Effects of vinblastine, podophyllotoxin and nocodazole on mitotic
advertisement

Journal of Cell Science 102, 401-416 (1992)
Printed in Great Britain © The Company of Biologists Limited 1992
401
Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles
Implications for the role of microtubule dynamics in mitosis
MARY ANN JORDAN*, DOUGLAS THROWER and LESLIE WILSON
Department of Biological Sciences, University of California, Santa Barbara, CA 93106, USA
*Author for correspondence
Summary
Inhibition of mitosis by many drugs that bind to tubulin
has been attributed to depolymerization of microtubules. However, we found previously that low concentrations of vinblastine and vincristine blocked mitosis in
HeLa cells with little or no depolymerization of spindle
microtubules, and spindles appeared morphologically
normal or nearly normal. In the present study, we
characterized the effects of vinblastine, podophyllotoxin
and nocodazole over broad concentration ranges on
mitotic spindle organization in HeLa cells. These three
drugs are known to affect the dynamics of microtubule
polymerization in vitro and to depolymerize microtubules in cells. We wanted to probe further whether
mitotic inhibition by these drugs is brought about by a
more subtle effect on the microtubules than net
microtubule depolymerization. We compared the effects
of vinblastine, podophyllotoxin and nocodazole on the
organization of spindle microtubules, chromosomes and
centrosomes, and on the total mass of microtubules.
Spindle organization was examined by immunofluorescence microscopy, and microtubule polymer mass was
assayed on isolated cytoskeletons by a quantitative
enzyme-linked immunoadsorbence assay for tubulin. As
the drug concentration was increased, the organization
of mitotic spindles changed in the same way with all
three drugs. The changes were associated with mitotic
arrest, but were not necessarily accompanied by net
microtubule depolymerization. With podophyllotoxin,
mitotic arrest was accompanied by microtubule depolymerization. In contrast, with vinblastine and nocodazole, mitotic arrest occurred in the presence of a full
complement of spindle microtubules. All three drugs
induced a nearly identical rearrangement of spindle
microtubules, an increasingly aberrant organization of
metaphase chromosomes, and fragmentation of centrosomes. The data suggest that these anti-mitotic drugs
block mitosis primarily by inhibiting the dynamics of
spindle microtubules rather than by simply depolymerizing the microtubules.
Introduction
ine significantly inhibits the exchange of tubulin at the
ends of in vitro reassembled microtubules without
exerting significant effects on the total mass of microtubule polymer (Jordan and Wilson, 1990). These
results indicated that vincristine and vinblastine inhibit
mitosis, not by inducing microtubule depolymerization,
but by stabilizing the dynamics of the spindle microtubules. We therefore wanted to determine whether
other microtubule-depolymerizing drugs inhibit mitosis
by depolymerizing spindle microtubules or by a more
subtle action on the microtubules.
In the present study, we characterized further the
effects of vinblastine, and we characterized the effects
of podophyllotoxin and nocodazole on mitotic accumulation, the mass of cellular microtubules, and the
organization of the spindle microtubules, chromosomes
and centrosomes in HeLa cells. We found that while
each drug blocked mitosis with very different effects on
microtubule polymer levels, all three drugs induced a
Inhibition of mitosis by several anti-mitotic drugs
including vinblastine, podophyllotoxin and nocodazole,
that can depolymerize microtubules in vivo and in vitro,
has been considered to occur by a mechanism involving
depolymerization of microtubules (e.g. see Malawista
et al. 1968; Wilson and Bryan, 1974; DeBrabander et al.
1976; Hoebeke et al. 1976; Zieve et al. 1980; reviewed
by Dustin, 1984). However, we found that inhibition of
mitosis in HeLa cells by low concentrations of vincristine and vinblastine occurs with little or no depolymerization of spindle microtubules (measured by enzyme-linked immunoadsorbence assay (ELISA) of
tubulin in isolated cytoskeletons; Jordan et al. 1991). By
immunofluorescence microscopy with an antibody to
tubulin, blocked metaphase spindles appear morphologically normal or exhibit only slight abnormalities in
microtubule organization. We also found that vinblast-
Key words: mitosis, microtubule dynamics, vinblastine,
podophyllotoxin, nocodazole.
402
M. A. Jordan and others
nearly identical concentration-dependent series of rearrangements of spindle microtubules, centrosomes
and chromosomes. The series was characterized by a
drug-concentration-dependent: (1) increase in the
length and number of astral microtubules; (2) decrease
in the length of the central spindle; (3) increase in the
number of chromosomes that were found near the
spindle poles rather than in the metaphase plate; and
(4) fragmentation of centrosomal material. With low
concentrations of vinblastine and nocodazole, these
changes occurred in the absence of any net depolymerization of microtubules.
The observations reported in the present study,
together with data on the effects of vinblastine,
podophyllotoxin and nocodazole on the dynamics of
microtubules in vitro (see Discussion), suggest that
these three antimitotic drugs, and perhaps others as
well, inhibit mitosis primarily by inhibiting microtubule
dynamics. The data support the idea that the dynamic
behavior of microtubules is crucial to the progress of
mitosis and the cell cycle.
Materials and methods
Cell culture
HeLa S3 cells were provided by Dr. Jeannette Bulinski
(Columbia University, New York, NY) or American Type
Culture Collection (Rockville, MD). Cells were grown in
monolayers at 37°C without antibiotics in 5% CO2 as
previously described (Jordan et al. 1991). Subcultures of cells
for assay of polymer mass were performed at a density of 1.5
X 106 cells/ml in 15 ml. Approximately 20 h later fresh
medium plus or minus drug was added. Cells were harvested
for assay of polymerized and soluble tubulin 18-20 h after drug
addition. Subcultures of cells for immunofluorescence microscopy and for assays of proliferation were plated at a
density of 4 x 104/2 ml in 35 mm dishes containing no. 1 glass
coverslips freshly coated with polylysine (50 /ig/ml, 2 h, 37°C,
followed by a rinse with water and a rinse with medium).
Approximately 40 h later, fresh medium containing drug (or
no drug) was added. At this time, cells were scraped from
some coverslips and counted by hemocytometer to determine
cell number at the time of drug addition. Cell viability was
determined by exclusion of trypan blue. At 18-20 h after drug
addition samples of control and drug-treated cells were
counted to determine the increase in cell number. Two to four
independent experiments were performed with each drug to
determine the concentration dependence for inhibition of cell
division. Simultaneously, parallel coverslips of cells were
fixed and processed for immunofluorescence microscopy.
Vinblastine was a gift from Eli Lilly and Co., Indianapolis,
IN. Podophyllotoxin was obtained from Aldrich (Milwaukee,
WI) and nocodazole from Janssen Pharmaceutical (Beerse,
Belgium).
Boulder, CO; Chu and Klymkowsky, 1989) that is specific for
/3-tubulin in HeLa cell extracts (data not shown), centrosomes
were detected using antiserum 5051, a human autoimmune
anti-centrosomal antiserum (a gift from Dr. S. Doxsey,
University of California, San Francisco, CA; Calarco-Gillam
et al. 1983), and chromosomes were stained with DAPI (4,6diamino-2-phenylindole; Sigma Chemical, St. Louis, MO).
Second antibodies were fluorescein isothiocyanate-conjugated goat anti-mouse IgG and rhodamine-conjugated goat
anti-human IgG (Cappel, West Chester, PA). The percentage
of cells arrested in metaphase was counted on preparations
double-stained with DAPI and for anti-tubulin immunofluorescence; at least 400 cells were counted at each drug
concentration tested; two or three independent experiments
were performed for each drug. At drug concentrations that
were just sufficient to induce metaphase arrest, metaphase
was clearly distinguishable from other stages of mitosis by the
characteristic compact metaphase plate of chromosomes.
However, at high drug concentrations associated with the
formation of types 111 and IV mitotic spindles (see Results for
description of types), cells with highly condensed masses of
chromatin were arbitrarily called "metaphase" as has been
the convention in the literature. Sufficient numbers of mitotic
cells were counted to acquire a minimum of 50 anaphase
and/or metaphase cells to determine the anaphase/metaphase
ratio. Between 50 and several hundred metaphases were
scored for each drug concentration to determine the frequencies of normal and types I-IV spindles.
The distance between the two poles was measured on
metaphase spindles that had been triply stained with DAPI
and with anti-centrosomal and anti-tubulin antibodies.
Measurement was done directly on the coverslip preparation
using a 40x Olympus oil immersion objective and an eyepiece
reticle. Only spindles that had both centrosomes in the plane
of focus were measured. A minimum of 22 spindles were
measured for each drug concentration. Photo-micrographs
were obtained using a Zeiss Photomicroscope III equipped
with an epi-fluorescence condenser and a 40x Olympus
UVFL oil immersion objective of numerical aperture 1.3 as
described previously (Jordan et al. 1991).
Quantitation of tubulin in microtubules
Cells were released from flasks by gentle scraping, collected
by centrifugation, and resuspended for counting and for
collection of stabilized microtubules in cytoskeletons as
described in detail previously (Thrower et al. 1991). Tubulin
in microtubules was determined using an enzyme-linked
immunoadsorbence assay (ELISA) (Thrower et al. 1991)
using a monoclonal antibody to beta tubulin (1-1.1, IgM,
kappa class; Ball et al. 1986). The tubulin standard was threetimes-cycled microtubule-associated protein (MAP)-depleted
bovine brain tubulin prepared as described by Farrell et al.
(1987). Between 2 and 6 independent determinations of
microtubule polymer mass were made for each drug concentration.
Results
Immunofluorescence microscopy and determination of
concentration dependence for metaphase arrest and
spindle reorganization
Cells grown on coverslips were prepared for immunofluorescence microscopy as described previously; fixation was in
formalin followed by methanol (Jordan et al. 1991). Tubulin
was detected with a mouse monoclonal antibody (E7, IG^ a
gift from Dr. Michael Klymkowsky, University of Colorado,
We incubated HeLa cells for a duration of one cell cycle
with a range of concentrations of vinblastine, podophyllotoxin and nocodazole. Cells were then fixed and
processed for fluorescence microscopy to determine the
arrangement of chromatin or chromosomes using
DAPI, the microtubules using anti-tubulin immunofluorescence, and the centrosomes using 5051, a human
Inhibition of mitosis by drugs
403
Table 1. Effects of vinblastine, podophyllotoxin and
nocodazole on mitotic block, the mass of microtubule
polymer and cell division
"•met
*^ana/met
Drug
Vinblastine
Podophyllotoxin
Nocodazole
0.01
0.1
1
10
100
1000 10000 100000
[ VinblastineJ (nM)
Fig. 1. Metaphase arrest and microtubule depolymerization
and spindle length after incubating HeLa cells with
increasing concentrations of vinblastine. (A) Percentage of
cells in metaphase (circles) and percentage decrease in
mass of polymerized microtubules compared with control
cells (squares). At concentrations of 10 ,uM and 100 ,uM
vinblastine, the polymer mass increased as a result of
formation of vinblastine-tubulin paracrystals. The relatively
large standard errors of polymer mass measurements at 10
and 100 fiM vinblastine are probably due to the small
number of assays carried out at these concentrations and to
cell death, which was prevalent in this vinblastine
concentration range (data not shown). (B) Percentage
decrease of interpolar distance of normal, type 1 and type
II spindles.
scleroderma autoimmune antiserum. The percentage of
cells in mitosis or in a mitotic-like stage after drug
treatment was determined from the stained preparations. In parallel, we isolated stabilized cytoskeletons and determined the total mass of tubulin in the
form of microtubules that remained after drug treatment as compared with control cells.
Effects of vinblastine on mitosis
Mitotic arrest and microtubule polymer mass
Incubation with vinblastine induced cells to accumulate
at a stage resembling mitotic metaphase in a concentration-dependent manner (Fig. 1A, circles). A total of
50% of the cells accumulated in metaphase (Kmet) at 0.8
nM drug (Table 1), and a peak of maximal accumulation occurred at 6 nM vinblastine (Fig. 1A). Vinblastine inhibited cell division concomitant with metaphase
arrest; half-maximal inhibition of cell division (Kdiv)
occurred at 0.45 nM vinblastine (Table 1).
The ratio of the number of cells in anaphase to the
"-dep
(nM)
0.80
30
54
0.40
5
12
11
15
600
0.45
20
22
14
0.5
11
Kmei, drug concentration that induced accumulation of 50% of
cells in metaphase after incubation for 18-20 h. From the data of
Figs 1A, 4A and 6A. Kima/mcx, drug concentration that induced a
50% decrease in the ratio of the number of cells in anaphase to
the number of cells in metaphase after incubation for 18-20 h (data
for vinblastine is from Jordan et al. (1991). Kdcp, drug
concentration that induced a 50% decrease of microtubule
polymer mass as determined by quantitation of tubulin in
cytoskeletons isolated from cells 18-20 h after drug addition. From
the data of Figs 1A. 4A and 6A. ^Cdiv, drug concentration that
induced inhibition of cell division by 50% after treatment of
exponentially growing cells for 18-20 h. The values were
determined from plots of the percentage inhibition of increase in
number of cells after incubation for 18-20 h in the presence of
drug vs the drug concentration (data not shown). For example, the
value for vinblastine was derived from Fig. 1 of Jordan ct al.
(1991).
number of cells in metaphase at each vinblastine
concentration was determined to ascertain whether
accumulation of cells in metaphase by vinblastine was
due to slowing of mitosis relative to the entire cell cycle,
or whether it was due to a block of mitosis at
metaphase. An increase in the number of cells in
metaphase and a proportional increase in the number of
cells in anaphase would indicate that mitosis was
slowed, whereas an increase in the number of cells in
metaphase and a decrease in the number of cells in
anaphase would indicate a metaphase block. Vinblastine induced a decrease in the ratio of cells in anaphase
to cells in metaphase in a concentration-dependent
manner, with 50% decrease in the ratio (Kana/met)
occurring at 0.4 nM drug (Table 1). At 1.6 nM
vinblastine, the anaphase/metaphase ratio was zero
(data not shown). Thus, vinblastine blocked cells at
metaphase of mitosis.
Vinblastine affected the total mass of cellular microtubule polymer in a complex fashion with respect to
drug concentration and with respect to metaphase
accumulation (Fig. 1A, squares). Metaphase accumulation occurred without any reduction in total microtubule polymer mass at vinblastine concentrations
between 0.1 and 6 nM; maximal metaphase accumulation occurred in the concentration range in which
microtubule polymer mass was unaltered. Microtubule
polymer mass was reduced by 50% (Kdcp) at 11 nM
vinblastine, 14 times the concentration required for
50% accumulation of cells at metaphase (Kdep/Kmct,
Table 1). Microtubules were completely depolymerized
at 100 nM vinblastine. Vinblastine at concentrations
greater than 1 juM induced formation of vinblastinetubulin paracrystals, resulting in increased polymer in
isolated cytoskeletons (described further below).
404
M. A. Jordan and others
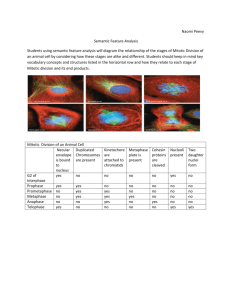
Fig. 3. Microtubule, chromosome and centrosome
arrangement in HeLa cells after 18-20 h incubation in
vinblastine at the specified concentrations. (A,a,B) 0.8 nM;
(C,c,c') 3.2 nM; (D,d,d') 6.4 nM; (E,e) 100 nM; (F) 1 (M;
(G) 10 (M\ (H) 100 [M vinblastine. (A,C,D,E,F,G,H)
Anti-tubulin immunofluorescence; (a,c,d,e) DAPI stain of
chromosomes; (B,c',d') anti-centrosomal
immunofluorescence. (A,a) Type I abnormal spindle
induced by incubation in 0.8 nM vinblastine, arrows point
to tufts of long astral microtubules and to chromosomes
located near the spindle poles. (B) Fragmented
centrosomal material induced by 0.8 nM vinblastine, the
microtubules and chromosomes of these three spindles
were in the normal metaphase configuration (not shown).
C,c,c') Types I, II and III abnormal spindles (labelled I, II
and III) induced by 3.2 nM vinblastine. Arrows point to
chromosomes located near the spindle poles. (D,d,d') Type
III abnormal spindles with accompanying peripheral
punctate aggregates of tubulin (in D) and fragmented
centrosomes induced by 6.4 nM vinblastine (in d').
(E,e,F) Absence of microtubules and presence of punctate
aggregates of tubulin induced by 100 nM and 1 fjM
vinblastine, respectively; the chromosomes of these cells
(type IV) were mitotic-like (condensed with no nuclear
membrane) except for the cell marked i which was in
interphase; each cell had one or two compact centrosomes
(not shown). (G,H) Vinblastine-tubulin paracrystals
induced by 10 JJM and 100 pM vinblastine, respectively; the
nuclei of the cells in (G) were in interphase (not shown);
the cells of (H) were judged to be dying from the pyknotic
appearance of their chromatin and their inability to exclude
trypan blue (not shown). Bars, 10 ^m; bar in a gives
magnification of A,a; bar in B gives magnification of all
other panels.
Fig. 2. Microtubule, chromosome, and centrosome
arrangement of two normal (control) cells in metaphase
(the cell on the left and the cell on the right). Other cells
(more dimly stained) are in interphase. (A) Indirect
immunofluorescence using anti-tubulin monoclonal
antibody followed by a fluorescein isothiocyanateconjugated second antibody on HeLa cells fixed and
stained as described in Materials and methods, (a) DAPI
stain of metaphase plates of chromosomes (arrows) in the
same cells, (a') Indirect immunofluorescence of
centrosomes (arrows) using 5051, a human scleroderma
autoimmune antisemm, followed by a rhodamineconjugated second antibody, in the same cells. Bar, 10 ixm.
In this and other micrographs, staining of the same cell or
group of cells using different antibodies and fluorochromes
is designated by one alphabet character, as A, a or a'.
Effects of vinblastine on spindle organization
Metaphase spindles of control cells contained primarily
kinetochore and interpolar microtubules; the few astral
microtubules that were present were typically very
short and were often barely detectable (Fig. 2A).
Condensed chromosomes (Fig. 2a, arrows) were in a
compact metaphase plate located midway between the
two spindle poles. A single compact mass of centrosomal protein was located at each pole (Fig. 2a',
arrows). The poles were separated by a distance of 7.9
± 0.4 pan.
After incubation with low concentrations of vinblastine (0.4-1.6 nM), between 12% and 69% of the cells
were blocked in metaphase (Fig. 1A). Yet, by immunofluorescence microscopy, many of the metaphase
spindles were morphologically indistinguishable from
metaphase spindles of control cells. (The proportion of
all metaphase spindles that appeared normal was 69%
after incubation with 0.4 nM vinblastine; the proportion
that appeared normal diminished to 9% of all metaphase spindles with 1.6 nM vinblastine.) However,
some spindles were clearly abnormal. The abnormal
spindles could be described in terms of four types (IIV), characterized by increasing degrees of disorganization (Jordan et al. 1991). Type I spindles were nearly
normal-looking bipolar spindles (Fig. 3A,a), except
that one or a few chromosomes were near one or both
spindle poles instead of being aligned with the majority
of the chromosomes at the metaphase plate (Fig. 3a,
arrows). Also, the astral microtubules of type I spindles
were typically more numerous and somewhat longer
than those of normal spindles (Fig. 3A, arrows).
Type II abnormal spindles were bipolar (Fig. 3C,c,c')
but the microtubules and chromosomes exhibited more
extensive rearrangement than those of type I spindles.
More chromosomes were located at one or both poles in
type II spindles than in type I spindles (Fig. 3c, arrows).
Astral microtubules were prominent and often were
very long (Fig. 3C). The average the distance between
Inhibition of mitosis by drugs
Fig. 3
405
406
M. A. Jordan and others
the two poles in type I and type II spindles was 26%
shorter than the distance between poles of normal
spindles (Jordan et al. 1991). Thus in both type I and
type II spindles the astral microtubules were longer and
the kinetochore microtubules were shorter than normal. The distinction between type I and II spindles was
subjective and was made primarily to emphasize the
qualitative continuum of changes that occurred with
increasing drug concentration. Type III spindles (Fig.
3C,c,c',D,d,d') were essentially monopolar and consisted of a ball of condensed chromatin (Fig. 3c,d), one
or more star-shaped aggregates of microtubules (Fig.
3C,D), and one or more masses of centrosomal material
(described further below) (Fig. 3c',d').
Between 0.2 nM and 1.6 nM vinblastine, the arrested
cell population contained a mixture of normal spindles
and abnormal types I, II and III spindles. For example,
12% of the cells contained metaphase spindles at 0.4
nM vinblastine; of those spindles 69% were normal,
16% were types I and II, and 15% were type III. As the
drug concentration was increased from 0.2 nM to 1.6
nM vinblastine, the frequency of normal and types I and
II spindles diminished and the frequency of type III
spindles increased. For example, 48% of the cells
contained metaphase spindles at 0.8 nM vinblastine; of
those spindles 16% were normal, 12% were types I and
II, and 72% were type III. Above 1.6 nM vinblastine, as
for example at 3 nM vinblastine, 82% of the cells
contained metaphase spindles, but there were no
normal-looking spindles; all spindles were types I, II or
III. At 6 nM vinblastine, 86% of the cells contained
metaphase spindles, but there were no bipolar spindles
and all spindles were type III (Fig. 3D,d,d').
Type IV mitotic figures (Fig. 3E,e,F) had no
microtubules and contained nondescript aggregates of
condensed chromosomes. Type IV mitotic figures
occurred only upon incubation with vinblastine at
concentrations of 50 nM and higher; at these concentrations no microtubule polymer was detected in
isolated cytoskeletons (e.g. 100 nM vinblastine; Fig.
1A, squares).
Small punctate aggregates of tubulin were observed
by immunofluorescence microscopy at vinblastine concentrations between 6 nM and 1000 nM. The aggregates
were present in very small numbers at vinblastine
concentrations of 6 nM (Fig. 3D); their number
increased after incubation of cells with vinblastine at
concentrations that resulted in loss of measurable
microtubule polymer. At 100 nM and 1000 nM
vinblastine they were the only tubulin-containing
structures visible by immunofluorescence (Fig. 3E,F).
The aggregates were located at the cell periphery in
cells displaying type III spindles (Fig. 3D) and were
scattered throughout the cytoplasm in cells displaying
type IV mitotic figures (Fig. 3E,F).
The centrosomal material of cells incubated with very
low concentrations of vinblastine (0.4 nM or less)
formed a single compact mass in interphase cells or two
compact masses in mitotic cells, one at each spindle
pole (not shown). Between 0.8 and 13 nM vinblastine,
centrosomal material in mitotic cells displaying normal
and types I, II and III spindles was fragmented into
multiple pieces that were located at or near the points of
convergence of microtubule arrays. The fragmentation
of centrosomal material induced by 0.8 nM vinblastine
is shown in three cells in Fig. 3B. The location of
fragmented centrosomal material at the foci of microtubule arrays is shown in Fig 3C,c',D,d'. Above 50 nM
vinblastine, mitotic centrosomes were compact (data
not shown); this occurred concomitant with total
microtubule depolymerization (Fig. 1 A). In contrast to
the effects of vinblastine on centrosomal material in
mitotic cells, the centrosomal material of interphase
cells was not fragmented at any vinblastine concentration examined (data not shown).
With increasing vinblastine concentration between
0.1 nM and 6 nM, the distance between the spindle
poles decreased. Central spindles were half as long as
central spindles in control cells after incubation with 3.6
nM vinblastine (Fig. IB).
Vinblastine-tubulin paracrystal formation
Tubulin paracrystals formed only at high vinblastine
concentrations (> 1 fjM), concomitant with an increase
in the mass of polymeric tubulin in cytoskeletal isolates
(Fig. 1A, squares). At 10 /JM vinblastine, cells
contained several long, large, polygonal tubulin paracrystals (Fig. 3G). The chromatin was either interphaselike (diffuse with an apparent nuclear membrane) or
mitotic (condensed and aggregated with no apparent
nuclear membrane) (not shown). At a vinblastine
concentration of 100 ^M, cells contained large numbers
of small, globular paracrystals (Fig. 3H), and the
chromatin often appeared extremely condensed (not
shown).
Effects of podophyllotoxin on mitosis
The effects of podophyllotoxin on mitotic arrest and
spindle organization appeared similar to the effects of
vinblastine in some ways, but there also were significant
differences in the mode of action of the two drugs.
Mitotic arrest and microtubule polymer mass
Like vinblastine, podophyllotoxin induced cells to
accumulate in mitotic metaphase (Fig. 4A, circles). A
total of 50% of the cells accumulated in metaphase
(Kmet) at approximately 30 nM podophyllotoxin (Table
1) and a peak of maximal metaphase accumulation
occurred at a podophyllotoxin concentration of 100 nM
(Fig. 4A). Incubation of cells with podophyllotoxin,
like incubation with vinblastine, decreased the ratio of
cells in anaphase to cells in metaphase in a concentration-dependent manner, with 50% decrease in the
ratio occurring at 5 nM podophyllotoxin (Kana/met,
Table 1). The anaphase/metaphase ratio was zero at 33
nM podophyllotoxin. Half-maximal inhibition of cell
division (Kdiv) occurred at 20 nM podophyllotoxin
(Table 1).
In contrast to the action of low concentrations of
vinblastine, metaphase accumulation by low concentrations of podophyllotoxin was associated with a high
degree of microtubule depolymerization (Fig. 4A,
Inhibition of mitosis by drugs
10
100
1000
10000
[Podophyllotoxin] (nM)
Fig. 4. Podophyllotoxin concentration dependence of
metaphase arrest and microtubule depolymerization (A)
and of spindle and cell reorganization (B,C) in HeLa cells
after treatment with podophyllotoxin at the stated
concentrations for the duration of one cell cycle.
(A) Accumulation of cells in metaphase was concomitant
with depolymerization of microtubules. Percentage of cells
in metaphase (open circles) and percentage decrease in
mass of polymerized microtubules (filled squares).
(B) Percentage of metaphases that were types I or II (open
triangles) and types III and IV (filled triangles). The
percentage of metaphase spindles that appeared normal
equals 100% minus the percentages indicated by the filled
and open triangles. (C) Percentage decrease of interpolar
distance measured on bipolar spindles (normal, type I and
type II).
squares; Table 1, Kdcp/Kmcl=0.5). For example, 50%
depolymerization of microtubules occurred at 15 nM
podophyllotoxin while only 8% of the cells were
arrested in metaphase (Table 1, Kdcp; Fig. 4A, circles).
Spindle organization
Between 3.3 nM and 18 nM podophyllotoxin, metaphase spindles were frequently types I and II, exhibiting
chromosomes located near the spindle poles and more
numerous, frequently longer, astral microtubules than
407
control spindles (Fig. 5A,a,b,C,c). The reorganization
of spindle microtubules, chromosomes and centrosomes induced by podophyllotoxin was qualitatively
similar to that induced by incubation with vinblastine
(see Fig. 3A,a,C,c). Type III spindles were prevalent at
18 and 32 nM podophyllotoxin. Small star-shaped
aggregates of microtubules were induced by podophyllotoxin (Fig. 5D,E), similar to but smaller than the starshaped aggregates induced by vinblastine (Fig. 3C,D).
Fig. 5b shows a particularly clear example of the
arrangement of polar chromosomes in a type I spindle.
Polar chromosomes were frequently distributed
unequally at the two poles after incubation with
vinblastine, podophyllotoxin or nocodazole (see below), suggesting that segregation of chromatids had not
occurred.
By contrast with vinblastine, podophyllotoxin
induced significant distortions of spindle morphology
without inducing accumulation of cells at metaphase or
inhibiting cell division. For example, only 2.2% of the
cells contained metaphase spindles at 6 nM podophyllotoxin, but 22% of the spindles were types I and II (Fig.
4B, open triangles). In addition, there was no net
microtubule depolymerization at this concentration
(Fig. 4A), and no inhibition of cell division. Even at 10
nM podophyllotoxin, only 5% of the cells were in
metaphase (Fig. 4A, open circles), and there was no
detectable inhibition of cell proliferation (data not
shown). However, spindle organization was significantly affected (Fig. 4B, 5C,c). Spindles were 23%
shorter than control spindles (Fig. 4C) and were often
abnormal (26% of the spindles were types I, II or III)
(Fig. 4B). In addition, the anaphase/metaphase ratio
was reduced by 60% as compared with controls.
The observation that 10 nM podophyllotoxin induced
a low frequency of cells in anaphase relative to cells in
metaphase and induced significant distortions of spindle
morphology without inhibiting cell division was initially
puzzling. A plausible explanation, however, is that the
rate of progression through metaphase was slowed by
low concentrations of podophyllotoxin, but metaphase
was not blocked, and anaphase and cytokinesis occurred normally. These data suggest that the mechanisms involved in proper construction of the metaphase
spindle are more sensitive to podophyllotoxin than the
mechanisms responsible for progression from metaphase to anaphase. The data also indicate that abnormal spindles do not necessarily result in metaphase
blockage, but that such spindles can proceed through
metaphase and the cells can divide.
The extent of spindle damage and the effects of the
damage on mitotic accumulation increased dramatically
between 18 nM and 33 nM podophyllotoxin (Figs 4A,
5D,d,E). At 18 nM podophyllotoxin, only 8% of the
cells were in metaphase (Fig. 4A, open circles). Of cells
in metaphase, 32% had spindles with normal organization, 23% of the spindles were types I or II, and 45%
were types III or IV (Fig. 4B). Bipolar spindles present
at this concentration were 63% shorter than control
spindles (Fig. 4C, open squares), and the mass of
microtubules was reduced by 52% as compared with
408
M. A. Jordan and others
Fig. 5
Inhibition of mitosis by drugs
Fig. 5. Microtubule, chromosome and centrosome
arrangement in HeLa cells after 18-20 h incubation in
podophyllotoxin at the specified concentrations. (A,a) 3
nM; (B,b) 18 nM; (C,c) 10 nM; (D,d) 18 nM; (E) 33 nM;
(F) 37 nM; (G,g) 110 nM podophyllotoxin.
(A,B,C,D,E,F,G) Anti-tubulin immunofluorescence;
(a,b,c,g) DAPI staining of chromosomes; (d) anticentrosomal immunofluorescence. (A,a) Type I abnormal
spindle induced by 3 nM podophyllotoxin; arrows point to
tufts of long astral microtubules in (A) and chromosomes
located near the spindle poles in a; (B,b) type I abnormal
spindle induced by 18 nM podophyllotoxin; arrows in b
point to chromosomes located near one pole, there were
none at the opposite pole; astral microtubules are not
prominent in this particular spindle but there are a few at
the arrow in B. (C,c) Two type II spindles induced by 10
nM podophyllotoxin; arrows point to long astral
microtubules in C and to large numbers of chromosomes
located near the spindle poles in c. (D,d) Three small
spindles induced by incubation with 18 nM
podophyllotoxin. All three spindles contain star-shaped
aggregates of microtubules. The upper two spindles contain
fragmented centrosomal material. The chromosomes of the
lower two spindles were condensed primarily into one
spherical mass characteristic of type III spindles. The
chromosomes of the upper spindle retained a slightly
increased density where one might expect the metaphase
plate, characteristic of a type II spindle (chromosomes not
shown). (E) Star-shaped aggregates of microtubules in type
III spindles (asterisks) and punctate aggregates of tubulin
in type IV spindles (arrows) induced by 33 nM
podophyllotoxin. (F) Microtubules of interphase cells
induced by incubation in 37 nM podophyllotoxin.
409
Effects of nocodazole on mitosis
The effects of very low concentrations of nocodazole on
mitotic arrest and spindle organization were similar to
the effects of vinblastine in several significant ways.
Mitotic arrest and microtubule polymer mass
Like incubation of cells with vinblastine and podophyllotoxin, incubation of cells with nocodazole induced
metaphase accumulation. A total of 50% of the cells
had accumulated in metaphase (/Cmet) at 54 nM
nocodazole (Fig. 6A, circles; Table 1), and maximal
accumulation occurred at 100 nM nocodazole. Unlike
vinblastine and podophyllotoxin, the percentage ac-
(G) Diffuse tubulin stain in both mitotic (labelled m) and
interphase (labelled i) cells induced by incubation with 110
nM podophyllotoxin; and (g) parallel DAPI staining of the
same cells showing either masses of condensed mitotic
chromatin or chromosomes (m) or diffuse interphase
chromatin (i). Bars, 10 fim. Small bar in a shows
magnification of A,a and C-g; large bar in b shows
magnification of B and b.
control cells (Fig. 4A, squares). After incubation with
33 nM podophyllotoxin, 65% of the cells were in
metaphase, and all of the mitotic figures were types III
and IV (Fig. 4B, closed triangles). Mitotic cells lacked
microtubules altogether (Fig. 5E, arrows), or contained
very small star-shaped aggregates of short microtubules
(Fig. 5E, asterisks). Short microtubules remained in
some interphase cells (Fig. 5F). Concomitantly, the
microtubule mass measured in isolated cytoskeletons
was reduced by 80% as compared with controls (Fig.
4A, squares). At podophyllotoxin concentrations
greater than or equal to 100 nM, no microtubules were
present in interphase or mitotic cells (Fig. 4A, closed
squares; Fig. 5G), and all mitotic figures were type IV
(Fig. 5G,g).
The centrosomal material was fragmented to some
degree at 10 nM podophyllotoxin and it was markedly
fragmented at 18 nM podophyllotoxin (Fig. 5d).
However, the centrosomal material was compact (data
not shown) at high podophyllotoxin concentrations (>
100 nM), which induced total microtubule depolymerization (Fig. 4A and Fig. 5G).
-20
10
100
1000
10000
[Nocodazole] (nM)
Fig. 6. Nocodazole concentration dependence of metaphase
arrest and microtubule depolymerization (A) and of
spindle and cell reorganization (B,C) in HeLa cells after
treatment with nocodazole at the stated concentrations for
the duration of one cell cycle. (A) Accumulation of cells in
metaphase was accompanied by little or no
depolymerization of microtubules. Percentage of cells in
metaphase (circles) and percentage decrease in mass of
polymerized microtubules (squares). (B) Percentage of
metaphases that were types I or II (open triangles) and
types III and IV (filled triangles). The percentage of
metaphase spindles that appeared normal equals 100%
minus the percentages indicated by the filled and open
triangles. (C) Percentage decrease of interpolar distance
measured on bipolar spindles (normal, type I and type II).
410
M. A. Jordan and others
cumulation did not diminish at high concentrations of
nocodazole (Fig. 6A; compare with Figs 1A, 4A). Like
vinblastine and podophyllotoxin, incubation with
nocodazole resulted in a concentration-dependent decrease in the ratio of cells in anaphase to cells in
metaphase, with 50% decrease in the ratio {Kana/met)
occurring at 12 nM nocodazole (Table 1), and a ratio of
zero at 100 nM nocodazole. Half-maximal inhibition of
cell division (Kdiv) occurred at 22 nM nocodazole (Table
1).
At concentrations of nocodazole (54 nM) that
induced 50% accumulation of cells in mitosis, there was
little or no detectable microtubule depolymerization
(Table 1, Fig. 6A). Some microtubule depolymerization
was associated with high levels of metaphase arrest, but
the degree of depolymerization was slight compared
with depolymerization associated with metaphase arrest induced by podophyllotoxin (Fig. 6A; compare
with Fig. 4A). For example, the microtubule polymer
level was reduced by only 30% at 100 nM nocodazole, a
concentration that induced maximal mitotic accumulation. By comparison, maximal metaphase accumulation with podophyllotoxin was accompanied by 100%
loss of polymer (Fig. 4A). An 11-fold higher concentration of nocodazole was required to depolymerize half
of the microtubule polymer than was required to induce
50% accumulation of cells at metaphase (Kdep/Kmet,
Table 1).
Spindle organization
Between 3 nM and 100 nM nocodazole, metaphase
spindles were frequently types I and II, exhibiting poleassociated chromosomes and lengthened, more numerous astral microtubules than control spindles (Fig.
7A,a,a',B,b,b'). At 33 nM and higher nocodazole
concentrations, spindles were frequently type III,
consisting of star-shaped aggregates of microtubules,
and type IV (Fig. 7B,b,b',C,c,c'). The reorganization
of spindle microtubules, chromosomes and centrosomes induced by nocodazole was qualitatively similar
to that induced by incubation with vinblastine and
podophyllotoxin (see Figs 3 and 5).
The organization of a typical type I spindle is clearly
visible in Fig. 7A,a,a'. It is evident that the chromosomes that are not included in the metaphase plate are
located at the ends of tufts of astral microtubules rather
than strictly at the centrosomes. Chromosomes with a
polar location were often located at the ends of astral
microtubules after incubation of cells with all three
drugs, but the relationship is easier to visualize in the
microscope than in micrographs. Types I and II spindles
occurred with relatively high frequency with nocodazole as compared with the other drugs. A maximum of
39% of all spindles were types I and II with nocodazole
(Fig. 6B, open triangles); whereas the maximum
induced by podophyllotoxin was 23% (Fig. 4B) and the
maximum induced by vinblastine was 17% (Jordan et
al. 1991). However, as with vinblastine and podophyllotoxin, some cells arrested in metaphase had normal
spindles. For example, after incubation with 33 nM
nocodazole, 22% of cells were arrested in metaphase.
Fig. 7. Microtubule, chromosome and centrosome
arrangement in HeLa cells after incubation with
nocodazole at the indicated concentrations for one cell
cycle. (A,a,a') 33 nM; (B,b,b') 100 nM; (C,c,c') 330 nM;
(D) 1 ,uM. (A,B,C,D) Anti-tubulin immunofluorescence;
(a,b,c) DAP1 staining of chromatin; (a',b',c') anticentrosomal immunofluorescence. (A,a,a') Two type I
spindles induced by incubation with 33 nM nocodazole.
The spindles with long astral microtubules (arrows in A)
and several chromosomes in the region of the poles
(arrows in a). It is clear that many of these chromosomes
are at the ends of long astral microtubules. The
centrosomes (arrows in a') are fragmented. (B,b,b') Type I
(I), FI (II) and III spindles (asterisks) induced by
incubation with 100 nM nocodazole. The types I and II
spindles have some chromosomes located near the spindle
poles (arrows in b), whereas the chromosomes of the type
III spindles are in spherical masses. Centrosomes are
fragmented (arrows in b'). (C,c,c') Several type III
spindles induced by 330 nM nocodazole. These differ from
the type III spindles induced by lower concentrations of
nocodazole (B,b,b'); they contain multiple star-shaped
aggregates of microtubules or other tubulin polymer (large
arrows). Their centrosomes (arrows in c') are compact and
located outside of the tubulin and chromosome-containing
structure. Centrosomes appear to have some tubulin
associated with them (compare centrosomal stain in c'
(arrows) with tubulin stain at small arrows in C). There are
also a few punctate aggregates of tubulin at the cell
periphery in C. (D) Punctate aggregates of tubulin and a
few short remnants of microtubules, 1 fiM nocodazole.
Bars, 10 ,um. Large bar in A shows magnification of
A,a,a'; small bar in D shows magnification of B-D.
Of these, 20% had normal spindles, 39% were types I
or II, and 41% were type III (Fig. 4B).
The interpolar distance was decreased by nocodazole
concentrations equal to or greater than 33 nM (Fig. 6C,
squares). Nocodazole at these same concentrations
induced formation of some monopolar spindles (type
III) (Fig. 6B, filled triangles). Incubation with nocodazole at concentrations that induced major rearrangements of spindle microtubules also induced fragmentation of centrosomal material. The centrosomal
material was, however, still located at the foci of the
microtubule arrays (Fig. 7A,a,B,b).
Mitotic cells at 330 nM nocodazole contained several
clusters of short, thick tubulin polymers (Fig. 7C). To
our knowledge, such structures have not been reported
previously with nocodazole. The tubulin polymers,
which were enclosed within masses of condensed
chromosomes (Fig. 7c), may be thick bundles of
microtubules or another polymeric form of tubulin. In
contrast to the fragmentation of centrosomes that
occurred between approximately 33 and 100 nM
nocodazole, the centrosomes were compact at 330 nM
nocodazole. The centrosomes were often near the
periphery of the cells, some distance from the tubulin
polymer-chromosome array (Fig. 7C,c,c'). At 330 nM
nocodazole, the cytoskeletal microtubule polymer mass
was reduced by 40% as compared with the polymer
mass in control cells (Fig. 6A), consistent with the
impression by immunofluorescence microscopy that
Inhibition of mitosis by drugs
Fig. 7
411
412
M. A. Jordan and others
nocodazole induced significant microtubule depolymerization.
By immunofluorescence microscopy, it was seen that
high concentrations of nocodazole (> 1 //M) induced
nearly complete depolymerization of microtubules.
Punctate aggregates of tubulin were scattered throughout the cytoplasm of the cells, and only a few interphase
cells contained short microtubule fragments (Fig. 7D).
However, some microtubule polymer was present in
isolated cytoskeletons (20-40% of controls; Fig. 6A).
The presence of measurable cytoskeletal tubulin
polymer but the nearly complete absence of stained
microtubule polymer in the corresponding microscopic
preparations in this concentration range was the only
inconsistency noted between the biochemical measurement of polymer mass and impressions of microtubule
polymer levels obtained by microscopy. The measurable cytoskeletal tubulin polymer after incubation with
nocodazole at concentrations ^ 1 [iM is probably
attributable to the tubulin aggregates and microtubule
fragments present at these concentrations.
Discussion
Motility of cilia and flagella occurs by the interaction of
the motor protein dynein with an array of stable
microtubules (reviewed by Warner, 1979). Such stable
microtubules clearly function as passive supports whose
assembly dynamics are not important in motility.
Studies from many laboratories have also documented
the probable importance of microtubule-associated
motor proteins in mitosis (e.g. see Pfarr et al. 1990;
Steuer et al. 1990; Hyman and Mitchison, 1991).
However, mitotic spindle microtubules are highly
dynamic, and it is reasonable to think that the dynamics
of the microtubules may be important in one or more
aspects of mitosis (e.g. see Saxton et al. 1984; Salmon et
al. 1984; Mitchison, 1989).
Many drugs that inhibit mitosis are thought to act by
destroying spindle microtubules, and the actions of
these drugs at high concentrations appear to result from
depletion of microtubule polymer (Malawista et al.
1968; Wilson and Bryan, 1974; De Brabander et al.
1976; Sluder, 1979; Zieve et al. 1980). However, we
found that at low concentrations, vinblastine, podophyllotoxin, nocodazole, colchicine and taxol inhibit
the exchange of tubulin at microtubule ends in vitro
without exerting significant effects on the total mass of
microtubule polymer (Jordan and Farrell, 1983; Wilson
et al. 1985; Wilson and Farrell, 1986; Jordan and
Wilson, 1990; R. Toso, M. A. Jordan and L. Wilson,
unpublished results with nocodazole). Thus, it is
conceivable that one or more of these drugs might
inhibit mitosis by affecting the dynamics of the
microtubules rather than merely by depolymerizing the
microtubules. In the present study, we found that
mitotic block by vinblastine and nocodazole in HeLa
cells occurred by a subtle effect on spindle microtubules, consistent with the idea that the drugs are
acting by affecting the dynamics of spindle microtubules
rather than by depolymerizing the microtubules. Low
concentrations of vinblastine and nocodazole arrested
cells at metaphase of mitosis in the presence of
apparently correctly assembled spindles with a full
complement of microtubules. The mechanism by which
podophyllotoxin induced mitotic arrest appeared to
involve depolymerization of microtubules. However,
podophyllotoxin also induced a reorganization of the
spindle that was similar to that induced by vinblastine
and nocodazole. It appears that podophyllotoxin induces alterations in spindle microtubule dynamics, but
that, at the lowest effective concentrations, the specific
alterations are not sufficient to block the transition from
metaphase to anaphase.
Vinblastine, podophyllotoxin and nocodazole depolymerize microtubules in vitro at micromolar concentrations (Wilson et al. 1976,1982; Hoebeke et al. 1976).
However, at low concentrations, all three drugs
stabilize microtubule ends in vitro. With in vitro
reassembled MAP-rich bovine brain microtubules, the
most sensitive effects of the three drugs on microtubules occur in the absence of significant changes in the
mass of assembled microtubules. For example, vinblastine inhibited exchange of tubulin at the ends of
microtubules half-maximally at 0.15 fiM, while the
polymer mass was reduced less than 3% as compared
with controls (Jordan and Wilson, 1990). Similar results
were obtained with podophyllotoxin (Jordan and
Farrell, 1983) and with nocodazole (R. Toso, M. A.
Jordan and L. Wilson, unpublished data). In addition,
all three drugs suppress dynamic instability behavior of
MAP-free microtubules in vitro. Specifically, we found
that vinblastine and nocodazole suppress rates of
growing and shortening at ends of individual microtubules and suppress transitions to phases of growing
and shortening as determined by computer-enhanced
video microscopy (R. Toso, K. W. Farrell, M. A.
Jordan, B. Matsumoto and L. Wilson, unpublished
results). Podophyllotoxin has been shown to inhibit
dynamic instability in microtubule suspensions using
radiolabel exchange methodology and electron microscopy (Schilstra et al. 1989). Thus, each of the drugs
can inhibit tubulin exchange at microtubule ends in
vitro without significantly altering the mass of assembled microtubules. In other words, at low drug
concentrations, the association and dissociation rate
constants for tubulin addition to one or both microtubule ends are suppressed in ways that stabilize
microtubule dynamics but do not alter the equilibrium
between microtubule polymer and soluble tubulin. It is
likely that the powerful capacity of vinblastine and
nocodazole to inhibit microtubule dynamics and stabilize microtubule ends in vitro and a similar alteration in
vivo is responsible for their ability to block mitosis.
With podophyllotoxin, inhibition of microtubule dynamics in concert with net microtubule depolymerization appear to be responsible for mitotic block.
Effects of vinblastine, podophyllotoxin and
nocodazole on mitosis
Effects on spindle organization
Metaphase accumulation by vinblastine, podophyllo-
Inhibition of mitosis by drugs
toxin and nocodazole was associated with concentration-dependent rearrangements in the organization
of spindle microtubules and chromosomes that were
similar for all three drugs. At the lowest effective
concentrations of all the drugs, many blocked spindles
were indistinguishable from normal (control) metaphase spindles (Figs 4B, 6B). The mildest perturbations
of spindle organization in spindles that snowed abnormalities were increasing numbers and lengths of astral
microtubules, decreasing lengths of the central
spindles, the presence of one or a few chromosomes
located near the spindle poles, and fragmentation of
centrosomal material (Figs IB, 3, 4C, 5, 6C, 7) (types I
and II).
Types I and II abnormal spindles appeared to result
from a redistribution of microtubule polymer from the
central spindle to the asters. The distance between the
poles decreased, and the number and length of astral
microtubules increased. Thus, the interpolar and
kinetochore microtubules must have shortened, while
the astral microtubules got longer. Such length changes
could occur by a common action of the three drugs on a
mechanism that controls the dynamics of tubulin
addition and loss at the ends of the microtubules. For
example, prevention of catastrophic depolymerization
of astral microtubules could result in increased numbers
and increased lengths of astral microtubules.
One of the most sensitive effects of the three drugs
was on congression of chromosomes to form the
metaphase plate. At the lowest effective concentrations
of the three drugs, most chromosomes congressed
normally, while in some cells one or a few chromosomes
appeared unable to congress. With increasing drug
concentrations, increasing numbers of chromosomes
were found at or near the spindle poles. Thus, the
correct attachment of microtubules to both kinetochores of a chromatid pair must be highly sensitive to
the drugs. During prometaphase, the astral microtubules repeatedly grow and shorten, apparently
probing the cytoplasm until kinetochore attachment is
achieved (Rieder et al. 1990; Hayden et al. 1990). One
can envision that interfering with growing and shortening of the astral microtubules in prometaphase might
result in the inability of the microtubules from one pole
to reach the kinetochore of a chromosome located near
the opposite pole. Alternatively, the attachment of the
microtubule to the kinetochore may be blocked by the
presence of the drug at the end of the microtubule.
Low concentrations of vinblastine, podophyllotoxin
and nocodazole produced high proportions (16-39%) of
spindles that were bipolar but contained imperfectly
congressed chromosomes (types I and II). This observation raises the question of whether a cell that
eventually overcomes mitotic block (either during
incubation with drug or after drug removal) will
undergo accurate chromosome segregation or not.
Little information appears to exist concerning induction
of chromosome nondisjunction by these three drugs.
However, other drugs (Colcemid, diethylstilbestrol)
that affect microtubule polymerization dynamics have
been found to cause aneuploidy (Kato and Yosida,
413
1970; Sharp and Parry, 1985; Wheeler et al., 1986). The
prevalence of abnormal bipolar spindles (types I and II)
observed with vinblastine, podophyllotoxin, nocodazole (Figs 4B, 6B), other vinca alkaloids (Jordan et al.
1991), and colchicine and taxol (M. A. Jordan, D.
Thrower and L. Wilson, unpublished results), suggests
that any drug that affects microtubule dynamics may
have the capacity to induce aneuploidy. Nicklas (1985)
proposed, in words that presage the observations
reported here, that "a little explored but perhaps very
important source of aneuploidy stems from the delicate
balance that must be struck between a spindle that is
too stable for reorientation to occur and one that is so
unstable that reorientation continues indefinitely".
At higher levels of metaphase accumulation with all
three drugs, the proportions of normal and type I
spindles decreased and types II and III became more
prevalent. Type III spindles were not bipolar. There
was no distinguishable central plate of chromosomes;
rather the chromosomes were arranged in a ball
enclosing one or more star-shaped aggregates of
microtubules. In type III spindles there was no
separation of centrosomal material into two distinct
masses. Rather the centrosome consisted of multiple
fragments that were generally located at the foci of the
microtubule array(s). The mechanism by which type III
monopolar spindles form may involve inhibition of
spindle pole separation during prophase. By whatever
mechanism they form, type III spindles appear to
represent an extreme in drug concentration-dependent
redistribution of microtubule polymer from the central
spindle to the asters.
Sufficiently high concentrations of all three drugs
caused microtubule depolymerization as determined by
ELISA (discussed below). The loss of microtubule
polymer in spindles was also clearly evident by
immunofluorescence microscopy (e.g. Figs 3F, 5G). At
sufficiently high concentrations of all three drugs, no
microtubules were visible. Depolymerization resulted
in the appearance of a type IV abnormal mitotic figure
consisting of a ball of condensed chromosomes and no
microtubules; centrosomes were compact and located
apparently randomly. Types III and IV spindles are
typical of the c-mitotic figures described by Levan
(1938) and Ostergren (1944) in their early studies on
colchicine.
Effects on the mass of microtubule polymer
All three drugs induced accumulation of cells in a
metaphase-like stage of mitosis. However, there was no
clear relationship between the extent of mitotic accumulation and microtubule depolymerization. For
example, upon incubation of cells with concentrations
of vinblastine or nocodazole that induced 50% accumulation of cells in metaphase, there was little or no
detectable microtubule depolymerization (Figs 1A,
6A). Thus, vinblastine and nocodazole appear to block
mitotic spindle function without changing the amount
of microtubule polymer. In contrast, metaphase accumulation with podophyllotoxin was accompanied by
depolymerization of microtubules. For example, at the
414
M. A. Jordan and others
podophyllotoxin concentration that induced 50% accumulation of cells at metaphase (30 nM), the drug
induced an 85% loss of microtubule polymer (Fig. 4A).
A possible error in the foregoing analysis may arise
by comparing the microtubule polymer mass in cultures
consisting predominantly of mitotic cells after incubation with drugs with the microtubule mass in control
cultures that consist predominantly of interphase cells.
It has not been previously determined whether mitotic
cells have an inherently greater mass of microtubules
than interphase cells. In the present study, we compared the microtubule content of interphase cells with
the microtubule content of a population of cells that was
enriched in mitotic stages but that had not been
incubated with any drug that induced metaphase block.
We measured isolated cytoskeletons from HeLa cells
that were synchronized in mitosis using a double
thymidine block (Rao and Engelberg, 1966) followed
by mitotic shake-off (Robbins and Scharf, 1966). The
mean mitotic index of the synchronized populations at
the time of cytoskeletal isolation was 45%, whereas the
mitotic index of the control populations was 2-3%.
Tubulin in polymer form comprised 0.79 (± 0.24)% of
the total protein in the synchronized mitotic populations (w=5) compared with 1.03 (± 0.04)% for
control populations («=117). In addition, Y. Zhai and
G.G. Borisy (University of Wisconsin, Madison, WI)
measured the mass of microtubule polymer in individual LLC-PK cells after microinjection of fluorescent
tubulin and found that, at 37°C, mitotic cells contained
90% ± 5% of the microtubule polymer mass of
interphase cells (personal communication). Thus, the
mass of microtubule polymer does not appear to
increase significantly, if at all, during mitosis.
In addition, two other lines of evidence suggest that
any mitosis-associated increase in microtubule polymer
mass must be small or nonexistent. First, Bulinski et al.
(1980) found that the total tubulin concentration in
synchronized HeLa cells in mitosis was the same as the
total tubulin concentration in a mixed cell population.
We found, after incubation with vinca alkaloids,
podophyllotoxin and nocodazole over the range of
concentrations used in this study, that the soluble
(unpolymerized) tubulin concentration remained constant as the concentration of tubulin in polymer form
was altered (Jordan et al. 1991, and unpublished data).
In other words, the soluble tubulin concentration
remained constant whether the cells were predominantly in mitosis or whether they were predominantly in
interphase. Thus, mitotic cells do not have a significantly higher fraction of tubulin in polymer form than
interphase cells, and from the results of Bulinski et al.
(1980) we know that mitotic cells do not have more total
tubulin than interphase cells.
Second, incubation of cells with the drug taxol
enhances microtubule polymerization (Schiff et al.
1979; Schiff and Horwitz, 1980). However, in a separate
study, we found that taxol at low concentrations can
induce approximately 33% mitotic accumulation in
HeLa cells without increasing the microtubule polymer
mass as determined by ELISA of tubulin in isolated
cytoskeletons (M. A. Jordan, D. Thrower and L.
Wilson, unpublished experiments). If mitotic cells
contained more microtubule polymer than interphase
cells, one would expect to measure higher polymer
levels in such a population of cells blocked in mitosis by
taxol compared with control cells that were predominantly in interphase.
Another possible error in the analysis of the
relationship between mitotic accumulation and microtubule polymer mass with vinblastine, podophyllotoxin
and nocodazole could arise if vinblastine or nocodazole
induced the formation of a non-microtubule tubulin
polymer. However, only trace amounts of small
aggregates of tubulin were observed after incubation of
cells with 6.4 nM vinblastine (Fig. 3D) or 100 nM
nocodazole (data not shown). These concentrations
were higher than the concentrations of vinblastine and
nocodazole that induced mitotic accumulation (Figs
1A, 6A). In addition, no paracrystals or macrotubules
(Bensch and Malawista, 1969) were found after incubation of HeLa cells with 2 nM or 10 nM vinblastine, as
determined by electron microscopy (K. Wendell, L.
Wilson and M. A. Jordan, unpublished data). After
incubation with 10 nM vinblastine the mean diameter of
microtubules was 24.9 nm. Some close alignment of
microtubules was observed by electron microscopy; this
may account for the thick appearance of the microtubule stain by immunofluorescence microscopy (Fig.
3D).
Effects on the distance between spindle poles
The concentration-dependent decrease in pole-to-pole
distance that occurred with vinblastine, podophyllotoxin and nocodazole (Figs IB, 4C, 6C) indicates that
all three drugs diminish the forces holding the two poles
apart. Suppression of dynamic instability or treadmilling of kinetochore microtubules (Mitchison et al. 1986;
Hamaguchi et al. 1987; Mitchison, 1989) or steric
hindrance of kinetochore/microtubule attachment
might lead to a disruption of the balance of forces in the
spindle and a resultant shortening of the spindle (for a
discussion of these forces, see Nicklas, 1988). It is
conceivable that the poleward forces inherent in
treadmilling or fluxing microtubules that are continually
adding subunits at their (+) ends (kinetochore and/or
interpolar microtubules) are instrumental in maintaining centrosomal separation. In support of the idea that
treadmilling of kinetochore microtubules is responsible
for pole-to-pole separation, Sawin and Mitchison
(1991a,b) induced formation of asymmetric halfspindles in vitro composed of treadmilling or fluxing
microtubules that extended between a centrosome and
a mass of chromatin. Thus, interactions among the
components of a single half-spindle are sufficient to
maintain chromosome/centrosome separation and,
therefore, in a whole spindle, centrosome/centrosome
separation. Perhaps vinblastine, podophyllotoxin and
nocodazole reduce the pole-to-pole distance by inhibiting the treadmilling dynamics that may be responsible
for maintenance of the separation between the poles.
Inhibition of mitosis by drugs
Effects on centrosomal organization
The mitotic centrosome appears to play a significant
role in mitosis. One component (p34 2 protein kinase)
of the mitotic control factor MPF is localized in
centrosomes of mitotic HeLa cells (Bailly et al. 1989).
Centrosomes nucleate microtubules (Kuriyama and
Borisy, 1981), and a cyclical phosphorylation-anddephosphorylation of centrosomal proteins parallels
the increase in microtubule nucleating activity of
centrosomes early in mitosis and the subsequent
decrease in nucleating activity at anaphase (Robbins et
al. 1968; Kuriyama and Borisy, 1981; Vandre and
Borisy, 1989). Using high concentrations of several
antimitotic drugs Kung et al. (1990) and Alfa et al.
(1990) obtained evidence suggesting that intact microtubules play an essential role in the activation and /or
inactivation of mitotic control proteins.
Sellitto and Kuriyama (1988) found that pericentriolar material was dispersed after treatment of CHO cells
with the antimitotic drug colcemid, but not after
treatment with high concentrations of nocodazole
(>330 nM). In agreement with their results, we found
that centrosomes of HeLa cells were compact after
incubation with nocodazole at concentrations greater
than 330 nM (Fig. 7c'). However, we found that, at
lower concentrations of nocodazole, as well as with
vinblastine and podophyllotoxin, the organization of
the centrosomal material was altered in ways that were
similar for all three drugs. Specifically, centrosomal
material was fragmented by concentrations of the three
drugs that affected the organization of spindle microtubules but did not totally destroy the microtubules.
Fragmented centrosomal aggregates were always
located at the foci of microtubule arrays (Figs
3C,c',D,d', 5D,d, 7A,a',B,b'). Curiously, at drug
concentrations that induced complete microtubule
depolymerization, the centrosomal material of mitotic
cells was compact, and the centrosomes of interphase
cells were compact at all concentrations of the three
drugs. These results support the idea that organization
of the mitotic spindle microtubules by centrosomes is
not simply a one-way street, but that intact microtubules can alter the organization of the centrosome. In
addition, they suggest that microtubule dynamics may
also exert an important role in regulating the structure,
and perhaps the function, of mitotic centrosomes.
Cell cycle
We have shown that vinblastine, podophyllotoxin and
nocodazole can arrest the cell cycle at metaphase in
cells having apparently correctly assembled spindles.
As the drug concentration is increased, the organization
of arrested metaphase spindles is altered in qualitatively
the same way with all three drugs. With vinblastine and
nocodazole, metaphase arrest occurs in the presence of
a normal complement of microtubule polymer. The
metaphase/anaphase transition appears to be selectively sensitive to drug effects on microtubule-dependent functions. Microtubule-dependent processes
required for progression of cells through interphase into
mitosis were not inhibited by drug concentrations that
415
induced blockage of mitosis at the metaphase/anaphase
transition. Our results support the idea that not simply
microtubules, but dynamic microtubules, are in some
way crucial to mitosis. Spindle microtubules are known
to be considerably more dynamic than microtubules in
interphase cells (Saxton et al. 1984; Salmon et al. 1984).
Murray and Kirschner (1989) postulated that a correctly
assembled spindle may be required for the degradation
of cyclin and the exit from mitosis. It seems reasonable
to think that the increased dynamics of spindle
microtubules may be critical to the correct assembly of
the spindle at a level that is not detectable by light
microscopy, or they may be critical for signalling the
transition from metaphase to anaphase.
We thank Dr. Roger Leslie and Dr. Teresa Burgess for
stimulating discussions during the course of this work, Dr.
Brian Matsumoto for the generous loan of his Olympus
objective, and Mr. Herb Miller for willing and patient
assistance with computer software. We also thank Dr. Teresa
Burgess, Mr. Rob Toso, and Ms. Kim Wendell for careful and
thoughtful reading of the manuscript. This work was
supported by the American Cancer Society, grant CH 381.
References
Alfa, C. E., Ducommun, B., Beach, D. and Hyams, J. S. (1990).
Distinct nuclear and spindle pole body populations of cyclin-cdc2 in
fission yeast. Nature 347, 680-682.
Bailly, E., Doree, M., Nurse, P. and Bornens, M. (1989). p34 cdc2 is
located in both nucleus and cytoplasm; part is centrosomally
associated at G 2 /M and enters vesicles at anaphase. EMBO J. 8,
3985-3995.
Ball, R. L., Carney, D. H., Albrecht, T., Asai, D. J. and Thompson,
W. C. (1986). A radiolabeled monoclonal antibody binding assay
for cytoskeletal tubulin in cultured cells. J. Cell Biol. 103, 10331041.
Bensch, K. G. and Malawista, S. E. (1969). Microtubular crystals in
mammalian cells. J. Cell Biol. 40, 95-106.
Bulinski, J. C , Rodriguez, J. A. and Borisy, G. G. (1980). Test of four
possible mechanisms for the temporal control of spindle and
cytoplasmic microtubule assembly in HeLa cells. J. Biol. Chem.
255, 1684-1688.
Calarco-Gillam, P. D., Siebert, M. C , Hubble, R., Mitchison, T. and
Kirschner, M. (1983). Centrosome development in early mouse
embryos as defined by an autoantibody against pericentriolar
material. Cell 35, 621-629.
Chu, D. and Klymkowsky, M. W. (1989). Experimental analysis of
cytoskeletal function in early Xenopus lacvis embryos. In The
Cytoskeleton in Differentiation and Development (ed. J. Arechaga
and R. Maccioni ), pp. 331-333. Oxford, UK.: IRL Press.
De Brabander, M. J., Van De Veire, R. M. L., Aerts, F. E. M.,
Borgers, M. and Janssen, P. A. J. (1976). The effects of methyl [5(2-thienylcarbonyl)—lH-benzimidazol-2-yl] carbamate (R 17 943:
NSC 238159), a new synthetic antitumoral drug interfering with
microtubules, on mammalian cells cultured in vitro. Cancer Res.
36, 905-916.
Dustin, P. (1984). Microtubules. Berlin: Springer-Verlag.
Farrell, K. W., Jordan, M. A., Miller, H. and Wilson, L. (1987).
Phase dynamics at microtubule ends: the coexistence of
microtubule length changes and treadmilling. J. Cell Biol. 104,
1035-1046.
Hamaguchi, T., Toriyama, M., Sakai, H. and Hiramoto, Y. (1987).
Redistribution of fluorescently labeled tubulin in the mitotic
apparatus of sand dollar eggs and the effects of taxol. Cell Struct.
Fund. 12, 43-52.
Hayden, J. H., Bowser, S. S. and Rieder, C. L. (1990). Kinetochores
capture astral microtubules during chromosome attachment to the
416
M. A. Jordan and others
mitotic spindle: direct visualization in live newt lung cells. J. Cell
Biol. I l l , 1039-1046.
Hoebeke, J., Van Nijen, G. and De Brabander, M. (1976). Interaction
of oncodazole (R 17934), a new anti-tumoral drug, with rat brain
tubulin. Biochem. Biophys. Res. Commun. 69, 319-324.
Hyman, A. A. and Mitchison, T. (1991). Two different microtubulebased motor activities with opposite polarities in kinetochores.
Nature 351, 206-211.
Jordan, M. A. and Farrell, K. W. (1983). Differential radiolabelling
of opposite microtubule ends: methodology, equilibrium exchangeflux analysis, and drug poisoning. Anal. Biochem. 130, 41-53.
Jordan, M. A., Thrower, D. and Wilson, L. (1.991). Mechanism of
inhibition of cell proliferation by vinca alkaloids. Cancer Res. 51,
2212-2222.
Jordan, M. A. and Wilson, L. (1990). Kinetic analysis of tubulin
exchange at microtubule ends at low vinblastine concentrations.
Biochemistry 29, 2730-2739.
Kato, H. and Yosida, T. H. (1970). Nondisjunction of chromosomes
in a synchronized cell population initiated by reversal of colcemid
inhibition. Exp. Cell Res 60, 459-464.
Kung, A. L., Sherwood, S. W. and Schimke, R. L. (1990). Cell linespecific differences in the control of cell cycle progression in the
absence of mitosis. Proc. Nat. Acad. Sci. USA 87, 9533-9557.
Kuriyama, R. and Borisy, G. G. (1981). Microtubule-nucleating
activity of centrosomes in Chinese hamster ovary cells is
independent of the centriole cycle but coupled to the mitotic cycle.
J. Cell Biol. 91, 822-826.
Levan, A. (1938). The effect of colchicine on root mitoses in Allium.
Hereditas 24, 471-486.
Malawista, S. E., Bensch, K. G. and Sato, H. (1968). Vinblastine and
griseofulvin reversibly disrupt the living mitotic spindle. Science
160, 770-772.
Mitchison, T. (1989). Polewards microtubule flux in the mitotic
spindle: evidence from photoactivation of fluorescence. J. Cell
Biol. 109, 637-652.
Mitchison, T., Evans, L., Schulze, E. and Kirschner, M. (1986). Sites
of microtubule assembly and disassembly in the mitotic spindle.
Cell 45, 515-527.
Murray, A. W. and Kirschner, M. W. (1989). Dominoes and clocks:
the union of two views of the cell cycle. Science 246, 614-621.
Nicklas, R. B. (1985). Mitosis in eukaryotic cells: an overview of
chromosome distribution. In Aneuploidy, Etiology and Mechanism
(ed. Dellarco, Voytek, Hollaender), p. 191. New York: Plenum.
Nicklas, R. B. (1988). The forces that move chromosomes in mitosis.
Annu. Rev. Biophys. Biophys. Chem. 17, 431-449.
Ostergren, G. (1944). Colchicine mitosis, chromosome contraction,
narcosis and protein chain folding. Hereditas 30, 429-467.
Pfarr, C. M., Coue, M., Grissom, P. M., Hays, T. S., Porter, M. E.
and Mclntosh, J. R. (1990). Cytoplasmic dynein is localized to
kinetochores during mitosis. Nature 345, 263-265.
Rao, P. N. and Engelberg, J. (1966). Effects of temperature on the
mitotic cycle of normal and synchronized mammalian cells. In Cell
Synchrony (cd. 1. L. Cameron and G. M. Padilla), pp. 332-352.
New York: Academic Press.
Rieder, C. L., Alexander, S. P. and Rupp, G. (1990). Kinetochores
are transported poleward along a single astral microtubule during
chromosome attachment to the spindle in newt lung cells. J. Cell
Biol. 110, 81-95.
Robbins, E., Jentzsch, G. and Micali, A. (1968). The centriole cycle in
synchronized HeLa cells. J. Cell Biol. 36, 329-339.
Robbins, E. and Scharff, M. (1966). Some macromolecular
characteristics of synchronized HeLa cells. In Cell Synchrony (ed.
I. L. Cameron and G. M. Padilla), pp. 353-374. New York:
Academic Press.
Salmon, E. D., Leslie, R. J., Saxton, W. M., Karow, M. L. and
Mclntosh, J. R. (1984). Spindle microtubule dynamics in sea urchin
embryos: analysis using a fluorescein-labeled tubulin and
measurements of fluorescence
redistribution after
laser
photobleaching. J. Cell Biol. 99, 2165-2174.
Sawin, K. and Mitchison, T. (1991a). Mitotic spindle assembly by two
different pathways in vitro. J. Cell Biol. 112, 925-940.
Sawin, K. and Mitchison, T. (1991b). Poleward microtubule flux in
mitotic spindles assembled in vitro. J. Cell Biol. 112, 941-954.
Saxton, W. M., Stemple, D. L., Leslie, R. J., Salmon, E. D.,
Zavortnik, M. and Mclntosh, J. R. (1984). Tubulin dynamics in
cultured mammalian cells. J. Cell Biol. 99, 2175-2186.
Schiff, P. B., Fant, J. and Horwitz, S. B. (1979). Promotion of
microtubule assembly in vitro by taxol. Nature 277, 665-667.
Schiff, P. B. and Horwitz, S. B. (1980). Taxol stabilizes microtubules
in mouse fibroblast cells. Proc. Nat. Acad. Sci. USA 77,1561-1565.
Schilstra, M. J., Martin, S. R. and Bayley, P. M. (1989). The effect of
podophyllotoxin on microtubule dynamics. J. Biol. Chem. 264,
8827-8834.
Sellitto, C. and Kuriyama, R. (1988). Distribution of pericentriolar
material in multipolar spindles induced by colcemid treatment in
Chinese hamster ovary cells. J. Cell Sci. 89, 57-65.
Sharp, D. C. and Parry, J. M. (1985). Diethylstilboestrol: The
binding and effects of diethylstilboestrol upon the polymerisation
and depolymerisation of purified microtubule protein in vitro.
Carcinogenesis 6, 865-87.
Sluder, G. (1979). Role of spindle microtubules in the control of cell
cycle timing. J. Cell Biol. 80, 674-691.
Steuer, E., Wordeman, L., Schroer, T. A. and Sheetz, M. P. (1990).
Localization of cytoplasmic dynein to mitotic spindles and
kinetochores. Nature 345, 266-268.
Thrower, D., Jordan, M. A. and Wilson, L. (1991). Quantitation of
cellular tubulin in microtubules and tubulin pools by a competitive
ELISA. J. Immunol. Meth. 136, 45-51.
Vandre, D. D. and Borisy, G. G. (1989). Anaphase onset and
dephosphorylation
of
mitotic
phosphoproteins
occur
concomitantly. J. Cell Sci. 94, 245-258.
Warner, F. D. (1979). Cilia and flagclla: microtubule sliding and
regulated motion in microtubules. In Microtubules (ed. K. Roberts
and J. S. Hyams), pp. 359-380. London: Academic Press.
Wheeler, W. J., Cherry, L. M., Downs, T. and Hsu, T. C. (1986).
Mitotic inhibition and aneuploidy induction by naturally occurring
and synthetic estrogens in Chinese hamster cells in vitro. Mutat.
Res. 171, 31-41.
Wilson, L., Anderson, K. and Chin, D. (1976). Nonstoichiometric
poisoning of microtubule polymerization: a model for the
mechanism of action of the vinca alkaloids, podophyllotoxin, and
colchicine. In Cell Molility (ed. R. Goldman, T. Pollard and J.
Rosenbaum), pp. 1051-1064. Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY.
Wilson, L. W. and Bryan, J. (1974). Biochemical and
pharmacological properties of microtubules. Advan. Cell Mol.
Biol. 3, 21-72.
Wilson, L. and Farrell, K. W. (1986). Kinetics and steady state
dynamics of tubulin addition and loss at opposite microtubule ends:
the mechanism of action of colchicine. Ann. N.Y. Acad. Sci. 466,
690-708.
Wilson, L., Jordan, M. A., Morse, A. and Margolis, R. L. (1982).
Interaction of vinblastine with steady-state microtubules in vitro. J.
Mol. Biol. 159, 125-149.
Wilson, L., Miller, H. P., Farrell, K. W., Snyder, K. B., Thompson,
W. C. and Purich, D. L. (1985). Taxol stabilization of microtubules
in vitro: dynamics of tubulin addition and loss at opposite ends.
Biochemistry 24, 5254-5262.
Zieve, G. W., Turnbull, D., Mullins, J. M. and Mclntosh, J. R.
(1980). Production of large numbers of mitotic mammalian cells by
use of the reversible microtubule inhibitor nocodazole. Exp. Cell
Res. 126, 397-405.
(Received 15 January 1992 - Accepted 2 April 1992)