Experiment 6: Determination of the Equilibrium Constant
advertisement

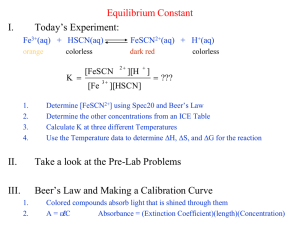
Experiment 6: Determination of the Equilibrium Constant for Iron Thiocyanate Complex The data for this lab will be taken as a class to get one data set for the entire class. I. Introduction A. The Spectrophotometer Substances are colored when they absorb a particular wavelength of light in the visible region and transmit the other wavelengths. Complex ions such as FeSCN2+ usually absorb light in the visible range; the color we see is the sum of the transmitted wavelengths. A spectrophotometer is an instrument that separates visible light into its component wavelengths and then measures the amount of light absorbed by the solution at a particular wavelength. The essential parts of a spectrophotometer are shown below: 1 Figure 1: Schematic of the important parts of the spectrophotometer The instrument has four main parts: 1. A light source that produces light with a range of visible wavelengths, 2. A diffraction grating and aperture that select one wavelength (λ = 447 nm) to pass through the sample, 3. The sample that is stored in a cuvette with a precisely known and reproducible path length, usually 1.00 cm and 4. A detector that measures the amount of light hitting its surface. The spectrophotometer that we will be using is a Spectronic 20-D. So what is it that the Spectronic 20-D is measuring? It is measuring the amount of light that is transmitted through your sample and hits the detector as a percentage of the light transmitted with a “blank” sample, %T= I ×100% Io (1) where %T is the percent transmittance, I is the intensity or amount of light that is transmitted through the sample and Io is the intensity or amount of light transmitted through the blank, a sample that doesn’t absorb at all. Since %T is not linear with concentration, what we would like to know is slightly different: how much light is being absorbed by our sample? 1 http://www.chem.vt.edu/chem-ed/spec/uv-vis/singlebeam.html accessed 8/12/05 Rev: 2013-2014 6-1 The absorbance is defined as, A = –log I = 2 – log (%T) Io (2) The absorbance, A, of an ideal solution is directly proportional to the concentration of absorbing ions or molecules in that solution. The equation that relates absorbance to concentration is known as Beer’s Law: A=εlc (3) Where: ε is called the molar extinction coefficient (aka molar absorptivity). It is an attribute that is specific to the absorbing species. It is a measurement of how strongly a chemical species absorbs light at a given wavelength (at a given wavelength, λ, ε is a constant) (We will use λ=447 nm.) l is the path length (width of the cuvette) (another constant) which contains the solution that is being analyzed and c is the concentration of the absorbing species expressed as molarity, M. (The absorbing species in this experiment is FeSCN2+. Hence, the concentration, c, of FeSCN2+ is directly proportional to the absorbance of that solution with a y-intercept equal to zero.) A = (constant) c (4) A graph of absorbance vs. concentration is known as a Beer’s Law Plot. The constant is the slope of the calibration curve made from the data in Procedure 1A. Once the constant is determined, you can measure the absorbance of any solution and then know its concentration of FeSCN2+. Let me restate this with direct reference to the procedure for this lab: once the calibration curve has been constructed using Procedure 1B, then any absorbance you measure in Procedure 2 can be used to calculate the concentration of FeSCN2+. It is the concentration of FeSCN2+ that is needed to then determine Kc for reaction (5). In this experiment, each group will use different cuvettes. It is imperative that the path length l is constant for all of these cuvettes. Therefore, each of these cuvettes is made with a width that is precisely 1.00 cm. Each cuvette is marked with a white line near the lip. This white line must be aligned precisely with the black groove in the sample holder to ensure that the path length is 1.00 cm. Operating instructions for the Spectronic 20-D are available at the end of this experiment. B. Determining the Equilibrium Constant The main purpose of this lab is to determine the equilibrium constant for the complex ion formed by the reaction of Fe3+ with SCN–, Fe3+(aq) + SCN–(aq) ↔ FeSCN2+(aq) (5) In this experiment, we will be calculating Kc for reaction (5) five different times. Each of those five times, we will get a different number for Kc. While Kc for this reaction is constant, our attempts to experimentally measure and calculate Kc will not be constant. There is experimental error, both procedural and human-made, in this experiment. Therefore, we will take the average of the five experimentally determined values of Kc. As noted in lecture and the text, when Kc is >> 1, a reaction is product-favored, goes essentially to completion and the concentrations of products at equilibrium are much larger than the concentration of reactants (essentially, there are no reactants left). When Kc is <<1, a reaction is reactant-favored, does not go at all and the concentrations of reactants at equilibrium are much larger than the concentration of products (essentially, no products form). Rev: 2013-2014 6-2 As a hint to the answer for this lab, the value of Kc for reaction (5) is somewhere close to 1 (0.001 < Kc < 1,000), meaning that the reaction gets stuck somewhere in the middle. What we will see is that we can manipulate the concentrations of reactants to make this reaction either go approximately 50% of the way to completion or as far as 99.5% to completion. This is the basis for this experiment. In Procedure 1B, the concentration of Fe3+ initially, after accounting for dilution, will be the equilibrium or final concentration of FeSCN2+ because the above reaction will go essentially to completion. We will use LeChatelier’s Principle (the subject of experiment 5-we’re just using it here) to drive the reaction to the right. We will add a known concentration of Fe3+. We will then add more than 100 times higher concentration of SCN– than Fe3+ to the reaction mixture. Using LeChatelier’s Principle, the excess SCN– will push the position of equilibrium far to the right and essentially to completion. The Fe3+ is the limiting reactant and all of the Fe3+ will be reacted to produce FeSCN2+. FeSCN2+ absorbs light at λ = 447 nm. We will construct a “calibration” or “standard” curve (a.k.a. Beer’s law Plot) that plots the known [FeSCN2+] on the x-axis and the absorbance A of FeSCN2+ on the y-axis to establish a linear relationship between these two variables. The power of the calibration curve is that for any solution containing FeSCN2+ as the only absorbing species, we can measure the absorbance and use the linear relationship to calculate the [FeSCN2+]. This is exactly why we are preparing the calibration curve. For Procedure 2, we add approximately equal concentrations of Fe3+ and SCN–. Under these conditions, the reaction will not go to completion. In fact, we will not know how far the reaction went to completion because we don’t know the equilibrium constant Kc. However, if we measure the absorbance of the unknown solutions in Procedure 3, then we can use the calibration curve prepared from Procedure 1 to calculate what the equilibrium [FeSCN2+] is in that solution. Once we have the equilibrium [FeSCN2+], we can fill in the rest of an ICE table to determine Kc. To summarize, in this experiment we will: 1. Create a calibration curve by measuring the absorbance of a series of solutions of known [FeSCN2+]. For these solutions, reaction (5) will be essentially complete. The calibration should yield a straight-line relationship between [FeSCN2+] and absorbance. 2. Measure the absorbance of a series of solutions for which [FeSCN2+] is unknown. For these solutions, reaction (1) will not be complete, but there is still the same relationship between absorbance and concentration. Use this relationship (equation of the line) and the absorbance to calculate [FeSCN2+]. 3. Determine the value of the equilibrium constant Kc for reaction (5). II. Experimental A. Equipment Needed: From stock room: 1 spectrophotometer cuvette, 10mL volumetric flask(s) Equipment in lab: Spectronic 20-D spectrophotometer, digital micropipettes, aluminum foil Chemicals in lab: 0.200 M KSCN in 0.500 M HNO3, 2.00×10–3 M Fe(NO3)3 in 0.500 M HNO3, 0.500 M HNO3 B. Disposal: Combine and collect all used chemicals from this experiment in a large beaker. At the end of the lab period, transfer waste chemicals to the provided waste container. Rev: 2013-2014 6-3 C. Experimental considerations 1. The product formed in this experiment is light sensitive. Direct sunlight will slowly decompose the product, but room light will not. Use the aluminum foil to wrap around any flasks with FeSCN2+ solution if you will be waiting for more than an hour to use the spectrophotometer (which will most likely NOT happen). 2. All of the ions in this experiment are dissolved in 0.500 M H HNO3 to prevent the side reactions that produce other complex ions. Henceforth, we will not mention this fact. 3. Please do not take any more of the standardized solutions than necessary. D. Before Starting Experimental Work (Before Class) 1. In your notebook, enter the experiment title, date, your name and name of partner. 2. Write the purposes of the lab. 3. Draw and label the major components of a spectrophotometer. 4. Write an executive summary of the procedures described in this lab. 5. Explain why the reaction goes to completion in Procedure 1 but does not go to completion in Procedure 2 even though the value of Kc is constant in both procedures. E. Procedure 1A. Preparing Standard Solutions for Absorbance Measurements. The data for this procedure will be pooled together as a class. 1. Turn on the spectrophotometer. It must warm up for 10–15 minutes. 2. Insert Table 1 in your notebook if you have not already done so. 3. Fill your smallest (10mL) beaker 2/3 full of 2.00×10–3 M Fe3+. Use the micropipettes to deliver the volumes listed in Table 1 of 2.00×10–3 M Fe3+ into each of five 10 mL volumetric flasks. (You may need to use a combination of micropipettes to obtain some volumes.) 4. Add 2.0 mL of 0.200 M SCN– to each volumetric flask, then dilute with 0.500 M HNO3 exactly to the 10.00 mL mark. Use a dropper to deliver the last few drops of HNO3. 5. Mix by alternately inverting and shaking for at least 30 seconds. F. Procedure 1B: Measuring the Absorbance of the Standard Solutions. 1. To standardize the Spectronic 20-D meter: a) Let the spectrophotometer warm up for at least 10 minutes. Set the wavelength dial (on top) to 447 nm. With the cell holder empty, adjust the left knob to zero transmittance. (When the cell holder is empty, the light path is blocked, so no light gets to the detector. We want the meter to recognize this electronic signal as 0%T.) b) Fill a cuvette with 0.500 M HNO3. Make sure you fill the cuvette more than halfway. Wipe any fingerprints from the cuvette with a Kim-Wipe (and not a paper towel–the paper towels scratch the cuvettes), and then place it in the cell holder. Ensure that the vertical white mark on the cuvette is aligned with the mark in the spectrophotometer. Close the cover for the sample holder. Adjust the lower right knob until the meter reads 100% transmittance. (This solution is considered a “blank” solution. It does not contain any of the species of interest, FeSCN2+. We want the meter to recognize this electronic signal as no light absorbed from the species of interest, or 100%T.) c) Switch from %Transmittance to Absorbance mode. To scroll between %Transmittance and absorbance, press the “Mode” button. Rev: 2013-2014 6-4 2. To gather the data for the Beer’s Law Calibration Curve: a) One at a time, starting with the most dilute solution, pour a small amount of each solution to be measured into the cuvette. Condition the cuvette by rinsing the walls of the cuvette with the solution by tilting and rotating much like it was a buret or pipette. Dispose of the liquid in a waste beaker. This is necessary if the cuvette is not dry so that the solution you are putting into the cuvette is not diluted by water or the previous solution left in the cuvette. b) Wipe off fingerprints from the cuvette with a Kim-Wipe. Place the cuvette into the sample holder, replace the cap, and read and record in Table 1 (which should be created in your notebook) the ABSORBANCE, A, (% transmittance may also be recorded but, for our purposes, A has a direct relationship to the quantity of interest, namely, concentration of iron thiocyanate). c) Measure the absorbance A for each of the standard solutions. d) Make a hand graph of your group’s data by graphing the diluted concentration of FeSCN2+ on the x-axis and the measured absorbance on the y-axis. (Note, the calculations for the diluted concentration of FeSCN2+ are described in section III A 1 on page 6.) Each student in the group must make their own graph. Check the linearity of your data by drawing a best-fit line through your data. Calculate the slope of your calibration line using two points on your line and dividing the rise over the run. e) Show your hand graph to your instructor. If any one or 2 point(s) are substantially off of the line, your instructor may require your group to re-prepare the solution and measure its absorbance. After approval of your data, discard the solutions into the container in the hood and record your data on the board to be part of the class data set. f) Record the class data set into your notebook. Perform any Q-tests requested by your instructor for suspect data points and then compute the average absorbance for each solution. G. Procedure 2: Absorbance of Unknown Solutions 1. Insert Table 2 in your notebook. 2. Each group should prepare 100 mL of 4.00×10–3M SCN- by adding 2.00mL of 0.200M KSCN (using a digital micropipette) to a 100mL volumetric flask, dilute with 0.500M HNO3 and mix well. 3. Prepare five solutions with the volumes of 2.00×10–3 M Fe3+ and the above prepared 4.00×10–3 M SCN– solution as indicated in Table 2 using the 10.0 mL volumetric flasks. Fill to 10.00 mL with 0.500 M HNO3 and mix well. 4. Determine the absorbance of each solution and record the value in Table 2 and on the board to be part of the class data set. This will be used to create an Excel graph. 5. Record the class data set into your notebook. Perform any Q-tests requested by your instructor for suspect data points and then compute the average absorbance for each solution. III. Further Instructions A. Required Calculations and Graphs Approach to calculations for the calibration curve. For the standard solutions, the number of moles of SCN– is much greater than the number of moles of Fe3+. Using LeChatelier’s Principle, the excess SCN– drives reaction (5) to completion (or >99% of the way to completion). Therefore, for the standard solutions used to prepare the calibration curve, Fe3+ is the limiting reactant and the initial concentration (accounting for dilution to 10 mL) of Fe3+ is converted entirely to FeSCN2+. Rev: 2013-2014 6-5 Calculating the concentration of a diluted solution. This calculation will be used any time you mix solutions together to calculate the concentration of ions in the new solution. Ex: If 0.100 mL of 2.00×10–3 M SCN– is diluted to a volume of 10.00 mL, what is the final concentration of SCN– ion? M1V1 = M2V2 (2.00×10–3 M) (0.100 mL) = M2 (10.00 mL) M2 = 2.00×10–5 M Units on concentration and volume can be any units, as long as they cancel out. 1. Calculate the diluted [Fe3+] in each of the standard solutions prepared during Procedure 1A using M1V1 = M2V2. Set this equal to [FeSCN2+] in the data table 1. Show an example calculation for the first solution and enter all values obtained into Table 1. 2. Do any required Q-tests first. Any values of absorbance that are considerably different than the average for a given solution should be tested using the Q test (see Statistical Functions in the introductory lab materials).Show your calculations even if they show that no data were required to be excluded from the data set. Be sure to include a comparison to the critical value of Q in the table and a conclusion to keep or discard the suspect data. 3. Determine the average of the absorbance measurements for each solution of FeSCN2+ in Procedure 1A. Only include values that were not discarded by the Q-test. Approach to calculations for Determination of the Equilibrium Constant. For the solutions in Procedure 2, the number of moles of Fe3+ is approximately equal to the number of moles of SCN–. In this case, we will observe that the reaction does not go to completion. When a reaction does not go to completion, we can count on setting up some kind of ICE table. The ICE tables and the equilibrium values of the concentrations will allow us to determine the value for Kc, the equilibrium constant. Ideally, all of our calculations will lead to similar values of Kc. 4. Create Table 3 in your notebook. a.) Calculating the concentration of each diluted solution. Calculate the initial concentration of Fe3+ and SCN-, accounting for dilution using C1V1 = C2V2, for each solution in Table 2. Show an example calculation for solution A for both the iron(III) and thiocyanate ions. Enter all calculated values into Table 3. b.) Creating the graph. Create a Beer’s Law Plot (a.k.a. calibration curve) using the class average data from Table 1. Use Excel to create this graph. The concentration should be plotted on the x-axis and the absorbance on the y-axis. Plot the best-fit line and report the equation of the line (y = mx + b) that relates conc. (x) to absorbance (y). This can be done by selecting Chart/Add Trendline from the menu. On the “Type” tab of the dialog box, select “linear”. On the “options” tab, be sure to include the equation of the line and the R2 value on the graph. Include all other items required for a good graph. c.) Determining “Final [FeSCN2+] M” for Table 3 from the calibration curve Use the equation from your Excel graph to solve for concentration of FeSCN2+ at equilibrium for each solution in Table 2 and enter the results in Table 3. (Remember that it is impossible for the conc. of FeSCN2+ to be greater then the initial conc. of either reacting species.) Show a sample calculation for the [FeSCN2+] from the equation of the best-fit line for Solution A. Rev: 2013-2014 6-6 d.) Calculating the value of the equilibrium constant from data in Table 3. Suppose that initially, after dilution,(using M1V1=M2V2) [Fe3+] = 8.0×10–4 M and [SCN–] = 8.0×10–5 M. Also suppose that you determine from your calibration curve (using conc = A/slope) that [FeSCN2+] = 3.0×10–5 M. At this point, your ICE table looks like this: Fe3+(aq) + SCN– (aq) ↔ FeSCN2+ (aq) I 8.0×10–4 C E 8.0×10–5 0 3.0×10–5 From the three columns in Table 3, there is enough information to determine the value of Kc. Because we know the initial concentration (we start with none) and the equilibrium concentration of FeSCN2+ (we do assume that the reaction is at equilibrium by the time we get it into the spectrophotometer, but this is a very good assumption born out by the fact that we don’t see the color of the solutions changing with time), we can figure out the change in concentration of FeSCN2+. Since the change must be the same for all of them (taking into account coefficients), we can fill in our table using arithmetic to make it look like this: Fe3+(aq) + SCN– (aq) ↔ FeSCN2+ (aq) I 8.0×10–4 C –3.0×10–5 E 7.7×10–4 8.0×10–5 –3.0×10–5 5.0×10–5 0 +3.0×10–5 3.0×10–5 From here, we need to use the Kc expression for reaction (5) to get the value of Kc for this reaction. Because reaction (5) forms complex ion FeSCN2+, it is also called Kf (even though it follows all the same rules as any Kc). Show the ICE table calculations for all 5 solutions. e.) Calculating the Average and Standard Deviation of Kc. Calculate the average and standard deviation for the 5 individual values calculated for Kc. B. In-Class Work (Before going home) 1. All dilution concentrations, Q-tests, and averages for absorbance values collected in Procedure 1A must be calculated. 2. Prepare a hand-drawn Beer’s Law plot (calibration curve) from your individual group’s data that takes up at least half a page in your notebook. If your notebook does not have quadrille squares, obtain a half sheet of graph paper from your instructor. (Put concentration FeSCN2+ on the x-axis and absorbance on the y-axis.) This graph is intended to identify if your group needs to re-prepare and/or re-measure the absorbance of any of the 5 solutions before sharing that data with the class. Be sure to use a ruler for your graph and that it includes all the standard requirements of a good graph. From the line of the hand-drawn graph, calculate the slope of the graph using a 2 point-slope formula: [slope = (y2-y1)/x2-x1)]. Have your graph checked by your instructor before going on to Procedure 2. Once your instructor has approved your graph, record your group’s data on the board to share with the rest of the class. If your notebook does not have graph paper in it, then obtain a piece of graph paper from the instructor, draw the graph on the paper and tape or staple the graph into your lab notebook. Your excel graph that you prepare at home (using class averages) should have a similar slope! C. Results: at home 1. Prepare a Beer’s Law plot (calibration curve) via Excel using the average absorbances for the class data set from Procedure 1. Include the equation of the best-fit line and R2 value on graph. R2 is a statistical function known as the coefficient of determination. The value of R2 for data in a straight Rev: 2013-2014 6-7 line is 1.000. As the data become more scattered, the value of R2 becomes lower. (For help with graphing in Excel, see the Chem 401 Lab Introduction section.) 2. Calculations to determine the initial concentrations of Fe3+ and SCN- for Procedure 2. 3. Calculations to determine [FeSCN2+] in the unknown solutions (A-E) from the equation of the best-fit line. (Rearrange equation to solve for concentration.) 4. ICE tables to calculate equilibrium concentrations of all species for solution A-e. 5. Calculation of Kc values for each of the 5 solutions from Procedure 2. 6. Calculate average and standard deviation values for Kc. D. Discussion Questions 1. What is the purpose of the diffraction grating in the spectrophotometer? 2. Visually estimate the path-length for the spectrophotometer that you used. 3. What is the primary difference between a cuvette and a test tube? 4. What is one advantage of using class average data over data from a single group to prepare the Beer’s Law Plot? 5. Why is it important to place the cuvette into the sample holder in the same orientation every time? E. Experimental Summary: after all calculations are complete In your notebook, after completion of all experimental work and calculations, create the heading Experimental Summary. 1. Write a summary paragraph describing what was done and what was learned in this experiment, including any issues that were encountered and dealt with. 2. List your average and standard deviation values for Kc. 3. A true value for Kc was reported in the literature (Footnote 2) as 133. Use this value to assess accuracy (% error) and the standard deviation to assess precision (%RSD) of your measurement of Kc. 4. List 5 potential sources of error when performing this experiment. 2 Cobb, C. L.: Love, G. A., J. Chem Ed. 75, 1 (1998) Rev: 2013-2014 6-8 Soln. 3+ mL Fe soln. (2.00×10–3 M) 1 0.100 2 0.200 3 0.400 4 0.600 5 0.800 Table 1: Absorbance of Standard Solutions Diluted Absorbance Values [Fe3+] M = at 447 nm [FeSCN2+] M Average Absorb. Note: All solutions contain 2.0 mL _______ M SCN- & final volumes were made up to _______ ml with ________ M HNO3. Soln. Table 2: Analysis of Solutions Used to Determine Kc mL of SCN– Absorbance Values –3 (2.00×10 M) (4.00×10–3 M) at 447 nm mL of Fe3+ A 3 1 B 3 2 C 3 3 D 3 4 E 3 5 Average Absorb. Note: All final volumes were _________ml made up with _________M HNO3. Soln. Table 3: Calculations for Determination of the Equilibrium Constant Initial [Fe3+] M Initial [SCN-] M Final [FeSCN2+] M Kc (accounting for (accounting for (determined from (solve for Kc using an dilution) dilution) calibration curve) ICE table.) A B C D E Rev: 2013-2014 6-9 Rev: 2013-2014 6-10 Operating Instructions for Spectronic 20-D The Spectronic ® 20D spectrophotometer shown below is a single-beam digital spectrophotometer with a wavelength range of 340 to 950nm. The nominal slit width of 20nm is constant over the entire range. Lever should be set to the proper filter for the wavelength setting. 9. Transmittance / Absorbance Control This control sets the display to 100%T (0.0A) when a cuvette containing a blank reference solution is inserted in the sample compartment. It must be reset whenever the analytical wavelength has been changed. When operating at a fixed wavelength for an extended period of time, check the 100%T (0.0A) readout and adjust if necessary. 10. Power Switch / Zero Control The ON-OFF main power switch is operated by the Power Switch/Zero Control knob. The Zero Control knob is used to set the display to 0%T readout when the sample compartment is empty and the sample compartment cover is closed. 1. Sample Compartment 2. Digital Readout The digital readout displays wavelength and data readings. The four LED status indicators, next to the labels TRANSMITTANCE, ABSORBANCE, CONCENTRATION, and FACTOR indicate the MODE currently active. 11. Filter Lever This control selects the filter to be used for the measurement. Red is used for measurements from 600 to 950nm. Black is used for measurements from 340 to 599nm 3. Mode Indicator 4. MODE Select Pushing this control sequentially selects the TRANSMITTANCE, ABSORBANCE, CONCENTRATION, or FACTOR mode. 5. & 6. Factor Adjust Controls The push-buttons labeled INCREASE (5) and DECREASE (6) are used in the CONCENTRATION and FACTOR modes. To set a lower CONCENTRATION or FACTOR value, press and hold down the DECREASE button until the desired value is displayed. To set a higher value, press and hold down the INCREASE button until the desired value is displayed. 7. Print Button If a printer is attached, this will print the value shown on the digital display. 8. Wavelength Control The wavelength control selects the desired analytical wavelength of the instrument. The selected wavelength appears on the left side of the LED display. The Filter Rev: 2013-2014 6-11 Rev: 2013-2014 6-12