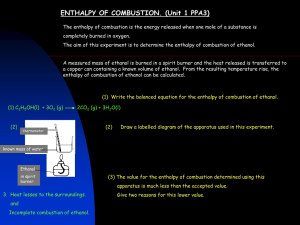
C2H5OH + 3O2 → 2CO2 + 3H2O ∆H = energy required for bond
advertisement

BRONZE MEDAL AWARD School: Participating Students: ∆Hcθ St. Paul's Convent School Koo Tien Tien Rosena Leung Yee Ling Winnie Ngan Yu Kuen Nancy A New Discovery of Car Fuel Project Title: = -1013 kJ mol-1 With this concept, we can then carry out the combustion process to determine the energy produced by different substances. Combustion equation: CXHYOZ + (x + y/4 - z/2) O2 → xCO2 + y/2 H2O Introduction In recent year, the government is very concerned about the type of car fuels to be used. This brings us the idea of investigating different substances through combustion in order to find out whether they can be used as alternative car fuels. The substances under investigation are being divided into three groups: a. b. c. = 5E(C−H) + E(C−O) + E(O−H) + 3E(O=O) + E(C−C) - 2[2E(C=O) + 3E(O−H)] common fuels: petrol, diesel oil, paraffin oil, methanol and ethanol; different percentage of petrol-ethanol mixture; food and fruit oil: lard, margarine, peanut oil, corn oil, canola oil, sesame oil, olive oil, ginger oil and citral. Experiments are being conducted to find out the amount of energy that they can produce. Energy produced per gram = mc∆T /mass of chemical used where m: mass of chemical used c: specific heat capacity of water ∆T: temperature change of water After calculating their energy produced in combustion, comparison can be made to determine the most suitable alternative car fuel. The Investigation Part I Principle Procedures of combustion: The chemical principle used in this project is based on the concept of bond enthalpy. The following is an example: 1. Weigh an empty aluminium can and pour 250 cm3 of water into it. 2. Reweigh the can with its content and measure the initial temperature of water. 3. Fill a burner with the substance investigated. Then light the wick and adjust it to give a flame about 1 cm high. 4. Weigh the burner with its content again and clamp the aluminium can above the burner. Then light the burner. 5. Shield the whole set up and stir the water at frequent intervals. After the temperature has risen for about 100 oC, record the highest temperature reached by water. Then extinguish the flame and remove the shield. Given the bond enthalpies of: C−H C=O O−H 413 kJ mol-1 740 kJ mol-1 463 kJ mol-1 346 kJ mol-1 497 kJ mol-1 360 kJ mol-1 C−C O=O C−O To calculate the enthalpy change of combustion for ethanol: C2H5OH + 3O2 → 2CO2 + 3H2O ∆H = energy required for bond breaking - energy required for bond forming St. Paul's Convent School Part II A) 1. 2. 3. B) 19.76 Part III Procedures of preparing ethanol-petrol mixtures: Weigh suitable amount of ethanol and petrol. Pour the two solutions into a beaker and stir the mixture. Fill a burner with the mixture. Follow the same combustion procedures as in Part I 15.29 Plant oil or Peanut oil animal fat Energy produced 15.59 (kJ g-1) 12.52 Corn oil 22.19 15.13 12.73 13.37 Canola oil Sesame oil Olive oil 21.93 21.53 25.32 Part III A) Procedures of changing solid fats into molten state: 1. Cut a piece of solid fat and put it into a beaker above a Bunsen flame. 2. Stir it until it is completely melt and transfer it to a burner. B)Extraction of orange oil: 1. Collect orange peels from six oranges and remove their outermost layer. Then cut them into pieces. 2. Transfer them to a round-bottomed flask which contains water. Then connect the flask to a steam generator and a water condenser to carry out steam distillation. 3. After about an hour, approximately 100 cm3 of distillate can be obtained. C) Carry out the same combustion procedures as in Part I Plant oil or animal fat Energy produced (kJ g-1) Lard 25.13 Margarine Orange oil Ginger oil 21.52 24.09 18.46 Citral 18.21 Discussion From the result in Part I and Part II, we can see that petrol and the mixture of 10% ethanol with 90% petrol give out the largest amount of energy respectively. The set-up for steam distillation of orange oil is not provided by the participants. However, we will not focus on these two parts. It is because they are non-renewable resources and will be run out pretty soon. Instead, we would like to pay more attention on the renewable source of fuels. This leads us to Part III. Results After conducting the experiment, we calculate the enthalpy change of combustion for the substances. The results are as follow: In Part III, several food oils are being investigated. From the result, we discover that most of the oil can produce energy of about 20 - 25 kJ per gram. Part I Common fuel Enthalpy change (kJ g-1) Diesel Oil Petrol Ethanol Methanol Paraffin oil 20.09 17.41 13.85 11.21 25.5 In order to be more effective in choosing the most suitable car fuel, we have converted the result to 'energy produced per dollar'. Part II Mixtures 100% petrol 90% petrol + 80% petrol + 70% petrol + 60% petrol + 10% ethanol 20% ethanol 30% ethanol 40% ethanol Enthalpy change 21.43 24.87 20.84 19.89 20.65 (kJ g-1) 50% petrol + 40% petrol + 30% petrol + 20% petrol + 10% petrol + 100% ethanol 50% ethanol 60% ethanol 70% ethanol 80% ethanol 90% ethanol From the graph, we can see that orange oil produces the largest amount of energy per dollar, followed by the margarine and the corn oil. St. Paul's Convent School Conclusion However, it is inconvenience to use margarine as a car fuel since it is a solid at room temperature. As a result, we only need to compare orange oil and corn oil. Orange oil produces more soot than corn oil when combusted. This can be explained by their structures. After taking careful consideration, orange oil is being chosen as the best car fuel. It is because orange oil is a renewable resource. It produces a large amount of energy per dollar and possesses good physical properties. It really has the potential to be developed. From their structures, we can see that orange oil has a higher degree of unsaturation. The carbon to hydrogen ratio is larger and hence the pollution problem caused by orange oil is greater than that caused by corn oil. However, we can solve this problem by installing a special device like catalytic converter into the car. Orange oil is a more volatile and non-viscous liquid than corn oil. These make it easier to be ignited. Thus the engine of the car can be started more easily. Besides, corn oil cannot be distilled at atmospheric pressure. It will choke the diesel engine of the car during combustion. Moreover, orange is a common fruit. Large amount of peels can be collected to make orange oil and this reduces the pollution problem. Besides, the government can collect peels from citizens by placing some 'orange peels collection box' on the street. With large amount of peels available, the price of orange oil will then be lowered. St. Paul's Convent School