen fixing. This ability to form colonies is a characteristic of many other types of prokaryotes besides cyanobacteria. When a surfacecoating colony of prokaryotes is formed, it is called a biofilm. Biofilms can cause a great deal of damage when they form on the surface of medical or industrial equipment or as plaque on human teeth. See Table 27.1, Figures 25.8, 25.9, 27.14 & 27.15 59 A bit earlier, some feats of bacterial survival in the form of endospores were described. Even more amazing, though, are some species in the domain archaea. Some of these do not have to go dormant to survive extreme environments. On the contrary, they thrive in extreme environments. Some archaea can live active lives in high-salinity environments such as the Great Salt Lake and temperatures beyond the boiling point of water. These organisms are called extremophiles. See Figure 27.17 60 LAB 22: Looking at Homologous Structures, III LECTURE 22: Protists, Plants, and Fungi; Chapters 28, 30, 31,35, 38 Most eukaryotes are unicellular. In older classification systems, these are considered members of the kingdom protista. Today, they are still called protists informally. There is biochemical evidence to suggest that protists aquired mitochondria by ingesting and establishing symbiotic relationships with ancient prokaryotes. Eukaryotic algae seem to have aquired chloroplasts by ingesting and establishing a symbiotic relationship with cyanobacteria. As mentioned in an earlier lecture, this process is called endosymbiosis. There are some motile eukaryotes which possess chloroplasts. These seem to have evolved by the engulfing of algae by larger eukaryotes – secondary endosymbiosis. An interesting example of the photosynthetic protist is the diatom. These algae consist of single cells surrounded by a crystal-like silica wall. A live diatom can withstand pressures equal to those under each leg of a table supporting an elephant. The silica walls of many species are almost jewel-like in shape. They form an important part of plankton on oceans and lakes, which provide food for larger eukaryotes – including whales. There are some 100,000 modern diatom species and many more in fossilized form known as diatomaceous earth. An interesting group of motile photosynthetic protists are the euglenids. When sunlight is unavailable, some euglenids can become heterotrophic – even predatory. Some species, such as those in the genus Euglena, have light detectors, enabling them to move toward light of appropriate intensity, thereby enhancing photosynthesis. Paramecia, amoeba, and many other organisms are part of the diversity of the protists. See Figures 28.2, 28.3, 28.7, 28.11, & 28.13 61 Plants are multicellular photosynthetic autotrophs. The cell wall of a plant is composed of the polysaccharide cellulose. The body plan of the typical plant consists of roots, stems and leaves. Roots anchor the plant in the soil, absorb minerals and water, and sometimes serve a storage function. Root systems consisting of one main vertical root that gives off smaller branches are called taproot systems. Taproots penetrate deeply into the soil, helping the plant adapt to habitats where water is not close to the surface. Nutrient storage is common in taproots; carrots, turnips, beets and other root crops have taproot systems. A fibrous root system has no main root, but consists of a mat of thin roots just below the soil surface. Most grasses have fibrous root system. Both taproots and fibrous roots often give off thousands of tiny root hairs, which vastly increase the root’s surface area for enhanced absorption. Stems in some species are modified for nutrient storage. This results in structures such as bulbs (as in onions) and tubers (as in potatoes). Leaves are the main photosynthetic organs, with a diversity of shapes. The typical plant has two types of vascular tissue: xylem for the transport of water and minerals, and phloem for the transport of organic nutrients such as sugars and amino acids. The first seed-bearing plants developed some 360 million years ago. In these plants, the male gamete is enclosed in a tough wall – a pollen grain. These can be carried long distances by wind or animals, greatly increasing the likelihood of fertilizing a female gamete. Some plants have specialized structures to support this process of sexual reproduction – the flower. The typical flower consists of green sepals enclosing petals, which are often brightly colored, especially when the plant is insect-pollinated. Pollen grains are produced by the male part of the flower, the anther. The female part of the flower consists of a sticky stigma that captures pollen. The style leads from the stigma to the ovary. The fertilized female gamete in the ovary develops into an embryo, and is surrounded by a food supply and a protective coat – the seed. Seeds can remain dormant for days, months, or even years until it lands in favorable conditions. Some plants develop a further layer of nutrition and protection around the seed – the fruit. See Figures 35.2, 35.3, 35.5, 35.6, 30.7, 38.4, 30.8, 30.9, & 30.11 62 Fungi are chemoheterotrophs that secrete digestive enzymes to break down organic nutrients in their surroundings. Unicellular fungi are called yeasts. The bodies of multicellular fungi are typically composed of tube-like filaments one-cell thick called hyphae. The cell walls of fungi are composed of chitin – the same substance found in the external skeletons of arthropods. An extension of many hyphae into the material the fungus is feeding on is called the mycelium. Some fungi have specialized hyphae capable of capturing and feeding on small prey, such as worms. The mycelium of some individual forest mushrooms cover the area of 1,800 football fields, and may be more than 1,900 years old – they are the largest living organisms by size and weight on the planet earth. Many fungi reproduce sexually. The cells of fungi are normally haploid. In sexual reproduction, hyphae of different individuals unite – a process called plasmogamy. After a period of time that varies between species, the haploid nuclei join to produce diploid cells. This is called karyogamy. The diploid cells then undergo meiosis, producing genetically unique haploid cells, which are released as spores. The wind or water can carry spores a great distance before they find favorable conditions and germinate. In asexual reproduction in multicellular fungi, spores are produced by mitosis of haploid cells. This mode of reproduction is common among molds. Asexual reproduction among yeasts also involves mitosis; the daughter cell is smaller in size than the parent cell – this is called budding. While some fungi cause disease in humans and other organisms, they serve many beneficial purposes. Some species of sac fungi and mushrooms are edible. Certain yeasts are crucial for brewing, baking and the ripening of certain cheeses. The decomposition of dead animals and plants by fungi is essential for the normal function of earth’s ecosystems. Some plants have symbiotic relationships with fungi that help the plant resist insects, head, drought, and certain toxins. Some animals have symbiotic fungi in their digestive systems to aid the digestive process. Some fungi form symbiotic relationships with cyanobacteria or green algae – these are called lichens. The first antibiotic – penicillin – was extracted from a mold, and many of today’s pharmaceuticals are fungal products. See Figures 31.1, 31.2, 31.4, 31.5, 31.6, 31.7, 31.9, 31.17, 31.18, 31.22, & 31.26 63 LAB 23: Looking at Homologous Structures, IV LECTURE 23: Exam 4 LAB 24: Lab Review, I LECTURE 24: Invertebrates, Chapters 32 & 33 There are some 1.3 million identified species of animals – multicellular heterotrophic eukaryotes. Different animals are characterized by different overall structures or body plans. One aspect of the body plan is symmetry. There is a type of symmetry in which any plane through the center of the animal will divide it into mirror images, but there is no left and right side – like a flowerpot. This is called radial symmetry – the sea anemone has this sort of symmetry. The other type of symmetry is the type in which there is a definite left and right side – like a shovel, and only one plane will divide the animal into mirror images. This is bilateral symmetry – the lobster has this sort of symmetry. Animals with bilateral symmetry also have a dorsal (top) and ventral (bottom) side, and an anterior (front) and posterior (back) end. Many animals with bilateral symmetry also have a concentration of sensory equipment at the anterior end. When this is part of the body plan it is called cephalization. During embryonic development, most animals develop distinct cell layers that will later form into various tissues and organs. Some animals have only two embryonic layers – an outer ectoderm and an inner endoderm. All bilaterally symmetrical animals – bilateralians – have a third embryonic layer sandwiched in between the ectoderm and the endoderm – the mesoderm. Most bilateralians have a cavity separating the digestive tract from the outer body – a coelom (“SEE loam”). When the cavity is formed entirely from mesoderm, it is a true coelom, and the animal is a coelomate. If the cavity is a mixture of tissues it is called a pseudocoelom, and the animal is a pseudocoelomate. A few bilateralians have on cavity – acoelomates. Animals without a backbone – invertebrates – constitute 95% of the known animal species. We will consider the major phyla (the next taxonomic classification down from domains and kingdoms) of invertebrates. Simple, asymmetrical animals with no true tissues, the sponges live out their lives in a fixed location. They capture and digest food particles suspended in the water in which they live. Sponges are hermaphrodites. Sperm are released into the water and are carried by the current to fertilize eggs in nearby individuals. A swimming larva is formed, and then it finds a place to settle down – for life. Taxonomists used to classify them in the phylum porifera, though that is currently undergoing revision. See Figures 32.4, 32.5, 32.7, 32.8, & 33.4 64 Animals in phylum Cnidaria (“nigh DARE ee uh”) have radial symmetry around a central digestive sac. A mobile cnidarian is a medusa. Jellies (jellyfish) are familiar examples. A sessile cnidarian is a polyp. Hydras, corals, and sea anemones are examples. Cnidarians have tentacles arrayed around the opening that is both mouth and anus. Prey is stung by specialized cells in these tentacles, and drawn into the digestive sac. The stinging cells, called cnidocytes, are unique in the animal kingdom; any animal that grows their own cnidocytes belongs to the phylum Cnidaria. Undigested remains are expelled through the mouth/anus. Many species of corals secrete a hard external skeleton of calcium carbonate. As successive generations leave behind their skeletal remains, coral reefs are built up. Coral reefs are critically important as habitats for many other species and for removing carbon dioxide from the atmosphere. Some coral reefs are so large, they included coral islands large enough to build towns on. The Great Barrier Reef in Australia can be seen from space. The phylum Platyhelminthes consists of flatworms. They are acoelomate bilateralians that have no respiratory or circulatory organs, depending on their flat shape to accomplish gas and water exchange by simple diffusion. There are both free-living species such as the planarian and parasites such as the tapeworm. Species within the phylum Mollusca are bilateralian coelomates with a three-part body plan. A muscular foot is used for movement, a visceral mass contains most of the internal organs, and the mantle is a fold of tissue protecting the visceral mass. The mantle in some species secretes a hard shell. Gastropods are the most numerous types of molluscs. The most familiar of these are snails and slugs, which are both cephalized, with eyes at the tips of tentacles. The foot of a gastropod ripples or moves by means of cilia. The mantle is the gas exchange area in land-based gastropods, while aquatic or marine species have gills. Most gastropods graze on algae or plants; a few are predators. Bivalves include such familiar animals as clams, oysters, mussels and scallops. The body plan of a bivalve includes a two-part shell that can be shut tight for defense. Most bivalves are suspension feeders, like the sponges. A few species have sensory organs such as eyes and tentacles along the outer edge of the mantle. Cephalopods are predators with tentacles and poisonous beak-like jaws and with welldeveloped sensory organs and complex brains. They include squids, cuttlefish, and octopuses. Most cephalopods travel by squirting water, creating a kind of jet propulsion. Many species of cephalopods can camouflage themselves by changing color. Only the chambered nautilus has an external shell. The now-extinct ammonites were shelled cephalopods, like the nautilus, and were sometimes the size of truck tires. There are giant species of squid that reach 18 meters in length. See Figures 33.5, 33.6, 33.7, 33.9, 33.10, 33.12, 33.15, 33.18, 33.20, 33.21 65 The phylum Annelida consists of worms with a bilateralian, coelomate, segmented body plan. They range from 1 mm to more than 3 m in length. One of the most familiar and important type of annelid is the earthworm. The earthworm has a specialized blood vessels pump – a primitive type of heart. Bristles composed of chitin help the worm anchor one part of its body as another part lengthens or shortens. It has a well-developed nervous system, including a brain-like formation at the head end. They are crossfertilizing hermaphrodites; even though each worm has both sets of sex organs, they have to align with another worm to mate. As worms eat and dig their way through the soil, they till and aerate it, making it much more valuable to farmers. In some marine annelids, the bristles are attached to leg-like extensions of its body. These extensions are rich in blood vessels, enabling them to function in gas exchange – a primitive type of gill. Some of these marine annelids live in tubes built from surrounding materials or their own secretions. Leeches are annelids that suck blood from other animals, including humans. Some species of leech can consume ten times their own body weight in blood, after which they do not have to eat again for months. The phylum Nematoda consists of species of bilateralian, coelomate, non-segmented round worms. Some species are free-living in the soil or water. A number of species live as parasites within the tissues of plants and animals. A nematode parasitic in humans is responsible for the disease trichinosis. This parasite is generally aquired from raw or undercooked meat, especially pork. More than one million species have been discovered in the phylum Arthropoda. These are bilateralian coelomates. An arthropod has a segmented body protected by an exoskeleton, with jointed appendages. Early arthropods, such as the trilobites, had little variation from segment to segment. In modern arthropods, there has been a diversity of specialization in these segments. The exoskeleton is composed of various combinations of protein and chitin, and becomes paper-thin over the joints for flexibility. While many aquatic and marine species exist, the exoskeleton provides the support and the protection from drying out that make life on land possible. The down side of the exoskeleton is that it limits the animal’s growth. Therefore, an arthropod must molt its exoskeleton and secrete a new one whenever growth is required – a process that uses much of the animal’s energy and renders it temporarily vulnerable. Arthropod antennae have sensory receptors for both touch and smell. Horseshoe crabs are arthropods which closely resemble their fossilized ancestors of hundreds of millions of years ago. Arachnids are predators and parasites, including spiders, scorpions, ticks and mites. Spiders have the unique ability to spin webs of silk. Millipedes and centipedes are notable for their large number of legs. See Figures 33.22, 46.1, 33.23, 32.24, 33.25, 33.26, 33.27, 33.31, 33.33, & 33.34 66 One group of arthropods – the insects – includes more known species than all other life forms combined. An insect’s wings are an extension of the exoskeleton. The ability to fly is a great advantage, either in predation or in escaping predation. Many insects develop into their adult form from a non-flying worm-shaped form called a larva. The change from the larva to the adult is a process called metamorphosis. Insects have a complex relationship with humans. On the one hand, we depend on bees, flies and other insects to pollinate agriculturally important plants. On the other hand, some insects are disease-carrying parasites. Also, plant-eating insects compete with humans for food crops; in some areas of the world, insects consume 75% of the annual crop. Crustaceans are mostly marine and aquatic arthropods. They are the only arthropods with two pair of antennae. They include crabs, shrimp, lobsters, crayfish, barnacles, and various types of krill. The phylum Echinodermata includes sea stars (starfish), sea urchins, sand dollars and sea cucumbers. Although many species have radial symmetry as adults, they are considered bilateralians, since they all have bilateral symmetry at least in their larval form. Although casual inspection would make you suppose they have an exoskeleton, echinoderms actually have a hard endoskeleton under a thin skin. They have a network of canals that pump water through their bodies – a water vascular system. They also have tube feet for locomotion and gas exchange. The water vascular system and tube feet are unique features of the echinoderm body plan. As different as echinoderms are from humans, biochemical evidence – including DNA evidence – suggests that the phylum Echinodermata is the closest relative to our own phylum – Chordata. See Figures 33.35, 33.36, 33.37, 33.38, 33.39, & 33.40 67 LAB 25: Lab Review II LECTURE 25: Vertebrates, Chapter 34 There is a small animal that spends most of its adult life with its tail buried in the sea floor, while its front end eats. It is shaped like a narrow blade-like fish – hence its name: the lancelet. When it leaves its burrow it even swims like a fish, with side-to-side undulations of its body – but it is not a fish. It has no vertebral column, yet it is a member of the phylum that includes vertebrates – the phylum Chrodata. Running down the lancelet’s back is a rod of fluid-filled cells encased in fibrous tissue – a notochord. During the lancelet’s embryonic development, a plate of ectoderm rolls into a neural tube that develops into a hollow nerve cord just dorsal to the notochord. The anterior portion of the lancelet has a series of narrow openings – pharyngeal slits – through which water passes, allowing it to filter food particles from the water. Unlike a worm, in which the anus is the most posterior structure, the lancelet’s body extends past the anus, forming a post-anal tail. These four characteristics – a notochord, a hollow nerve cord dorsal to the notochord, pharyngeal slits, and a post-anal tail – are present in all members of the phylum Chordata, although some chordates only have these characteristics during the embryonic stage of development. Lancelets, sea squirts, and hagfish are the only invertebrates in this phylum. Most chordates have a vertebral column. In the majority of vertebrates, the vertebral column encloses a major portion of the nerve cord, which in vertebrates is called the spinal cord. In many vertebrate species, the vertebral column (in fact, the skeleton as a whole) contains little or no bone, but is composed of cartilage. This is a primary characteristic of a group of vertebrates whose name – chondrichthyans (“con DRIK thee anz”) – means “cartilage fish”. These include rays, skates, and one of the most successful groups of predators on the planet – sharks. Sharks are heavier than water; therefore, they swim almost all the time to avoid sinking to the bottom. This constant swimming also keep water moving into their mouth and out of their gills (a special adaptation of the pharyngeal slits). During rare periods of rest, sharks work their jaws to keep water moving over the gills. In addition to eyes, the shark has a good sense of smell as well as specialized organs to detect electrical fields – including those fields generated by the muscle contractions of prey animals. There are no eardrums. Instead, the shark’s entire body conducts sound vibrations to its inner ear. True fish usually have bony skeletons. They do not need to move constantly, because the typical fish has an air sac called a swim bladder, which enables it to control its buoyancy. Thanks to the swim bladder, a fish can hover motionless without sinking – a tremendous advantage for a stalking predator animal or for a hiding prey animal. Fish are very diverse and numerous, including more than 27,000 species. The vast majority of vertebrates are fish. See Figures 34.4, 34.3, 34.5, 34.15, 34.16, & 34.17 68 Water is the most important compound in the body of a living thing, so the development of vertebrates that can leave the water was a momentous evolutionary event. There are fish that have lungs and can live out of the water for long periods of time – a handy ability when a pond or creek dries up. There are not many species of these lungfish, however. Today, it is mostly the amphibians that have mastered this life style of sometimes being in the water and sometimes being out of it. This group of animals includes salamanders, apodans (legless amphibians) and frogs. While most amphibians have lungs, their closeness to water enables them to rely on a thin, moist skin for most gas exchange. The species with lungs move air into them with movements of their mouth and throat. Frogs have a fish-like larval stage, complete with gills, called a tadpole. It undergoes a metamorphosis during which it develops legs, lungs, eardrums, and a digestive system adapted to a carnivorous diet. Various species of frogs have evolved anti-predatory features in their skin, such as distasteful or poisonous mucus, as well as protective coloration. In frogs and most other amphibians, the male grasps the female and spills his sperm over the eggs as the female sheds them. Fully land-based animals evolved ways to take their water with them, both during embryonic development and adult life. One adaptation of reptiles and mammals is the development of the amniotic egg. The amnion is a membrane, which acts as a shock absorber and also bathes the embryo. In a sense, the amniotic egg surrounds the embryo in a miniature pond. Shells surround the amniotic eggs of many species, further protecting the egg from dehydration. Rather than using motions of the mouth and throat to move air into the lungs like amphibians, reptiles and mammals use their rib cage to do so – a much more efficient mode of breathing. This frees them from the necessity to breathe through the skin, allowing a thicker, water-resistant skin to develop See Figures 34.22 & 34.25. 69 Reptile skin has scales composed of the protein keratin – the same protein found in human skin and nails. Many reptile species make use of external heat – seeking the sun when they are too cold – to maintain optimal body temperature. An animal that relies strongly on external heat to maintain body temperature is ectothermic. This is a very good energy-conservation system; an ectothermic reptile can survive on 10% of the food energy required by an endothermic (warm-blooded) animal such as a mammal. Lizards range in adult size from 16 mm (small enough to stand on a dime) to 3 m (the Komodo dragon). Snakes are, in a sense, legless lizards. In fact, some snakes have vestigial pelvic and limb bones. Elastic skin and loose jawbones enable most snakes to swallow prey much larger than their head. Some snakes have specialized heat sensors, improving their ability to detect hidden prey at night. Turtles are reptiles with shells fused to their vertebrae, clavicles and ribs. Fossilized turtles have been dated at approximately 220 millions years old. Closely related to the turtles, and appearing in the fossil record at around the same time are the alligators and crocodiles. There is an exhibit in the Smithsonian Museum of Natural history in which the skeleton of a fossilized crocodile is shown side-by-side with the skeleton of a modern crocodile. If you are not a specialist in these species, you probably can’t tell the skeletons apart. Birds are now classified as endothermic reptiles. Most birds fly, and flight involves a number of adaptation. Acute vision and fine muscle control are required for flight, and these abilities require a larger brain (relative to overall body size) than the brains of amphibians or non-flying reptiles. A form of keratin, the same protein in the snake’s scales, is the main protein in feathers. Birds lack a urinary bladder, teeth, and their gonads remain small except during mating season – all adaptation to save body weight to make flight easier. Another weight-saving adaptation is the existence of air spaces in the bones of many birds. Some birds soar, with little flapping of their wings. The hummingbird, on the other hand, flaps its wings continually when flying. Some birds engage in elaborate courtship dances. The oldest bird fossil has been dated at 150 million years old. See Figures 34.26, 34.27, 34.28, & 34.29 70 Mammals have mammary glands, skin covered with hair or fur with a fat layer underneath, a four-chambered heart, and a diaphragm to improve the efficiency of breathing. Also characteristic of mammals is a variety of teeth for chewing a variety of foods. Like reptiles, mammals surround their embryos with an amnionic membrane, but only a few species lay eggs (the platypus and the spiny anteaters). A few species complete the embryo’s development in a pouch called a marsupium. These animals include the possum, the kangaroo, and a number of other species. In most mammals, embryonic development is completed within the uterus of the mother. Primates include lemurs, tarsiers, monkeys and apes (humans are classified with the apes). Primates are mammals with digits that have flat nails instead of claws or talons. They have thumbs that are separate from and more moveable than the other fingers. The fingers have skin ridges (fingerprints in humans). See Figures 34.32, 34.33, 34.34, 34.35, 34.36, & 34.38 71 LAB 26: Final Lab Exam LECTURE 26: Human Evolution (Some information is this lecture is from The Origin of Humankind by Richard Leakey, Basic Books, New York, 1994.) In the late 1960’s, two biochemists from the University of California at Berkeley – Allan Wilson and Vincent Sarich – compared the structures of certain blood proteins in chimpanzees, gorillas and humans. These proteins have known rates of mutation. Therefore, they can serve as a sort of “molecular clock”. The more different the blood proteins are, the longer ago the species diverged. Based on this molecular evidence, they concluded that the common ancestor of humans and apes lived some 5 million years ago. More recent molecular clock evidence has revised this estimate to more like 7 million years ago. No fossils of this common ancestor have been discovered so far. Skeletal remains from the time of ape-human divergence are hard to find, and are usually very incomplete. Most finds are a skull fragment here, a bit of pelvis there. However, a very fortunate find was made in 1974 in Ethiopia. A 40% complete skeleton of a 3-foot tall female that walked erect was found, and was dated at approximately 3.2 million years old. The fossil was named “Lucy”. Lucy is a member of the species Australopithecus afarensis. Her brain was not particularly large – about one-third the size of the brain of a human of equal size. There have been several species grouped in the Australopithecus genus, with the oldest fossilized remains having been discovered in East Africa, particularly Ethiopia, Tanzania, and Kenya. Australopiths are believed to have been bipeds – that is, they were capable of walking upright on two legs. Bipedalism frees the upper limbs to grip tools and weapons – it is an important evolutionary step. There are several methods of determining whether or not a fossilized skeleton belonged to a biped or not. For example, the large hole in the skull through which the spinal cord exits – the foramen magnum – is located at the back of the skull of a quadruped. In a biped, it is located in a more central location, so that the skull is balanced over the spine in the upright position. Fossilized Australopithecus skulls were consistent with bipedalism. Also, footprints have been found associated with the Australopiths. Combined with evidence from the pelvic bones, the evidence of bipedalism in the Australopiths is reasonably strong. However, there are a number of characteristics of Australopithecus more ape-like than human-like. Their lower limbs were short in comparison to their upper limbs. Their shoulder joints were oriented more upward than forward. Their phalanges (finger and toe bones) were long and curved rather than short and straight. All of these characteristics are more useful for tree-dwelling quadrupeds than surface-dwelling bipeds. When CAT scans were done of Australopithecus skulls, the structure of their balance organs in their inner ears was more ape-like than human-like. Although Australopiths had developed human- 72 like bipedalism on the ground, they may have spent a significant amount of their time living an ape-like life in the trees. The earliest fossils belonging to our own genus Homo are those of Homo habilis. These fossils have been dated at approximately 2.5 million years old. Homo habilis had a much larger brain than any members of the Australopithecus species, almost half the size of a modern human brain. CAT scans of the skulls inner of Homo habilis fossils reveal human-like inner ears. Their legs were substantially longer than their arms, and their phalanges and shoulders were similar to those of a modern human. Clearly, Homo habilis was more completely adapted than the Australopiths to a bipedal life on the ground. Besides bipedalism and increased brain size, another milestone on the way to the modern human was the use of relatively complex stone tools. Simple stone tool use is known among apes. For example, chimpanzees have been observed to place hard nuts on flat rocks and open them with smaller rounded rocks. However, these are “found” tools. The chimpanzee uses the rocks as he or she finds them – there is no sculpting of the stones to a particular shape. The first deliberately formed tools appeared approximately 2.5 million years ago. While it is not clear whether or not Australopiths made stone tools, Homo habilis definitely did (thus the name, which means “handy man”). Approximately 1.9 to 1.7 years ago, a species of Homo with a larger brain capacity appeared – Homo erectus. Some authors use the term Homo ergaster to describe this species; others consider them to be separate. In this lecture, I will stick with the name Homo erectus. This new species of Homo made more sophisticated stone tools than Homo habilis. Richard Leaky and Kamoya Kimeu discovered a 1.5-million-year-old fossil of a nearly complete Homo erectus skeleton near Lake Turkana, Kenya in 1984. It is not known whether one of the Australopithecus species gave rise to the early Homo species, or whether they were separate lines of evolution from a common ancestor. No Australopithecus fossils have been found dated earlier than 1 million years ago. From this, it is assumed that the Australopiths were extinct by 1 million years ago. Some late version of Homo erectus is presumed to be the direct ancestor of Homo sapiens. Neanderthals were considered a separate species – Homo neanderthalis – when first discovered in 1856. Today, most scientists consider the Neanderthals to be an early subspecies of Homo sapiens (Homo sapiens neanderthalis). The Neanderthals had a better-developed tool kit than previous hominids, and also buried their dead. At least one 100,000 year-old Neanderthal burial site has been found. Different scientists place the appearance of the first Neanderthals at different points in time, but it was approximately 200,000 years ago. No Neanderthal remains have been found dated more recently than 28,000 years ago. Modern humans – Homo sapiens sapiens – appeared some 195,000 years ago and migrated out of Africa some 115,000 years ago. Modern humans and Neanderthals coexisted in many parts of the world up to 28,000 years ago, but DNA studies show little 73 evidence of interbreeding. Art objects began to appear some 77,000 years ago, and the cave paintings of France and Spain began to appear approximately 36,000 years ago. XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX) LECTURE 27: Course Review, I LECTURE 28: Course Review, II LECTURE 29: Final Lecture Exam (Next: Protein synthesis=ch 17=2-28 lecture; Origin of Species = ch 24 = 4-1 lecture; (Test III?) Phylogeny=ch 26 (no lecture); Bacteria and Archaea = Ch 27 = part of 4-17 lecture; Protists = ch 28 = 4-22 lecture; Plants = ch 29 (no lecture); Fungi = ch 31 =part of 4-24 lecture; (Test IV?) Invertebrates (2?) = ch 33 = 4-3 & 4-15 lecture + handouts; vertebrates (2?) = 4-15 & 4-17 lecture + handouts (Final) Total 25 lectures) 74