CORR.ELATIONS BETWEEN Si_O BOND LENGTH, Si-O
advertisement

Anerican Min*olooist
Vol. 57, pp. t57S-1613 (J922')
CORR.ELATIONS BETWEEN Si_O BOND LENGTH, Si-O-Si
ANGLE AND BOND OVERLAP POPULATTONS
CALCULATED USING EXTENDED HUCKEL
MOLECULAR ORBITAL THEORY
G. V. Gmns,M. M. Hernr,l aNDS. J. LoqrsxlrHaN,
D ep'artmentof Geoto'Eiaa.lSciences,
Virginia Polytechnic Institute ond State Uniuersitg,
B lacksburg, Vir girui,a2406I
AND
L. S. Benrcr,r, ANDHsruKANcYow,
D epartnzentof Ch,emistrg,
Uniu ersity o,f Miehig an,
Arm,Arbor, Miahigo"n4810!
AssrRecr
Extended Htickel molecr:lar orbital calculations have bee=ncompleted fof isolated
and polymerized tetrahedral ions for ten bilicate mineral! in which a(O) s 0.0
using ob.servedO-Si-O and Si-O-Si valence angles, assuming all Si-O bond lengths
are 1.63A and including silicon 3s and 3p and oxygen 2s and.2p i,tdmic orbitals as the
basis set. The resultant Mulliken bond overlap populations, z(Si-O),,correlate with
observed Si-O bond lengths, the shorter bonds being associated with the larger
n(Si-O). If the five Si(3d) atomic orbitals are also included in the basis set, the
observedSi-O(br) and Si-O(nbr) bond lengths correlate with n (Si-O) as two separate
trends with overlap populations for the Si-O(nbr) bonds exceedingthose for Si-O(br)
by about 20 percent, even if the Si-O-Si angle is 180o. Calculation of z(Si-O) as q
function of the Si-O-Si angle for the Si-O bo"ds irr Sir6ro- (assuming Si-O : 1.634
and { G-Si-O : 109.47o)first excluding and then including the 3d-orbitals, predicts
in each case that the Si-O(br) bond lengths should decreasenon-linearly and that
the Si-O(nbr) bond lengths should increase slightly as the Si-G-Si angle increases.
Scatter diagrams of observed Si-O(br) bond lengths to two-coordinated oxygens
versus the Si-O-Si angle appear to va,ry non-linearly with the shorter bonds tending
to occur at wider angles.If these bond lengths a,rereplotted against - 1/cos(Si-O-Si),
the scatter diagrams show improved linear trends. Multiple linear and stepwise
regression analyses of the bond lengths as a function of -1/cos(Si-O-Si), Af(O)
and (CN) indicate that each makes a significant contribution to the variance of the
Si-O(br) bond length. The conclusion by Baur (1971) that the Si-O(br) bond length
is independent of Si-O-Si angle in silicates for which Af(O) : 0.0 can be rejected at
the 99 percent confidence level.
IwrnonucnoN
Pauling (1939) has concludedfrom the electronegativitydifference
between Si and O that the Si-O o--bond has about 50 percent ionic
l Now at the Geology Department, Rutgers-The
Brunswick, New Jersey 08903.
1578
State University, New
MO CALCULATIONSFOE SILICATE IONS
1579
character, attributing a charge of about '*2 on the silicon atom.
Becausethis charge exceeds*1, thereby violating his electroneutrality principle, he (1952) postulatedthe formation of.d-p.-bonds
betweenthe two atoms to neutralizethe charge.Using an empirical
equatio r relating interatornic distanceto the amount of
char"'-bond
acter, he then computeda Si-O distanceof 1.63Ain closeagreement
with reported values. Subsequently,Fyfe (1954) calculated semiempirical bond energiesfor the SiO+tetrahedronas a function of the
Si-O separation,and concludedthat the relatively short Si-O bond
can be explainedwithout resorting to extensivedouble bonding.On
the other hand, Cruickshank (1961) has assertedon the basis of
group-theoretic arguments,approximate molecular orbital calculations, and group overlap integralstha;t of the five 3d-orbitals, only
the two e orbitals on silicon form strong z'-bondswith the appropriate
2pr lone pair orbitals on oxygen.The Si(3d) orbital involvementin
the Si-O bond has since been substantiated by all-electron a,birui.tio
SCF molecularorbital calculationsfor the silicate ion and orthosilicic
acid (Collins, Cruickshank, and Breeze, lg72). These calculations
show (1) that there is considerableSi(3d,)-orbital participation in
the wavefunctions with the e orbitals forming
and the f2
"'-bonds
orbitals forming o-bonds with the appropriate valence orbitals on
oxygen; (2) that the calculatedl"e,sX-ray fluorescence
spectrausing
Sd-orbitals are in remarkably good agreementwith the experimental
spectra of silica glass; the agreementis notably poorer when the
3d-orbitals are not included in the calculation (seeFig. 1); and
(3) that the residualchargeon Si is not *4 as assumedin an electrostatic modelbut is closeto zeroin reasonableagreementwith Pauling's
electroneutralityprinciple and the resultsof a Si(K)-spectrochemical
shift study by Urusov (1967).
Within the last decade a large number of silicate and siloxane
structures have been carefully refined using precise difiraction data
obtained by modern techniques. These refinements have revealed a
numbet of systematic variations in bond lengths and bond angles:
(1) the Si-O(nbr) bonds are usually shorter than the Si-O(br) bonds;
(2) shorter Si-O bonds are usually involved in wider O-Si-O and
Si-O-Si angles;and (3) the tetrahedral anglesusually decreasein the
order
d [o (nbr)-Si-o (nbr)] > { [O(nbr)-Si-O@r)] > { [o (br)-Si-o(br)].
Several investigators have arg ed that these variations can be rationalized in terms of a covalent m del that includes3d-orbital involvement
between silicon and oxygen cl. Cruickshank, 1961; Lazarev, 1964;
GIBBg, HAMTL, T,OUISNATHAN,BARTELL, AND YOW
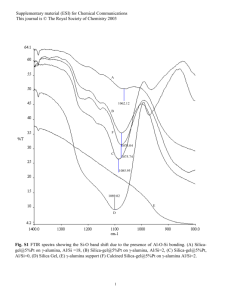
Frc. 1. Comparison of (q) the
experimental L." X-raY fluorescencespectra for silica"glass
with that calculated by Collins
et aI, (1972) from rmults obtained in all-electron ab ini'tin
SFC MO calculations on the
silicate ion (b) using an extended basis set that includes
Si(3d) orbitals and (c) using a
minimum basis set that excludes Si(3d) orbitals.
E,
o
F
(J
lrJ
F
lrJ
o
ltJ
I
F
F
o
UJ
UJ
(J
lrj
E.
a
F
z
l
o
()
l!
o
o
z
MO CALCULATIONSFOR SILICATE IONS
1581
McDonald and Cruickshank, 1967a; Cannillo, Rossi, and Ungaretti,
1968;Bokii and Struchkov,1968;Noll, 1968;Brown, Gibbs,and Ribbe,
1969;Brown and Gibbs, 1970;Louisnathan and Smith, 1971;Mitchell,
Bloss,and Gibbs, 1971).On the other hand, consistencywith a covalent
model that includes3d-orbital involvement doesnot, of course,preclude
other bonding models. For example, Mitchell (1969) notes that the
sametrends are implicit both in Gillespie's(1960)valence-shellelectronpair repulsion model and in a molecular orbital model involving a
valence basis set of atomic orbitals (without the 3d-orbitals of Si).
Despite the systematic variations observed between Si-O bond
length and the Si-O-Si and O-Si-O anglesin silicatesand siloxanes,
no attempt has yet beenmade to rationalizethesetrends in terms of
modernvalencetheory. An attempt at such a classificationshouldnot
only serve to improve our understanding and interpretation of such
observables
as the elasticconstants(Wiednerand Simmons,1972),the
bond polarizabiliiies (Revesz,1977), the diamagneticsusceptibilities
(Verhoogen,1958), the Raman and infrared spectra (Griffith, 1969)
and the X-ray emissionand fluorescence
spectra (Dodd and Glen,
1969;Urch,1969;Collinset a1.,1972)of silicatesin generalbutshould
also clarify our understandingof the long-range and short-range
forces involved in A/Si ordering (Stewart and Ribbe, 1969; Brown
and Gibbs,1970).
Recently,Baur (1970,1971)attempted to rationalizethe Si-O bond
length variations for a large number of silicatesin terms of an electrostatic model by showing that the Si-O bond lengths predicted by his
so-called extended electrostatic valence rule correlate well with those
observed for structures where LfQ) + 0'. However, he notes that
the rule fails to predict bond length variations in silicateslike quartz
and forsterite where Af(O) : 0.0. In spite of this shortcoming,Baur has
rejected Cruickshank'sdoublebonding model in favor of an electrostatic
I For Si-O bond length variations in the tetrahedral portion of a silicate, the
extended electroStatic valence nrle states that the deviation of an individual Si-O
bond from the mean tength, (Si-O), in a silicate ion is proportional to Af(O). The
actual bond length is predicted by the empirical equation
Si-op*a. : (si-o) + 0.091 ^f(o)
where af(O) : f(O) - f(O), l(O) is the sum of the Pauling (1929; 1960)bond
strengths received by oxygen andf(O) is the mean f(O) for the silicate ion under
consideration.Baur (1970, 1971) usesp6 instead of f(O) to denote the sum of the bond
strengths received by oxygen. However, we prefer to retain Pauling's symbolism for
two reasons: (1) f(O) is an accepted symbol used by many investigators and (2) p
denotes bond-order or bond-number in modern valence theory (c/. Coulson, 1939;
Pople el al., 1965; and Mulliken, 1955).
T582
GIBBS,,
HAMIL, LOUISNATHAN,,BARTELL,
AND YOW
model (l) becdusehe could r;Lotprouea dependencebetweenSi-O bond
suggeststhat irnoreof the variation in bond length may be explained
in terms of Af(O) than in terms of the angle, it cannot be concluded
a priori that fhe bond length variation is independent of the angle
without appropriatestatistical tests.
Accordingto Baur (1971),the main differencebetweenhis electrostatic model and Cruickshank'sdouble bonding model is that the
latter stressesthe importance of the intrinsic electronic structure of
the tetrahedral ions whereas his electrostatic model stressesthe extrinsic effects from the the non-tetrahedral cations. Although Baur
is aware of the empirical nature of his model and the fact that it
cerbainly cannot replace a model basedon electronic structure, he has
stated that future models for the Si-O bond (1) should incorporate
the electronic structure of the constituent atoms as well as the effects
of the non-tetrahedralcationsand (2) shouldbeginwherehis electrostatic model breaks down (for minerals like forsterile, quarbz,etc.).
The Si-O bond length variations in forsterite were recently examined
in terms of extendedHiickel molecular orbital (EIIMO) theory by
Louisnathan and Gibbs (1971) who found that the observedbond
length variations show a close correspondence
with bond overlap
populations,n(Si-O), calculated (1) for the silicate ion alone and
(2) for the silicate ion with its nearest neighbor non-tetrahedral
cationsand their ligands.
r T'he relationship between Si-O distance and
Si-O-Si ahgle for the silica
polymorphs was recently re-examined by D. Taylor (lWZ); SeeMineral.:' Mag.
38, 62H31. After correcting the $i-O distances in the polymorphs for thermal
motion of the oxygen atoms, Taylor calsulated a regression line for the corrected Si-O bond lengths and their associated Si-O-Si angles. Ile found the resulting line to be less steep than those calculated earlier by Brown et aI. (1969)
for the framework silicates but statistically identical to the one calculated in. our
study (see Table 3). Moreover, Taylor notes the probability the slope of the line
might be zero is a little less than 0.05, a result at va.riance with the findings by
Baur (1971) who believes that a dependence between Si-O distance and Si-O-Si
angles does not exist for minerals like the silica polymorphs where Af(O) - 0.0.
MO CALCULATIONSFOR SILICATE IONS
1583
??(Si-O)calculatedwith Si(spd) valencebasis
set for the silicate ion in forsterite
(1) For the silicate ion (2) With non-tetrahedral
alone
cations and their ligands
Bond Length
n(Si-O)
n (Si-O)
Si-O(1) : 1.6134
Si-O(2) : 1.654
Si-O(3) : 1.635
0.935
0.878
0.892
1.003
0.931
0.968
The n(Si-O) calculatedfor the silica;teion alone (seecol. l above)
were made using the observedO-Si-O angles but with all Si-O distances set equal to 1.634. Despite the assumptionin the carculation
that all si-o bond lengths are constant, it is clear that the resurting
n.(Si-O) can be used to order the observedSi-O bond lengths in
forsterite. Encouraged by this result, the present study was undertaken to learn whetheror not EHMO theory can serveas a model for
classifyingand ordering si-o bond length variations when the nontetrahedral cations are ignored and a((O)=0.0'. Moreover, in the
event that these calculationsprove successful,they should improve
o'ur understandingof the nature of the si-o bond in terms of modern
valence theory and provide plausible reasonsfor the variation of
individual si-o bond lengthsin silicatesand siloxanesin generar.rn
addition,new scatterdiagramsof si-o (br) bond lengthplotted against
Si-O-Si angle will be presented for data from Bl carefully refined
silicate and several siloxane structures in which A((O) is zero or
small.The relative contributionsof Si-O-Si, -l/cos(Si-O-Si), A((O),
and (CN) (the mean coordination number of oxygen bonded to
silicon) to the Si-O(br) bond length in these structureswill be assessedusing multiple linear and stepwiseregressionmethodsl (see
appendix).The outcomeof the analysiswill show that we can reject
the hypothesisthat a relationshipbetweenSi-O(br) and Si-O-Si angle
might not exist for silicateswhereA((O) = 0.0.
Trrn Cer,cur,ATroN
emn SrcNrrrcANcEor Boxl Ovnnr,epporur,lrroys
Osrarxnu rN THEExruxopn Hiicrnr, Mor,ncur,enOnsrrAr,Trrrony
SincePauling (1929) formulatedhis famousset of rules for predicting stable atom configurationsin ionic crystals,many silicate structures haVe been investigated by X-ray methods and have been found
'Linear, multiple linear,
and stepwise linear regression computations presented
in our paper were completed using the UCLA BIOMEDICAL
PROGRAM
PACKAGE,1967.
1584
GIBBS,HAMIL, LOUISNATHAN,BABTELL,AND YO'W
to conform remarkably well. Nevertheless,the steric details of the
tetrahedral ions in many of thesesame silicatesalso appearto conform with a model that includescovalentbonding (cf. Brown et al',
1969;Brown and Gibbs, 1970).In an attempt to learn whetherthese
details are indeed consistentwith covalent theory, extendedHiickel
molecular orbital calculationswere made for the tetrahedral ions in
ten silicatemineralsspecificallychosensuchthat A((O) is either zero
or small. In this way the importanceof the intrinsic electronicstructure of the tetrahedral ions can be emphasizedto the reader (Hamil,
Gibbs,Bartell and Yow, 1971).Silicatesshowinga larger variation in
a((O) will be consideredelsewhereby Louisnathanand Gibbs in a
study of the disilicates (1972d).EHMO calculations,although very
inexpensiveand crude by ab initio'standards(Richardsand Horsley,
1970),have beenmoderatelysuccessfulin generatingWalsh-Mulliken
diagrams (molecular orbital energiesplotted against bond angle)
(c/. Hoffmann, 1963; Gavin, 1969; Allen, 1970; 1972) and' in delineating trends in variations of bond length with bond overlap populations or bond.order for a number of relatively large moleculesin
their ground states (c'1.Dallinga and Ross,1968; Gavin, 1969;Boyd
and Lipscomb, 1969). Recently, Bartell, Su, and Yow (1970) calculated EHMO bond overlap populationsfor the tetrahedral bonds in
selectedsulfates and phosphatesand found that they show strong
correlationswith (1) the simple valence bond orders assignedby
Cruickshank (1961) and (2) the observedtetrahedral bond lengths'
To learn whethersi-o bond overlappopulationsfor the silicatesshow
similar trends, calculationswere undertaken using an EHMO program originally written by Hoffmann (1963).In the calculations,oneelectronmolecularorbitals, MOs, are constructedas a linear combination of atomic orbitals,LCAO,
vo : t, co,6,
where c16i&r-the linear .oum.i.ot.- und 41 the Slater type single exponent atomic valence orbitals. The energy associated with an MO,
- .Stil =
ea,is obtained by diagonalizing the secular determinant lHot
(explicitly
evaluated
0, where Sri are the two center overlap integrals
from the positional coordinates of the constituent atoms) , a,nd'Hai a're
the elements of the Hiickel Hamiltonian matrix. In the rigorous
Roothaan-Hartree,Fock method, the Ha1are complicated functions of
the linear coefficients and of two-, three-, and four-centered integrals,
and this fact requires an iterative solution of the secular equation
(Richards and Horsley, 1970). On the other hand, in EHMO theory
MO CALCULATIONSFOR SILICATE IONS
1585
lhe H15terms are not explicitly evaluated but are chosento simulate
spectroscopicallyobtained quantities, namely the negative of the
valence orbital ionization potentials (VOIP) whereasthe I/ai terms
are approximatedby the Wolfsberg-Helmholz(1952)parametrization
Htr:Bno(//',*H,,).
When one-electronMOs are constructedand their energiesobtained,
the Pauli exclusion principle is applied as an afterthought and the
MOs are filled with electrons pair-wise starbing from the lowest
eigenstate.By inserbingeigenvalues,
e;,,into the set of secularequations,
i
(t,o - els;;)c6,.: o,
the linear coefficientscpi &ra evaluated but they are not in any way
refined to self-consistency.It is important to note that trends in these
linear coefficientsdependin large part on the geometryof the tetrahedral ions modeledin our calculations.Finally, a Mulliken (1955)
population analysis is cornpletedto obtain the desiredSi-O bond overlap populations,
n(Si-o) : 2 X N(/r) t
I
co,coiS,i,
using the overlap integrals, ,ri. ,rrr"urt"to"*lr"n* and the number of
electrons,ff(k), (0, 1, or 2) in MO rI,7,.
The bond overlap populations
provide numdrical estimates of the strength of covalent bonding and
anti-bonding betweentwo atoms. Two atoms are consideredto be
bondedwhen the overlappopulationis positive,nonbondedwhen it is
zero, and antibonded when it is negativel short bonds are usually
involvedwith large overlappopulationsand longerbondswith smaller
ones.If observedSi-O bond lengths are used in the calculation of
n.(Si-O),the shorterbonds,which usually have largeroverlapintegrals,
S,;y,will tend to have larger bond overlap populat'ionsthan longer
bonds. As it is important to be able to discriminate betweenthis induced correlationand that inducedby the geometricalaspectsof the
tetrahedral ions, we have undertakentwo separatecalculations (1)
using the observedO-Si-O and Si-O-Si anglesand clampingall Si-O
distancesat 1.634 and (2) using the observeddistancesand angles.
By clamping all the Si-O at 1.&34,the correlationbetweenz(Si-O)
and the observedSi-O bond length induced by the overlap integrals
is effectively removed and what remains should be related to the
intrinsic electronic structure of the ions induced in large part by such
1586
GIBBS;HAM'IL, LOIIISNATHAN,BARTELL, AND YOW
geometricalfactors as the o-si-o and si-o-si angles.Becauseof the
very crude nature of the EHMO method intrinsic in'the very daring
approximationsoutlined aboveand becauseof the utter neglectof the
screeningpotential producedby the cotilomb and exchangeinteractions, little significancecan be attached!,o Lheactual'nu,rnbersobtained
for zr,(si-o). However,it is the trends betweenthe calculatedra(si-o)
and the exp,erimentallydetermined si-o bond lengths that ale consideredsignificant,not the absolutenumbersfor any one tetrahedral
ion. Thus, EHMO results can be useful in ordering and cl'assifging
observedbond lengthswith bond overlappopulationsbut it cannotbe
used to proue the observedbond length variations nor can it be used
Lo pt'o,uethe participation of the 3d-orbitals of si in the wave functions
calculatedfor the ions studied.
The YOIP and orbital exponentsused as input to the program are
given in Table 1. For the s- and p- orbitals the free-atorn optimized
orbital exponentsof clementi and Raimondi (1963) were chosen
together with voIP similar to the zero-chargevalues of Basch, viste,
uoa Gruy (1965). For the 3d orbitals of silicon, an orbital exponent
significantly higher than the Slater-rule value was adoptedfor reasons
was adjustedto -5'5 eV
discussedby Bartell et al,.(1970), and'H,s6,sa
to yield orbital and overlap populationscomparable,in the caseof
SiHa, to ab ini'tio populationsfound by Boer and Lipscomb (1969)'
Theseparametersgave 3d populationsin sio+* which turned out to
be of the same magnitude as Lheab initio poptiations reporbedsubsequentlyby Collins e'tal. (1972).
A fundamental weaknessof the extendedHiickel scheme(which is
not based on u b.ormfi'd,eHam\ltonian) is that its energiesand wave
functions are sensitive t0 the somewhat arbitrary parameterization
adoptedand, accordingly,have only a qualitative significance.On the
other hand, the EHMO trendsin chargedistributionsand bond popuT.cnr,n 1. Ver,pNcp Onsrrer, Ioxrzerron
PornNtrar,s (VOIP) eNo OnsttaL
ExpoNprrs (€).
$tom
Oxygen
Silicon
Atomic Orbital
VOIP (eV)
2s
-32.33
2.246
2p
-r5.79
2.227
.JS
- 14.19
1.634
-8 .15
t.428
3p
MO CALCALATIONSFOE SILICATE IONS
1587
lations in a seriesof related moleculeshave been found to be insensitiveto the exact parameterization;they are also roughly independent of whether the VOIP are frozen at plausiblevalues or &re
adjusted to self-consistencywith aiornic charges (Basch et at., L}BS).
Therefore, since the present investigation is a preliminary exploration
of. trenils in a series of structures, it seemedadequateto choosethe
simplestvariant of the extendedHiickel methodwith fixed VOIP and
exponents.Finally, it is important to note that one of the biggest
deficienciesof the extendedHtickel methodis its failure to take adequate account of the Coulornb,interactions. The method works the
best for moleculeswhere the electronegativity difference,Ax, between
bondedatoms is small. However,it starts to break down when Ax is
greaterthan approximately1.3, the breakdownbeing completewhen
L1a2 2.5 (Allen, 1970).Thus, when heteropolarsubstances
with intermediatebond type (like the Si-O bond in a silicate whereAx = 1.2)
are studied, EHMO theory may be used to qualitatively diagnosethe
covalentaspectsof the bonding.Accordingly,it is of particular interest
to apply the EHMO method to silicate structureswhich have been
found to conform remarkably well in the past with Pauling's rulesl
for ionic crystals yet which also conform in many instanceswith a
modelthat includescovalentbonding.
Tnp Rnr,erroNsurpBprwnuN Si-O (br) BoNo Ovpm,epPopur,errox
ANDrHE O-Si-O aNo Si-O-Si ANcr,ps
In a study of severalorthosilicates,Louisnathanand Gibbs (1g72a)
have found that the tetrahedralvalenceanglesin hypothetically distorted SiOetetrahedrahave a pronouncedeffecton n(Si-O), the wider
O-Si-O anglesbeing associatedwith bonds of larger overlap populations. They also showed for a number of silicates (1g72b) that a
correlation can be made betweenthe mean of three O-Si-O angles,
(O-Si-O)3, and the length of the Si-O bond commor to these three
angles,shorter bonds tending to be associatedwith larger values of
(O-Si-O)a.McDonald and Cruickshank (l967a) and more recenily
Brown and Gibbs (1970) have also shownthat shorberSi-O distances
are typically involved in wider O-Si-O angles.Although Baur (1g20)
has also indicated that shorter Si-O distancestend to be involved in
wider O-Si-O angles,his geometricalmodel of a Si atom rattling
lAccording to Bent (1968)
Pauline's mles ma5.rhave a more universal application as structural principles than heretofore realized because when suitably rationalized they may apply to covalent as well as ionic compounds, perhaps even
to metallic compounds.
1588
GIBBS,HAMIL, LOAISNATHAN,BARTELL, AND YOVT
within a rigid"tetrahedronof oxygenspheresis not realizedin general
in the silicates (Louisnathanand Gibbs, L972b).
The Si-O-Si angles in silicates (L'iebau,1961) and in siloxanes
(Noll, 1956) are usually between130o and 140o,although anglesas
wide as 18Ooare not,uncornmon.Except for a,nangle of 93" in Si2Oz
(Andersonand Ogden,1969),angleslessthan 120" are rare. A bonding
model that includes Si (3d) orbital participation predicts that the
length of the Si-O(br) bond shouldincreaseas the Si-O-Si anglebecomesnarrower (Cruickshank,1961).Moreover,as the Si-O-Si angle
narrows,increasingelectrostaticrepulsionsbetweenthe si-atoms and
decreasings-characterin the O(br)-orbitals (i.e.,a decreasingo-bond
strength) are additional factors that are predicted to lengthen SiO(br) (Cruickshank,1961;Brown et a1.,1969)fn a re-investigation of the crystal structure of zunyite, Louisnathan
and Gibbs (1972c)have found that the sizeof the tetrahedral angleshas
a pronouncedeffect on zr[Si-O(br)] in Si,Ou- ions with linear Si-O-Si
linkages.Thus, if {to(br)-Si-O(nbr)l > {[O(nbr)-Si-O(nbr)], then
nlsi-O(br)l
{[O(nbr)-Si-O(nbr)], then n'[Si-O(br)] = n'[Si-O(nbr)] whereas if
{ tO(br)-Si-O(nbr)l
n[Si-O(nbr)] (seeFig. 2). These results calculated with all Si-O bond
Iengths clamped at 1.634 imply that the magnitude of the tetrahedral
angles plays an important role in establishing n'[Si-O(br)] in the
Si-O(br)-Si linkage. In order to assessthe nature of the relationship
between the Si-Oftr) bond length and Si-O-Si angle, we calculated
n[Si-O(br)] as a function of Si-O-Si angle for the three Si,O"z6-ions
shown in Figure 2. The results calculated with Si-O : 1.63A and a
Si(sp) valence basis set are presentedin Figure 3a. Regardlessof the
size of the tetrahedral angles,n'[Si-O(br)] increasesnon-linearly wil}r
increasingSi-O-Si angle,the largestoverlap population being associated
with the 1800 angle. Analyses of the linear coefficient, c6;, and the
overlap, 8o;, matrices indicate that the changein z[Si-O(br)] is related
to concomitant changesin both the o- and zr-bonding potentials. In
other words, widening of the Si-O-Si angle increasesboth the o- and
r-bond. strengths as proposed by Cruickshank (1961), Brown et q,I.
(1969), and Brown and Gibbs (1970). Using results provided by the
theory of atomic orbital hybridization, Louisnathan and Gibbs (1972c)
have proposedthat the Si-O(br) bond length should vary as a function
of -l/cos (Si-O-Si). When the n'[Si-O(br)]are replotted as a function
of -l/cos (Si-O-S;), a well-defined linear relation (Fig. a) is realized.
The trends predictedby EHMO theory are similar whetheror not
the Si (3d) orbitals are includedin the calculation (Figs. 3a and 3b),
1589
MO CALCALATIONS FOR SILICATE IONS
(o)
(b)
(c)
Frc. 2. A comparison of Si-O bond overlap populations (the decimal fractions
listed adjacent to the Si-O(br) and Si-O(nbr) bonds) calculated for three Si:Oz6-ions
(Da,, point symmetry with Ca along the Si-O-Si linkage) where (o) {[O(br)-Si-O
(nbr)l > dlo(nbr)-Si-O(nbr)1, (b) *[O(br)-Si-O(nbr)] : d[O(nbr)-Si-O(nbr)]
and (c) {[O(br)-Si-O(nbr)]
< {lO(nbr)-Si-O(nbr)1. The calculation was made
using a Si(sp) valence basis and with all Si-O : 1.63,i. Note that z[Si-O(br)] >
n[Si-O(nbr)] for the conformation in (o), nlsi-O(br)l - z[Si-O(nbr)] for (b) and that
n[Si-O(br)] < z[Si-O(nbr)] in (c). (See Louisnathan and Gibbs, 1972c).
suggesting that
the observed steric details of a tetrahedral
ion in a
silicatemay not be usedto establishthe involvementof the 3d-orbitals
in the formation of the Si-O bond.This is in agreementwith Mitchell's
(1969) assertionthat "one cannot,. . . expectexperimentsto establish
whether d-orbitals are (really' used anymore than experimentscan
show s and p orbitals definitely occur in bonding of the first row
elements"(seealsoBartell et aI., l97A).
Si-O Bono LrNcrn Venrerrolv rN Low-QuARrz,
I"ow-cnrsrosAr,rrn,ANDCopsrrp
The silica polymorphsare possiblythe most appropriatestructures
for examiningthe dependenceof Si-O (br) bond length on both the
O-Si-O and Si-O-Si angles,because(1) there are no bonds other
than Si-O(br), (2) all oxygensare two-coordinatedand bridge two
tetrahedra,and (3) A((O) : 0. For our analysis,we have chosendata
from three preciselyrefined structures,all with e.s.d.'sless or equal
to 0.0064: low-quartz (Zachariasenand Plettinger,1965),low-cristobalite (Dollase,1965),and coesite(Araki andZolLai,1969)(GroupA
GIBBS, HAMIL, LOU ISNATHAN, BARTELL; AND YOW
(b)
(o)
o7a
o50
o
o48
I
oo?4
o
./
@
of?
o46
.t'
t40
ts
si-o-si(.)
si-o-si(.1
Fro. 3. Si-O(br) bond overlap populations calculated as a function of the
Si-O-Si angle for the three pyrosilicate ions depicted in Figure 2. (a) Si(sp)
basis with atl Si-O = 1.63A and (b) Si(spd) basis with all Si-O - 1.63A.
Si-O(nbr) bond overlap populations for each ion increase only slightly with
Si-O-Si and accordingly are not shown.
in Table 2). The data for keatite and low-tridymite werenot included
in the analysis for reasonsgiven in the next section.The three structures chosenprovide twelve individual Si-O(br) bond lengthsvarying
from 1.598to 1.631A.Figure 5c is a scatterdiagram of Si-O(br) bond
length versus (O-S,i-O)3 angle; tr'igure 5b shows Si-O(br) versus
[-|/cos(Si-O-Si)]. Using the estimatedstandarderror of the slopes
for the lines fitted to the distributionsin Figures5c and 5b (seeTable
3), the null hypothesiscan be testedthat the true slopein each case
is zero; that is, that the Si-O(br) bond length is independentof
(O-Si-O)3 or -1/cos(Si-O-Si). Student's ltl calculates tn be 3.3
and 2.3 for the data in Figures5c and 5b respectively(Table 3). Since
these values exceedthe 9O percent confidencelevel (c/. Draper and
Smith, 1966)of t(10,0.90) - 1.4,we can rejectthe hypothesisLhaLa
relationship between Si-O(br) and -1/cos(Si-O-Si) or (O-Si-O)3
might not exist.
We have usedthe function [-l/cos (Si-O-Si)] rather than the angle
itself becausez[Si-O(br)] vary non-linearly with the angle but
MO CALCULATIONSFON ilLICATE
IONS
1501
(o)
o.50
F
I o.cs
o
I
'=
ir O.48
c
o.47
t30
t40
r50
r70
160
t80
si-o-si (.)
(b)
o.50
I
-
o
0.49
I
'=
;J
c
O.48
o.47
t.6
t.5
t.4
t.3
t.2
t.l
l.o
-llcos(Si-O-Si)
Frc.4. Plots of ztSi-O(br)l as a function of (a) Si-G-Si angle and (b)
-1/cos(Si-O-Si)
where the bond overlap population was calculated for the
conformation in Figure 2b and a Si(sp) basis set.
7592
GIBBS, EAMIL, LOUISNATHAN, BARTELL, AND YOW
linearly with [-1/cos(Si-O-Si)] (Fig. a). However, even if the
relationshipbetweenSi-O (br) bond length and Si-O-Si angle is ascan again be
sumedto be linear, the hypothesisof no interdependence
rejected at the 90 percent confidencelevel (seeTable 3). When the
obSi-O(br) lengthsare estimatedusing the interceptand coefficients
tained from a multiple linear regressionanalysis,using both (O-Si-O)3
and [-1/cbs(Si-O-Si)] as independentvariables,a reasonablecorrespondencebeiween the experimental and estimated distancesis
observed(Fig. 5c and Table 2). Finally, two commentson Figures
5o and 5b are worth making: (1) the quantity [-1/cos(Si-O-Si)]
may be a better definedvariable ihan (O-Si-O[ becausethe former
includesa more accurateaccountof the hybridization characteristics
on O(br) than the latter doesof the hybridization characteristicsof
Si, and (2) the calculatedslope [-0.05(2)] for Figure 5b is statistically identical with that [-0.0E4(1)] obtained for n"[Si-O(br)]
deversus [-l/cos(Si-O-Si)] (Fig. ab), an interestingcoincidence
2
Table
Sr-o(bt)
rengths,
bond
ad
Group
Ar
s14(br)-sr
to.
A!(O)
3111c6
bond
Si-o(b!)
PolForPhe
s1-0(b!)
sr(1)4(r)
sr(2){(2)
s1(r)-0(3)
s1(2){(3)
sr(r){(A)
sr(2)-o(4)
sI(r)4(5)
51(2)-0(5)
sr-o(l)
sr-o(l)'
Cloup
r.600
r.6oa
I:607
r.619
r.598
r.625
1.63r
1,511
1.603
1.616
Dr
s14(4)
L.627
l:3i3
sr4(r)
r.6052
t
t.r95l
to!
slllc'
<cN>
a<(o)
20
0.0
1.600
l. @8
$00
145.E
r @00
L 2392
I 611
145-0
r,2204
2.O
20
20
l.6115
150.4
1.1501
2.0
0.0
I 5240
1!6. r
1.3874
20
00
I 6095
r41.5
I 246C
2.O
0 0
dl.tdc.
.tlosE6
s1-o(l)
s1-o(t)l
;;;Gi
vh.r.
v!.
d.t.
.41.
Sl{(bt)-Sl
ar(o)-0.0
tor
au
<d>'
Etldtne'
00
OO
00
bond
S1{(br)
ad
0 and
146,8
1 6045
and
data
sngte
<cN>'z
0
Sl{(br)-St
a((o)io
s1-GSt
1.601
r.608
sl-o(r)
sr-o(2)
w.
truober'
.ooldldtlon
sUleates
PolrrPhs,
dt.tsnce
qhere
tr€an
orygen
angles,
for
.Ulc.t..
Dorrade (1965)
Arakl and zoltar (1969)
Zacbrkden t?lettlnger
(1965)
oqg€u
a crulck.Mnk(1967b)
l-527
rto
l.l5l2
2.8
0.0
&|'oald
r.6re
r32.e
r.4690
2.1
00
rr.ch.r(1969)
1.6052
UO.0
LO
2.9
0 0
Prdtt
3
(P.r.oul
l:?31
r.5e4s r6s.6
sr-0r(2)
s14I(l)
i:S
r 5e4r
r5e.!
I 01t7
2.7
00
l.2t4t
2.0
r,0194
H3S1-O-SIB3
1,6s.
I 634
1a{.1
0.0
rl4Dlg@
(q3)6(sro)3
1.63t
1.63t
13r.6
r.s62
2.0
00
ob.r@t(1e72)
(cB3)E(sro)4
L.622
L.522
144,0
1.223E
2-o
o.o
(cH3)ro(sio)5
L 620
I 620
t46.2
r.2034
2.o
o.o
(cff3)r2(sio)6
r,622
t.622
149.6
l.1594
zo
o'o
sr4(1)
1.626
1.626
1E0.0
l0
2.9
0.0
sr{(r)
I 6t2
r 632
180.0
l0
29
0.0
lbzs12o7
Er2Sr207
cd.)
Gtbb., E!cc& .od
(196!)
e.sh.!
s1{r(2)
sr-or(r)
srolh
4.r!.,
r shepel.v
(1963)
(1970)
MO CALCULATIONS FOR SILICATE IONS
Tab]e
Group
Cr
s1-o(br)
bond
dlrtance
aher€ o(br)
8"3s14&5o25
Lw rbrte
LN Cordlerrce
sr-O(r)
1,599
t6O,O
16r.2
t:8li
i'.211 r.6185 B'.7
data
tor
l.mOO
1.0564
t 7
elltcare!
2.s
-.lO
sbmoo
-'04
Ese re'4"
r.603
1.620
:l[i]*[:]
i.3li
r.5o5s
1.614
1.614
r36.E
t.37l8
X.7
r3r.e
L.4st4
1.4
.02
.o2
Lts 5
r @oo
oz
2.7
ctbb. (t965)
hsher
(1e67)
-.25
phrulp. !.!.f!.,
-:33
**
!!'q!"
i:331
i:133
i:3i3
r.63r
r'6125
rs'8
r55'e
r.5304
r'@$
2.s ::ff "*
2.e -'.31
3i[i]:3[i]
i.z:rt
r'534
r4o.r
1.303s
(reIr)
2.1 -'os hutid
:1[li:3[1i
i:33i
s1(r)-o(r)
s1(2)_o(6)
slG){(E)
r.617r
r5r.5
r.r37e
z.E ::3i 'o**
1.66
r.629
1.633
r.06
r.oz9
1.613
143.E
143.0
8t,O
L,2392
L,z52L
r.3250
.O
.07
,Ol
6d !r''v (re64)
'rd !'4 (re7o)
krltullr.
s1(r){(7)
1,621
1.521
141.1
r.2850
3.2
-.13
c'l61Eat6rt.
s1(r){(7)
I.613
1.513
ta2.2
L.2622
3.0
_.03
:l[i]][;i
i:!ii
t.5z6s r3e.7
r.3rr2
s1(I){(7)
1.613
1.613
r44.!
L.223E
3.0
-.@
i:lli
r.5re
142.4
1.2622
-:31
rr{.r
:|[]li[;i
r.ptb
!!.!1.,
cibbr
(1969)
sr(I){(7)
1.616
r.616
r39.!
r.3l9o
3.2
;.8
Gbucopbo.
Sr(1){(7)
1,6t1
l.6rt
L17.2
t.IA97
3.2
-.tl
s1(r){(7)
I 616
I 616
lat.O
r.2m
j.O
-.03
:l[i18]
i..232
r.628
r&.0
r.ss
t,52A
t39.I
1.32S
3.0
-.03
t.622
r3e.8
r.sez
t.@3
1.603
l4l.O
t.a$
i.fll
r.6345
136.r
r.3373
1.3651
h-
?rtdtlve
b-
c@ln8toa1t.
Sl(r){(7)
1.626
:l$lil[;]
i:31
51(r)s(7)
:i[]1i8]
protryhlbot.
hthophylllte
s1(r){(7)
1.524
:l$11[ii
i.!ll
t.624
137.1
r.62r
140.6 r.2$r
r.6$
l4l.a
L.27%
r.6,6
140.6
r.2e6
1.6t7
r.617
t3r.g
1,3a99
i:|?i
r.6275
r&.3
i.orar
si(I){(7)
1,fl5
3i$ifl[:]
i:fli
sr(t){t(7)
:i[ili[3]
H&bU
!!.q!,,
(r9tr)
-:33
1r@llr.
c@ltrst@lt'
(f97r)
(re67)
3il[;]:3:3i
:i?[:i:333]
(lnort€
c-c6te!.d
(re6e)
1.644
:1[l]1[1]
ctun.rltc
(1970)
6!d et.
L.3et2 2.s ::3X
st(3)-o(6)
sr(4)_o(6)
si{(l)
EPidrdFlt€
aqle
ruo coordl@ted
:ii[:]-8S[:]
KlsuaroPlrte
*"lll#,,."
r.610
(cont.)
s1{(br)-S1
1.
3ll[3i:3:[:]
tudd& cordlerltG
Danburlrc
r.599
2
v.
1593
(1969)
(1969)
-:3;
-:83
-.03
-:B:
-.Ot
3.1
-:Bl
3,2
-.07
-.31 r l q . r
(t970)
-.Il
3.5
-'03 brb'c (re6)
,",.-"""
:::..
,::,,
;fi :.,.:,,.::,-.;.:::
il;i;fi
spite the fact that all n(Si-O) were calculated assumingall Si-O
equal 1.634 and all O-Si-O angles: 109.47"in Figure 4.
Tnu Connpr,erroNBnrwnnu Si-O (br) BoNo LnNcmr eNo Si-O-Si
Axcr,p rN Srnucrunns WHpnn a((O) rs ZERo
In structures of low-quartz, low-cristobalite, coesite,hemimorphite,
thortveitite, beryl, emerald, benitoite, YbsSizO,T,Er2SisO7,and five
siloxanes,all the oxygen atoms receive the same bond strengttr, i.e.
GIBB!, HAMIL, LOAISNATHAN, BARTELL, AND YOW
1594
(o)
(c)
r64
r64
!
a
a
=
a
-
'ez
162
o
ooo
a'
I
a
1I
U'
6
t.
r60
to
t60
o
a
t60
r50
at
(o-si-o)t
si-o-si(")
(b)
(d)
r64
r64
a.
3 t62
a
!
,ez
a
o
o
a
o
I
lo a
oo
t60
(,)
t60
a
| 60
t4
r5
t2
to
| 62
1.64
si- o(.st.l(l)
-llcos(Si-O-Sil
bond length data from Table 2, group
Frc. 5. Scatter diagrams of si-o6r)
A, plotted as a function of (a) Si-O-Si, (b)-1lcos(Si-O-Si)' (c) (O-Si-O)s:
the average of the three o-si-o angles involving a common si-o bond and
(d) si-o(est.)A using the results of a multiple linear regression analysis with
-1rlcos(Si-O-Si) and (O-Si-O)" as independent variables'
((o) = 2.0 and a((o) = 0.0.The si-o(br) bond lengthsand si-o-si
anglesin thesestructuresare listed in Table 2 (GroupsA and B)' The
scatter diagram of Si-O(br) versus Si-O-Si angle and [-l/cos(SiO-Si) ] are given in Figures 6o and 6b, respectively.For lhe 27
individual si-o(br) bond lengthsin this data set, the hypothesisthat
the true slopes of (l) Si-O(br) versus Si-O-Si arrgle and of (2)
Si-O(br) versus [-l/cos(Si-O-Si)] are zero was t'ested.The calcuand Itl- values
lated intercepts,slopes,partial correlationcoefficients,
2-5 and 3.6 for
ate
values
are given in Table 3. The calculatedltlthe critical
exceed
these
Figures6o and 6b, respectively.Because
lfls
that
null
hypothesis
the
1.3, we can reject
value of ,(25,0.90)
not
be
correlated
might
Si-O (br) bond length and Si-O-Si angle
(actually 2.5 alsoexceedst (25,0.90)) . This resultis contraryto Baur's
(1971)conclusionthat a correlationcannotbe madebetweenSi-O (br)
and Si-O-Si anglefor silicateswherea((O) : 0. He reachedhis conclusion using data from keatite, high-tridymite, low-quartz, low-
MO CALCULATIONS FOR SILICATE IONS
1595
The correlations presentedin our Figures 6a and 6b are somewhat
improved (seeTable 3) when the data from ybrSizOT,Er2Si2O7,and
Table 3
Ln[ercepts, a^r slopes, brr and correlation
coefficieDts,
r, for.]-inear
regresslon eqdatlons fittad
ro dara in TabLe 2 and Flgs, 5-7. ltls calc.
for Ho:Bl = O; all ltl's
exceed a two-slded 902 1evel test
Group A of Table
2
bt
S1-0(br) bond length vs.
S1-O-S1 angle for slllca
polynorph data (Fig, 58)
L,677
Sl-O(br) bond length v6.
-1lcos(Si-0-S1) for sllIca
pollnorph data (Flg. 5b)
1.539
0 . 0 6( 2 )
Si-O bond Lengtha vB,
<O-Si-o>a for s1l1ca
polynorpH dats (Ftg. 5c)
4,094
- 0 . 0 2 3( 6 )
S1-0 bond length ve.
SL-o(est) for sLllca
pollnorph data (F+g. 5d)
-0.168
- 0 . o o o 4( 2 ) I
rL. tq)
I. O6J
S1-0(br) bond length v€.
-Ucos(sl-O-Si)
; Flg. 6b
{' 1
1 .. 5
54
15
6
Itl
-0 .48
1.8
0.57
2.3
-n
1 . 1( 3 )
Groups A and 82 of Table
S1-0(br) bond length vs.
Sl-o-St angle; Flg. 6a
t
sanple size
77
0.76
3.7
-o,72
-0,4s
4.8
2.s
24t
27'
0.7s
0.58
5.3
3,6
24't
27'
-0,51
-0.44
5.1
4.3
78r
81',
0.53
o.49
5.5
5.0
78r
81'
2
-0.0009(2)
-o,oo04(2)
0 . 0 8( 1 )
0 . 0 6( 2 )
Groups A, B, and C2 of Table 2
Si-O(br) bond length vs,
S1-0-Sl angle; Fig, 7a
1' 11.,578040I8
Si-O(br) bond length ve.
-1lcos(Si-o-Sl);
Fle. 7b
1' 11..556514
re,s.d,rs
-0.0005(1)
-0 ,0004
( 1)
0 . 0 s 3( 9 )
0 , 0 4 (79 )
are glven 1o parentheses and refer
2The data fron thortveitite,
Errsiroil
glven in the flrst
atatlstlcs
iow'of
to the last
digit.
and ybaslroT rrere not uaed to obtaln the
thc dati sEtb enclosed by braces (see text).
1596
GIBBSTHAMII" LOU-I&NATHAN' BARTELL, AND YOW
(o)
t.66
'a
t64
a\o
.cl
r.62
a
o
t'
--.1
I
a
t-a-
taa\-
r.60
o
o
--
\.-= . O
_ _l
a
r.58
r30
t40
r50
r80
170
160
si-o-si(")
(b)
r.66
\-o
--
r.54
L
-o
o
-
o
1.62
.o
--o- \
a
\.
ao
-O\ !r'
I
6
oa o-'-r
'
t.60
.
--
a
-O
- - -t \f.,.
a
t.58
1.6
1.5
t.4
1,3
t.2
-llcos(Si-O-Si)
to
l.l
:
,
Frc. 6. Scatter diagrams of Si-O(br) bond'length data from Table 2; groups
A and B, plotted as a function of (a) Si-O-Si angle and (b) -1lcos(Si-O-Si)'
The dashed line in (b) is a least aquaxesline. The dashed line in (a) is transgenerated from (b). The open circle plots are data for thortveitite, Ybosiroz
and ErgSLOn.
.
MO CALCALATIONSFON SILIAATE I.ONy
I8S7
thorbveititeare excluded(open'symbolsin Figs.,6aa,nd.'6b).In-these
Rm,errvp ConrnrsurroNs ol. -1/cos(Si-O-Si), a((O) exn (Cff)
To rHE Venr,lnrr,rryoF TrrESi-O (br) BomuLnxcru
As' a((O) and the. mean coordination number, (CN), of oxygen
bonded to silicon have also been suggestedas determining factors of
the Si-O(br) bond length (Baur, lg7l), an examination oieach in the
The results are listed in Table 4 in the order ranked by the stepwise
Table
Analysls
of varlance:
Multiple
the data used to prepare Fig.
the dependenr variabte;
t(74,
iodependent
variable
was found
fndependeot
-1lcos
(Si-O:Sl)
AE (o)
<cN>
llhe
Varlable
data
stat.lsties
ltl
4
linear
regression
analysls
for
7 wlth Si_O1try
bond length
as
=
O.9O)
1,7,
The order of the
by scepvise
reglessioD
oethocls,l
Partial
r
Addeal Regres6ion
Su of Squares
Sdple
Slze
t' 1
6 '. o4
0'63
0;59
0,0043
0.0038
81',
t4'2
o.44
0.41
0,0020
q..0020
78r
81,
U.J)
0.0005
0.0006
78,
81'
F,2
from thortveitite,
ErrSi,Oa,
glven ln the flrst
iow'of
0,34
and yb^Si"o,
the datf
s6tC
were not
e.rclosed
uaed to obtaln
by braces_ (see
the
text).
1598
GIBBS,IIAMIL, LOAISNATHAN,BARTELL, AND YO'W
regressionmethod (c/. Draper and Smith, 1966).The ltl- valuesindicate that all three variables contribute significantly to the varia-
a random sample in the sensethat the data are omitted for structures
where A((O) is relatively large and where O(br) is coordinatedby
more than two atoms. Therefore the regressioncoefficientcalculated
for our data probablywill not apply to an expandeddata set.Howevet,
sinceA((o) and actual coordinationnumber of o(br) are moderately
correlated,we cannot,concludea priori whether the magnitude of the
slope associatedwith (cN) will be larger or smaller than 0.006 or
whether (cN) witl make a significant contribution to the regression
sum of squaresin the presenceof a((O).
Plots of Si-O (br) bond length versus Si-O-Si angle for a wide
variety of silicates (Cannillo et a1.,1968; Brown and Gibbs, 1970)
exhibit the sametrend as Figures7,aor 7b (seeTable 3) irrespective
of the coordination number of the bridging oxygen' Accordingly, it
appearsthat the length of the si-o (br) bond is related in part to the
$i-O-Si angle regardlessof the value of a((O) or (CN), with the
shorter bonds being involved in the wider angles.Although this result
is consistentwith a covalentmodel that includessi (3d) orbital participation, one cannot concludeas indicated earlier that theseorbitals
are definitely used in the compositionof the si-o bond (Bartell et al.,
1970).
Si-O
BntwnnN n(Si-O) ANDTHEOssunvED
Tnn Rnr,erroNSHIP
BoNp LnNcrn
As both O-Si-O and Si-O-Si angles appear to exert a pronounced
role in establishingrz(si-o), we calculatedthe bond overlap populations for isolated and polymerized tetrahedral ions in ten silicates
(Table 5) using the observedO-Si-O and Si-O-Si anglesand clamping
all si-o at 1.63a.The calculationfor a valencebasis set consisting
of silicon 3s and 3p and oxygen2s and 2p atomic orbitals yields bond
overlap populations which, when plotted against the observedsi-o
distances(Fig.8), show an apparentlinear relation with longerbond
lengthsbeing assoeiatedwith smaller overlap populations.As expected,
(o)
t.66
a
o
t.64
o
o
3 r.ez
o
I
an
o foo
ao
a
t.60
.
.
a
a
a
o
I
r.58
r30
r70
160
r50
r80
si-o-si(")
r.66
(b)
t.64
1.62
ll
o
I
6
r.60
t
a
r58
t6
r5
t4
t3
1.2
tl
t.o
-llcos(Si-O-Si)
Fra. 7. Scatter diagrams of Si-O(br) boad length data from Table 2, groups
A, B and C, plotted as a function of (a) Si-O-.Si angle and (b) -1lcos(Si-O-Si)The dashed line in (b) is a least squares line. The dashed line in (a) is transgenerated from (b). The open circles are data for thortveitite, Yb"StOr and
Er"SLO". The reported e.s.d.'s of ihe Si-O bond lengths for the structures given
in Table 2 are all less or equal to 0O1A.
1600
GIBBS, HAMIL, LOUISNATHAN, BARTELL, AND YOW
Table 5
an
b!'
r1-0(l)
sr{(r),
si4(2)
sa-0(3)
st-0(t)
si-o(2)
sr-o(4t
mortv.lrtr.
tosilltn.
hrrld
l.ryl
1.66
l.t4t
1.517
1,500
Le21
sl-o(l)
st-0(2)
sr-0(t)
t.60t
sl-0(4)
sr_0(t)
st_0(6)
ar_0(7)
1.623
l.a2E
Sl-O(r)
st-o(l)l
8r-0(2)
l.6s
l.t8t
sr-o(I)
sr-0(I)l
st-0(2)
l.!94
t.59t
3r-0(r)
sr-o(3)
sr{(l}
s!-o(r)l
8r{(2}
sr(r){(r)
st(r)-o(2)
sr(r)-0(3)
sr(r)-0(a)
sr(2)-0(4)
sr(2)-0(5)
s1(2){(6)
s1(2){(7)
sr(3)-0(r)
51(l)-0(6)
st(3)-o(9)
st(t)-0(3)
C.st0l
!.!:!,(lrl
sr (r)-0(l)
sI(r)-0(2)
sr(!)-o(3)
s1(l)-0(9)
sr(2)-0(r)
s!(2)-o(.)
sr(2)-0(5)
sr(2)-0(a)
s!(3)-o(5)
sr(r)-0(7)
s!(3)-0(6)
sro)-o(9)
sr(2)-0(2)
sr(2)-0(3)
sr(2)'0(8)
sr(3)-0(6)
sr(3)-0(3)
lLeLit
o.(8!
o.as
0.t06
0,511
|,626
1,63r
b..t.
.!d
t ="*
0,4t5 "=","t
O,rLl
0.478
0,155
0 942
0.515
0,946
0.530
0.5@
0.505
0.495
b..!t
t*
0.734
0,739
0,955
0,97E
.4!s
h!ll, c!!b. .nd ilbba
(r97r)
o,922
0,93!
o.?56
(1967)
C!!rct.h.d
t,627
l a28
0.494
0.501
0.!03
0.506
0.t0I
0.502
0.?61
0.9?t
0.926
0.115
0,925
o.926
1.616
r,st
o.{91
o,a6E
0,510
0,50E
o.(i2
0.405
0.515
0.t2t
o ?5E
0,7t1
0,941
0,944
0,161
o,752
0.960
0,96!
1,621
0.505
0,50E
0.50!
0.5rr
0.t30
0.503
0,tt8
0 742
0,9!9
0.7E2
0,8r5
0,912
0.t22
0.51t
0,t0{
0,rr7
0,182
0 9t9
0, t95
0 800
1.620
0.505
0.50t
0.t0!
r.603
1.6t6
0,56
o;til
0,t16
0.t06
0 1A2
0,162
0.78t
0,7aE
rld
acbrt...r
(1965)
Pl.rcrnr.r
r.6x
1.646
0.4s
0.4t6
o.!or
0.499
o.a?l
0.521
o,?75
0.7tt
0,9{o
0,162
o tx'
0 9n{
lt.ch.r
0.50!
0.509
0,464
0 4!6
0,115
0.52r
0.506
0,411
0.48!
0.512
0,501
o.att
0:531
0 514
0.4t0
0,165
0.41!
0.545
0.523
0.454
0.462
0.t23
0.t16
0 4(5
0,941
0.940
o,ttt
0.761
O 1Lt
0,959
0.934
0.741
0.t45
0.9{t
0.919
0.751
0.98t
1.60t
1.507
l.a2l
I 6t6
L,612
t.6t9
1.596
I 606
1.460
1.678
1.517
r.595
1.698
l.6ot
1.603
0.521
0 52!
o.462
0.488
1.589
t.609
1.503
1.626
1.701
L65t
1,669
t.600
I t5l
1 561
1,504
L6lr
t.649
t.6tl
L,641
Ttpp. .Ed fldllton
(19?1)
h8h.!
(1968)
b.ib.r
(1968)
lr.d
(!969)
.!d
rx.o!
(rt69)
o 142
0,7t0
0. trt
0.999
0 960
0 7l!
o,t2t
0.960
0.98t
0 69!
(1969)
rrol.r
0.549
o 522
0 469
0.461
o 525
o 555
0.45!
0,96!
o 964
0 703
0.17L
0.693
1 00r
0 959
0. t18
0,724
0 97r
LOl6
0 tor
0.521
0.499
0.451
0.515
o.a6E
b 950
0.918
0.t02
0.951
0 723
Donily rtrd Allqn
(1968)
Fo.rt.!tr.
sr-O(t)
sr-o(2)
sr-0(3)
1,614
1 654
r.615
0 515
0,400
0,469
0.525
0,4t0
0 487
0 935
0 878
0.898
0.952
0.862
o a90
8106.d
Cllb. (1972);
but.n.rh.n
.nd c1bb.
(r972.)
D.tolrr.
sr-o(l)
st-0(2)
s1-0(!)
sr-o(4)
r t70
r 648
r,551
1.661
0.506
0.{93
0,492
0,487
0.545
0 484
0 4!2
0,{69
0,918
0.900
0.899
0.890
o 98t
o €65
0sl
0.861
Fort, Phlutp. .nd Orb6.
(rn Dr.pr..!ron)
the overlap populationsobtained using observedtetrahedral angles
and bond distancesin the calculationsshow a similar but better developedtrend when plotted againstthe observedbond length (Fig. 9).
Theslopes,intercepts,corelation,coefficients,and It |- statisticscalculated:for the data in"Figures8 and g are given in Table 6 and Show
that both trendsare highly significant.
The curvesof Bartel"let at. (1970)which relate bond length to bond
overlap population are similar to those obtained.,inFigures 8 and 9
t70
W I T H O U Td O R B I T A L S ;
o
Si-O = 1.53A
r.69
tr:
A
D
V
O
o
c
,O
Morgorosonile
Beniloile
Bcryl
Emerold
Quorlz
Tourmoline
0ioplose
Thorfveilile
+ Forslerile
x Dololile
r68
t67
r66
r65
164
E r,63
I
o
-lU'
v
| 62
t6l
r60
>o
159
T
v
r58
r57
r56
r55
o47
048
049
o50
051
o 52
0.55
n( s i - o )
Fro. 8. Scatter diagram of Si-O bond length data plotied against; z(Si---O),
calculated using observed valence angles, assuming'Si-O = !63 A; aud excludilg
Si(3d) orbitals for the minerals Iisted in the upper right. Si-O(br) bonds aie iq
dicated by open symbols and Si-O(nbr) bonds by bolid ones.'Data takbn from
Table 5.
GIBBS, HAMIL, LOAISNATHAN, BARTELL, AND YOW
l?o
wrTHOUt d OREIIALSi
S i - O ( o b e)
t69
s
t6t
r57
o
O Nigh 9r.nur.
o
CaSiOr
t66
t65
-
d\,
-" ..\
164
o
o
o
r6s
"o
o
o
t62
o\.'
l5l
OeO
t60
DD
\
-
'
r59
r56
r5c
n(Si-Ol
Frc. 9. Scatter diagram of Si-O bond length data plotted against n(Si-O)'
ealculated using the observed distances and angles and excluding Si(3d) orbita.ls
for the minerals listed in the upper right of this figure and Figure 8. Si-O(br)
bonds are indicated by open symbots and Si-O(nbr) bonds by solid ones. Data
taken.from Table 5.
suggestingthat the overlap populations calculated for the sulfates'
phosphates,and the silicatesare comparablewhen the 3d basisfunctions are neglected.Correlations can also be made betweenthe Si-O
bond lenglh, the net non-bonded geminal populations (c/. Barbell
et o,1.,1970), and the net chargedifierenceson Si and O; however,
inasmuch as the non-tetrahedral cations were not included in the
calculations,such correlations are not appropriate here.
When the valenceorbital basis set of silicon is extendedto include
the five Si(3d) orbitals, the EHMO calculation gives n,(Si-O) that
again correlate with observedSi-O bond lengths but as two separate
trendswith the overlappopulationsfor the Si-O(nbr) bondsexceeding
those for the Si-O(br) bonds by about 20 percent (Figs. 10 and 11,
Table 5). The overlap populations calculated using the observed
1603
MO CALCULATIONS FOR SILICATE IONS
tetrahedralanglesand all Si-O = 1.63Aare plotted againstSi-O(obs)
in Figure 10 whereasthose calculatedusing observeddistancesand
anglesare plotted againstSi-O(obs) in Figure 11.Thetrends in both
scatter diagramsare highly significantas evincedby the ltl -values
(Table 6). The correlationsare much better developedin Figure 11
wherez(Si-O) were calculatedusing the observedSi-O distancesand
angles.In addition,the slopescalculatedfor the Si-O (br) bond trends
(Figs. 10 and 11) are steeperthan thoseof the Si-O(nbr) bonds.The
Si-O(br) bond trends for the data given in Figures8 and 9 alsoappear
to be steeper,indicating that the Si-O(br) and Si-O(nbr) bond overlap populationscharacteristicallyconstitutetwo distinct populations
evenwhen a Si (qp) valencebasisset is used (Table 4). The difference
calculatedin n.(Si-O) for the Si-O(br) and Si-O(nbr) bondsis due in
Table
Intercepts,
regresslon
a^, slopes, bil
e{uatlons
fitted
6
and correlation
coefficients,
ro dara ln Flgs. 8-11; ltl
linear
= o
o:Bl
for
r,
for
tt
l.l
bl
o
observed S1-0 bond length vs.
n(Si-O) calc. f,or Si-0 = 1,63fr;
F1g. 8
2.763
-z.zdL
-0.84
9 ,I
Observed S1-0 bond length vs.
glsl-0(br)1
calc. for Si-O =
1.63; Flg. 8
3,025
-t
-0,89
8.3
-L,tt6
-0.77
5.3
Observed Sl-O bond length vs.
nlsi-o(nbr)l
calc, for S1-0 =
1,63 ; I'tg. 8
9., t
Observed S1-0 bond length vs.
n(Si-o)
calc. for obs. S1-0;
Fig. 10
2.L39
-7.024
-0,94
20.0
Observed S1-O bond length vs.
ntSt-O(br)l
ca1c, for Si-O =
1,63; Fig.8
3,220
- 2 . O 76
-0,77
6.0
-0.816
*0.70
5.5
-0. 906
-0.94
13.4
-0.548
-0,94
L0.2
observed S1-0 bond length
vs.
n[Sl-O(nbr)]
calc. for S1-0 =
1,63; F1g.8
observed Si-o bond length
vs.
nIS1-0(br)]
calc. for obs.
si-O; F1g, Il
observed Sl-0 bond length vs,
nIS1-O(nbr)] eale, for obs.
st-0;
Fig. 11
2.3L9
1604
GIBBS, HAMIL, LOAISNATHAN, BARTELL, AND YOW
t72
WITH d OREITALS;
S i- 0 . | 6 l l
r70
j! r64
o
o
I
t62
o
v,
o
t60
r58
r56
154
o74
076
078
080
082
084
0S
O8a
O90
092
094
096
098
n(si-o)
Frc. 10. Scatter diagram of Si-O bond length data plotted against ??(Si-O),
calculated using observed valence angles, assuming Si-O - 1.68,4.and including
Si(3d) orbitals for minerals listed in upper right of Figure 8. Data taken from
Table 5.
part to the very different environmentsimposedon these bonds by
our modeling of the tetrahedral ion in each silicate as an isolated
molecule.Accordingto Bartell et aL. (1970),part of the differencemay
also be steric and part may be due to electrostaticforcesnot reflected
in the bond overlap populations.
The a(Si-O) valuesusedto prcpareFigures8 and 10 werecalculated
with the Si-O distancesclampedat 1.68Abut with observedO-Si-O
and Si-O-Si angles.Clamping all Si-O distancesat l.68A may eliminate the bias inherent in using the observedpositional parameters,
but it can introducebias becausethe bond lengthsare not allowedto
respondto differencesin the bond overlap populationsestimatedby
Hiickel theory. Moreover, for many complexpolymerizedSiOa ions,
the Si-O bond lengths cannot,always be clampedat 1.68A and still
retain the observedanglesand the continuity of the polymerizedunit
at the sametime. In thesecases,it may be possibleto obtain a reasonableestimateof the ra(Si-O) by using the observeddistancesand
anglesin the calculations.This is becausewell-developedcorrelations
exist betweenn (Si-O) ealculatedfor Si-O = 1.68Aand those calcu-
fl"t"i
MO CAI,CULATIONS FON yLICATE
IONS
1605
ht)
NF
qE
sE
@
o=
OP
o
r
.E;
-x
XQ
c
o
D
o
8
Oaa
al,
J
+.
F-
E8
EO
9l
o
B
o
tO
ts
oQ
'
6f
ar
.b90 tAr
EE
YA
>,r4
HS
.od
d
.Eq
a
6
xd
!E
;i6
o
q
o
3co
*Q.
o/
a:
b.-
?€
D
o/o
oJ
0
0 o
0
$
o
I- t^ t s
o
f h v
P
ct
'Ho
:S
-
uor,
dai
Fd
0
9a
vF
! . i
o€
h
o
.P
iEg_EEq_EiEi
(sqo)
o - !s
o
H
d
1606
GIBBS,HAMIL, LOAISNATHAN,BAR,TELL,AND YO'W
Iated usingthe observedpositionalparameters(Fig. 12) (seeCameron,
1971).
Concr,usroNs
(1970)
Allen
has implied that EHMO results may be useful in
determininga relationshipbetweenbond overlappopulationand bond
length relations like that obtained for the hydrocarbons(c/. Schug,
1972) but this is Lhemost he believesthat it can do. Our calculations
appearto have been successfulin demonstratingsuch a relationship
and suggestthat at least part of the Si-O bond length variations observedin the silicatescan be rationalizedin terms of a covalentbonding model. Our results also imply that the steric details of a silicate
cannot be used f,o proue the participation of the Si (3d) orbitals in a
Si-O bond formation becausesimilar structural trends are predicted
a
when they are omitted and a valencebasisset is used.Nevertheless,
covalent bonding model including d-orbital participation permits an
understandingof the X-ray emission(Dodd and Glen, 1969;O'Nions
spectra (Urch,
and Smith, 1971;Collins et aI., L972) and fluorescence
1969,1971;Collinset aL,,1972)and the bondpolarizabilities(Revesz,
1971). In addition, it gives a reasonablygood accountof the trends
in Si-O bond length variations. Consequentlya covalent bonding
model that includes Si (3d) orbital involvement should not be dismissedas chemicallyinsignificant.SinceEHMO theory fails to take
explicit accountof electrostaticeffects,the seniorauthor and his students are presentlyundertakingMO calculationsat the next level of
sophisticationwhich involve the inclusionof the two electronintegrals
and refi.nementof the linear coefficientsto self consistencyas embodied in the CNDOI2 approximationof Pople, Santry, and Segal
(1965). These calculations do take ordinary Coulornb interactions
into account.It will be of interest,therefore,to comparethe CNDO/2
results with those obtained in the present study as well as with those
obtained by the ab iruitio method.
Finally, the ultimate goal of any bonding theory for the silicates
is to provide some insight into the physical laws that govern atom
arrangements,physical properties and stabilities. Molecular orbital
theory in its simplest form has been used with moderatesuccessby
spectra,bond
the organicchemistto rationalizereactionmechanisms,
length variations,and shapesfor a large number of organicmolecules
(Streitwieser,1961). The trends obtained in our study suggestthat
extendedHiickel molecularorbital theory, unlike the extendedelectrostatic valence rule, may be used to classify and order the bond
length variations in the tetrahedralportion of a silicate when A((O)
o54
o
o52
o48
o46
o80
lf)
(D
I
o.48
050
052
b
o78
a
o
!
o
O1
o76
o
J
o
a'
o74
U)
c
o72
o96
070
c
o,94
laa
a
.).-'
a
o92
o 88
0,90
0.92
0 94
096
098
rOO
n ( S i - O 6 6 )e
Frc. 12. Bond overlap populations, rr(Si-O), calculated with Si-O = 163
A us. ra(Si-O) calculated with observed distances, both with observed
valence angles.
(a) For Si-O(br) and Si-O(nbr) bonds without Si(3d) orbitals; correlation coefficientr - 0.94 (Data from Figs.8 and 10).
(b) For Si-O(br) bonds with Si(3d) orbitals; r := 0.90 (Data from
Fies.9 and 11).
(c) For Si-O(nbr) bonds with Si(3d) orbitals; r '= 0.90 (Data from
Figs.9 and 11).
1608
GIBBS,HAMIL, LOAISNATH.A.N,
BARTELL, AND yOW
- 0. Moreover, similar calculationsby Urch (1971) have provided
valuable insight into the L2,s X-ray fluorescencespectra recordedfor
silica glass. As the rewards are great, earth scientists are urged to
considerthe use of MO theory in their interpretation of spectra,
physicalproperties,order-disordermechanisms,
and phasetransformations of minerals.Even if applicationof the theory provesonly moderately successful,it should, for example, improve our ability to
evaluate evidencebearing on the physical conditionsthat prevailed
when a mineral or a mineral assemblage
forrned.
AcxNowtgocupNts
We are grateful to Drs. G. E. Brown, Jr., R. G, Burns, J. R. Tossell, and
D. R. Waldbaum for reviewing the manuscript and making a number of helpful
suggestions.Professors F. D. Bloss, D. W. J; Cruickshank, P. H. Ribbe, and J. C.
Schug, and Dr. M. TV'.Phillips are thanked for very helpful discussions and for
critically reading the manuscript. G.V.G. gratefully acknowledges the National
Science Foundation for its generous support in the form of grants GU-3192,
GA-L?D?, and GA-30864X, and Virginia Polytechnic Institute and State University for providing funds to defray the relatively large computer costs incurred in the study. M.M.E. and SJ.L. gratefully acknowledge the NSF departmental $art (GU-8192) support in the form of research associateships.
Appor.rrrx
Stepwise regression analysis is a systematic and objective procedure for
recognizing those variables that contribute most significantly to the variation
in the response,regardless of their actual entry points into the model. As a first
step, a correlation matrix is computed and the va.riable most highly correlated
with the response variable is used to compute a linear regression. The second
variable to be entered into the regression is the one whose pa.rtial correlation
with the response is the largest, i.e., the one that makes the greatest reduction
in the error sum of squares. Next, the method examines the contribution the
first variable would have made if the second one had been entered first and
the first variable second. If the partial F' of the first variable is statistically
significant at some preselected confidence level, the fust variable is retained;
otherwise it is rejected. Assuming that both the first and second variables make
a significant contribution, the method selects the next variable to be entered, i.e.,
the one that now has the largest partial correlation with the responsegiven that
the first and second variables a,re in the regression. A regression equation is
calculated with all three variables and the third variable is tpsted for significance.
Moreover, partial F' tests are again made for the first and'second variables to
leanr whether they should be retained in the regression model. If their partial F's
exceed the preselected level, they are retained; otherwise they are rejected. The
procedure is continued and the next most highly correlated variable is entered
into the regression. Significance tests a.re made as before and the method continued with each variable incorporated into the model in a previous step being
tested. The method is terminated when all the variables have been considered
and no more are rejected (cl. Draper and Smith (1966), p. 178-195for numerical
example).
MO CALCULATIONSFOR SILICATE IONS
1609
Rnnnnpwcns
Ar,r,nw, L. C. (1970) Why three-dimensional Hiickel theory works and where it
breaks down, 1z O. Sinanoglu and K. B. Wiberg, eds.,SigmntMolecular Orbital
Theory. Yale Univ. Press, New Ifaven, Connecticut, ZZ7-245.
(1972) The shape of molecules. Theoret. Chim. Acta 24, ll7-l}l.
Al,unwurwourv, A, O. BesrreNsn, V. EwrNc, K. Ilnonnnc, eNn M. Tneorrnsnnc
(1963) The molecular structure of disiloxane, (SiIL),O. Acta Chem. Scand.
r7,2455-2t60.
Awnnnsow, J. S., aNo J. S. Oconw (1g6g) Matrix isolation studies of Group-IV
oxides. I. Infrared spectra a:rd structures of SiO, SirOr, and SLO".,1. Chem.
Phgs. 5r,4189-4196.
Anerr, T., ewn T. Zor,rar (1g69) Refinement of a coesite structure. Z. Kristal,Iogr.
129,381-387.
Benrnr,l, L. S., L. S. Su, enn H. Yow (1g70) Leneths of phosphorus-oxygen and
sulfur-oxygen bonds. An extended Hiickel molecular orbital examination of
Cruickshank's d,rpn piclure. Inorg. Chem.g, 1908-1912.
Bescu, 8., A. Vrsrn, exo H. B. Gney (1965) Valence orbital ionization potentials
f rom atomic ryectra dala. T heor et. Chi,m.Ac t a 3, 459-464.
Beun, Wnnxun (1970) Bond length variation and distorted coordination polyhedra
in inorganic c-rystals.Trans. Amer. Crgstallngr.,4.ss. 6, 12b-15b.
(1971) The prediction of bond length variations in silicon-orygen bonds.
Arner. M'ineral. s6, lSZz-1599.
Bnwt, H. A. (196S) Taqgent-sphere models of molecules: VI. Ion-packing models
of covalent compounds.J. Chem. Ed.45,Z6g-728.
Bonn, F. P., ervn W. N. Lrpscorus (196g) Molecular SCF calculations for SiII. and
H"S. J. Chem. Plrys. So,98g-992.
Borrr, N. G., arn Y. T. Srnucnrov (1g68) Structural chernistry of organic
compounds of the nontransition elements of Group IV (Si, Ge, Sn, Pb).
Zh. Struht.: Kluinu9,63M72 [transl. J. Struct. Chem. 9, 618-6Z2].
Boro, D. R., exo W'. N. Lrpscorrs (1g6g) Electronic structures for energy-rich
phosphates.J. Theoret. BioI. 2s, 40Z4ZO.
Bnoww, B. E., aNn S. W. Barr,ny (1964) The structure of maximum microcline.
Acta Crystall.ogr. t7, l}gl-1400.
Bnowr, G. E., eNn G. V. Grsss (196g) Oxygen coordination and the Si-O bond.
Amer. Mi,neral. s4, l128-l1zg.
-,
(1970) Stereochemistry and ordering in the tetrahedral porAND tion of silicates.Amer. Mineral.55, 15gZ-1607.
aNn P. H. Rrnnu (1969) The nature and variation in length of the
Si-O and Al-O bonds in framework silicates. Amer. Minerar. 54, 1044-1001.
BusrNo, W. R., aNn II. A. Lnw (1964) The effect of thermal motion on the estimation of bond lengths diffraction measurements.Acta Crystallogr. 17, 142146.
CeruunoN, M. (1971) The Crystal Clrcmi,strg ol Tremolite anil Richteri,te: A
Studg of Selected, Anion and, Cati,on &tbstituti.on. Ph.D. thesis, Virginia
Polytechnic Institute and State University, Blacksburg, Virginia.
CaNwrr,r,o,E., G. Rossr, eNn L. Urvcennru (1968) The crystal structure of macdonaldite. Accad,.N,az.Lincei 4s,gW4L4.
CmuoNrr, E., aro D. L. R"rruornr (1968) Atomic screening constants from
SCF functions. .I. Ch em. Phgs. 38,2686-2689.
1610
GIBBS, HAMIL,
LOAISNATHAN,
BARTELL,
AND YOW
Cooe, A., A. Der, Nncno, ewn G. Rossr (1967) The crystal structure of krauskopfite. .dccod.Naa. Dei, Lincei 42,859-873.
Corr,rr.rs,G. A. D., D. W. J. Cnurcrsnewr, eNn A. Bnnuzn (1972) Ab i,nitio
calculations on the silicate ion, orthosilicic acid and their Zr, a X-ray spectra.
J . C hern. Soc., F aradny Trans. I I, 68, I 189-1195.
CmtoN, A. F., awo G. Wrr,rrNsor.r (L969) Ad.uam,ed,Ircrgoni'c Chemistra. John
Wiley and Sons,New York, N. Y.
Corrr,sor.r,C. A. (1939) The electronic stmcture of some polyenes and aromatic
molecules. VII. Bonds of fractional order by the molecular orbital method.
Proc. Rogal Soc.,Ser.A,169,413-428.
CnurcrsnaNr, D. W. J. (1961) The role of 3d-orbitals in r-bonds between (a)
silicon, phosphorus, sulfur, or chlorine and (b) oxygen or nitrogen. J. Chem.
Soc.7077,5486-5504.
Der,r,rxca, G., eNo P. Ross (1968) Semi-empirical prediction of bond lengths and
conformations . R ec. Trau. Chi,rn. 87,906-915.
Donn, C. G., ewo G. L. Gr,nll (1969) A zurvey of chemical bonding in silicate
minerals by X-ray emission spectroscopy. Amer. Mi.neral. 54, 1299-l3ll.
Dor,rAsn, W. A. (1965) Reinvestigation of the structure of low cristobalite. Z.
Kristallo gr. 721, 369-377.
(1968) Refinement and comparison of the structures of zoisite and
clinozoisite. Amer. M,i,nerar.$, 1882-1898.
(1969) Crystal structure and cation ordering of piedmontite. Amer. Mineral. 54,71tu_-717.
(1971) Refinement of the crystal structures of epidote, allanite and hancockite. Amer. M ineral. 56, 447464.
DoNNev, G., rwo R. Ar,r,rvrew(1968) SLOrc groups in the crystal structure of
ardennite. Acta Crystallngr. B24, 845-855.
Dnarnn, N. R., exr I[. Surrn (19ffi) AWIieiI Regression Analysis. John Wiley
and Sons,fnc., New York.
Frrcnn, L. W. (1969) The crystal strusture of grunerite. Mineral. Soc. Amer.
Spec.Pap.2, 95-100.
(1970) Refinement of the crystal structure of anthophyllile. Carnegie Inst.
W aslui,ng
ton Y ear B ooh, 68, 283-288.
Frscnnn, K. (1969) Yerfeinerung der Kristallstruktur von Benitoit BaTitSilOJ.
Z. Kristall,o gr. 729,369-377.
Fnnno, R. L., eNr D. R. Poecon (1969) Determination and refinement of the
crystal structure of margarosanite, PbCaoSi*;On.
Z. Kristallngr. 728,213-228.
Fyru, W. S. (1954) The problem of bond !,ype. Amer. M'ineral.39, S1-1004.
Gevrw, R. M., Jn. (1969) Simplified molecular orbital approach to inorganic
stereochemistry.J. Chetn. Ed. 46,413422.
Gruus, G. Y. (1966) The polymorphism of cordierite. I, The crystal structure of
low cordierite. Amer. Mi,nerar. 51, 106&1087.
(1969) Crystal structure of protoamphlbole. Mi'neral. Soc. Amer. Spec,
Pap. z, l0l-109.
D. W. Bnocx, eNo E. P. Mnecnnn (1968) Structural refinement of hydrous
and anhydrous synthetic beryl, AL(Be"Si")Oreand emerald, Al'.nCr".'(Be"Si')Or.
Lithos, 1,275-285.
Grr.r,nsrrc, R. J. (1960) Bond angles and the spatial correlation of electrons.
J. Amer. Ch.em.Soc.82,5978-5983.
Gnrrrrtn, W. P. (1969) Raman studies on rock-forming minerals. Part 1. Orthosilicates and cyclosilicates.J . Chem.Soc. (A), 1372-L377
.
MO CALCULATIONS FOR SILICATE
IONS
1611
Eeuu,, M. M., G. Y. Grsss, ewo P. H. R"rsse (1971) The location of the hydrogen atoms in the water molecule of dioptase. [abstr.]. Geol. Soc. Amer.
Abstr. Programs,3, 315.
-,
G. V. Grsns, L. S. Benrnrr,, eNn I[. Yow (1971) A comparison of Si-O
bond lengths wiih EIIMO bond overlap populations. [abstr.]' Geol. Soc.
Amer" Abstr. Programs, 3, 589.
Horrrvreww, R. (1963) An extended Eiickel theory. I. Ifydrocarbons. J. Ch'em.
Phgs.39, l3W-L412.
Laucnorv, R. B. (1971) The crystal stmcture of kinoite. Amer. MhwraL 56,
193-200.
Lezennv, A. N. (1964) Polymorphism of molecules and complex ions in oxygen
compounds of silicon and phosphoms. Report 1. Nature of the Si-O-Si bonds
and values of the valence angles of oxygen. Izu. Akail. iVauk ,$SSE, Ser.
Khi/m.2.235-241.
Lmueu, F. (1961) Untersuchungen iiber die Griisse des Si-O-Si Yalenz-winkels.
Acta Crgstallogr.14, 1103-1109.
LoursxerneN, S. J., awo G. V. Grsns (1971) A comparison of Si-O distances in
the olivines with the bond overlap populations predicted by the extended
Hiickel molecular orbital (EHMO) theory. [abstr.]. GeoI.$oc. Amer. Abstr.
Prog:rams,3,686.
(1972a) The efrect of tetrahedral angles on Si-O bond overANDlap populations for isolated tetrahedra. Amer. M,i,neral s7,16l+-1642.
(1972b) Variation of Si-O distances in olivines, soda melilite
AND-and sodium metasilicate as predicted by semiempirical molecular orbital
calculations. Amer. M ineral. 57, 1643-1663.
(1972c) Aluminum-Silicon distribution in znnyite. Arner.
AND Mineral. s7, 1068-1087.
(1972d) The Si-O bond length variation in pyrosilicates.
AND labstr.l GeoI. Soc.Amer. Abstr. Prograrns,4,580-581.
aNu J. V. Surrn (1971) Crystal structure of tilleyite: Refinement and
coordination. Z. Kri*tallo gr. 132,288-306.
McDoNer,o, W. S., exn D. W. J. Cnurcrsrrexr (1967a) A reinvestigation of the
structure of sodium metasilicate, Na"SiO". Acta CrystalX.ogir,22, 3743.
_
(1967b) Refinement of the structure of hemimorphite. z.
AND _
Kris tallo gr. 124, 180-191.
Meagher, E. P. (1967) Polym,orphism ol Cord:ieri,te. Ph.D. thesis, Penn. State
University, University Park, Pennsylvania.
Mrtcnnll, J. T., F. D. Br,oss, ervn G. V. Grsss (1971) Examination of the
actinolite structure and four olher C2/m a.mphiboles in terms of double
bonding. Z. Kri*tall,ogr. 133,?73-300.
Mrrcnnrr,, K. A. R. (1969) The use of outer d orbitals in bonding. Chem. Rea.69,
157-r78.
Mur,r,rrow, R. S. (1955) Electronic population analysis on LCAO-MO molecular
wave function s. l. J. C hem. Phgs. 23, 1833-1846.
Nor,r,, W. (1956) Characteristika der Siloxan-bindung in polymeren Anionen
(Silikaten) und polymeren molekiilen (Siliconen). Fortschr. Mirrc,ral. 34,
63-&1.
(1968) Chemistry and, Technologa of Si.Itcunes,2nd ed.. Academic Press,
Ine., New York, N.Y.
OmnueMrtnn, I{. (1972) The molecular structures of cyclosiloxanes. latrstr.]
Fowth Austi,n Sym.posium on Gs,s Phase Molewlar Structure, Ll-12.
t6l2
GIBBS, HAMIL,
LOAISNATHAN,
BA&TELL,
AND yOW
O'IrTroxs, R. K., exo D. G. W. Snrrrn (1971) Bonding in SiO, and Feno" by
oxygen KaX-ray emissionspectra.Natwe,231, 130-133.
Partxo, J. J., ewl J. R. Cr,enr (1968) The crystal structure and cation distributi,on of glaucophane.Amer. Mineral.s3, 1156-1173.
-:,
M. Rnss, ewn J. R. Cunr (1969) Crystal chemical characterization of
clinoamphiboles based on five new structure refinements. Mineral Soc.Amer.
Spec.Pap. 2,177-186.
Petrr,rrvc,LrNus (1929) The principles determining the structure of complex ionic
crystals. J. Amer. Chem. Soc. 51, 101G-1026.
(1939) The Natwe of the Chemical Bond, lst ed,. Cornell Univ. Press,
Ithaca, N. Y.
(1952) Interatomic distances and bond character in the oxygen acids and
related substances.J. Plrys. Chern. 56,361-365.
(1960) Th.e Nattne ol tlte Ch.emical Bond", 9rd ed. Cornell Univ. Press,
Ithaca, N. Y.
Punr,res, M. W., P. H. Rrssp, ewn G. V. Grsss (1971) Refinement of the crystal
structure of danburite. [abstr.] Geol. Soc. Amer. Abstr. Programs 3, 338.
Ponr,n,J. A., D. P. S,rxrnr, aNl G. A. Sncer, (1965) Approximate self-consistent
'molecular
orbital theory. I. Invariant procedures.J. Chem. Phgs,43, L29135.
Rnvnsz, A. G. (1971) r-bonding and delocalization effects in SiO, polymorphs.
Phys. Reu. Lett. 27, 1573-1581.
Rrnnn, P. H., H. D. Mecaw, alro W. H. Tavr,on (1969) The albite structures.
A ct a Cr y stal.Iogr. zS, 1503-1518.
Rrcnenns, W. G., ervo J. A. Honsr,nv (1970) Ab initio Molecular Orbi,tal CaIcuIations for Chemists. Clarendon Press,Oxford.
RorrNsow, P. D., awn J. E. FeNc (1970) The crystal structure of epididymite.
'
Amer. Mineral.55, 1541-1b49.
Scnuc, J. C. (1972) Introd,uctory Quanturn Chemistrg. Eolt, Rinehart and
Winston, Inc., New York, N. Y.
SrraNrvox, J., awn L. Kerz (1970) The structure of barium silicon niobium oxide,
Ba"SLNbuOgo:A compound with linear silicon-oxygen-silicon groups. z4.cto
Cry stall,ogr. 826, 105-109.
Snewrvor, R. D., ervn C. T. Pnowrrr (1969) Effective ionic radii in oxides and
fluorides. Acta Crystallogr. B25, 925-946.
SuolrN, Y. I., euo Y. F. Srrnpnr,nv(1970) The crystal structure of the rare earth
pyrosilicates. Acta Crgstalln gr. 826, 484492.
Stnwenr, D. B., ewo P. H. Rrssn (1969) Structural explanation for variations in
cell parameters of a,lkali feldspar with AllSi ordering. Amer. J. Sci., Schai,rer
VoI.267-A, 444-462.
Srnnrrwrosnn, A. (1901) Molecular Orbital Theorg lor Organic Chernists. John
Wiley and Sons,Inc., New York, N.Y.
Ttren, A., eNn W. C. Harvrrr,roN (1971) A neutron diffraction study of the ferric
tourmaline, buergerite. Amer. M,i,nerar.56, 101-113.
TRolnn, F. J; (1969) The crystal structural of a high-pressure polymorph of CaSiO".
Z. Rr1"stallogr.130, 185-206.
Uncn, D. S. (1969) Direct evidence for S(1,-2p
r-bonding in oxy-anions. J. Chem.
Soc, A, 3026-3028.
(1971) X-ray emission spectroscopy.Quart. Reu. 25,343-364.
Unusov, V. S. (1967) Chemical bonding in silica and silicates. Geolth'i,miya, 4,
399-412[Eng. trans. Geochem.Int. 4,350-362(1967)].
MO CALCALATLONSFOR SILICATE IONS
1613
YnnuoocnN, J. (1953) Physical properties and bond type in Mg-Al oxides and
silicateir.Amer. M'ineral. $, 5EZ-879.
Wrnnren, D. J., elln G. SrrmoNs (lg72) Elastic properties of alpha quartz and
the alkali halides based on an interatomic force model. J. Geophus. Res.77,
826-856.
Wor,rsnunc, M., eNn L. Hnr,lrnor,z (19b2) The spectra and electronic structure
of the tetrahedral ions MnOr-, CrOn- and CIO;. J. Ch,em.Ph,ys. 20,837-843.
Zecnanresow, W. H., aNn H. A. Pr,orrrxcEn (1965) Extinction in quartz, Acta
Crystallo gr. tB, 7l0-7 14.
Manuscrtpt recei,ued,Janunry 11, 197P; accepted,tor publi,cati,on,Jw,e 6, 1n2.
Note add,eil i,n proof ba GVG: The electrostatic valence rule proposed by
Pauling (1929) and extended by Baur (1970) was originally.conceivdd to chaftircterize strengths of ionic-type bonds and local charge balance in stable iohic
crystals. It is evident, however, that the model seems to apply equally well to
compounds in which the bonds have a relatively large arriount of covalent character. For these compounds,Pauling (1960,S4Z-548,Foohrote 64) has indicated
that "if the bonds resonate among the alternative positions, the valence of the
metal atom will tend to be divided equally among the bonds to the coordinated
atoms, and a rule equivalent to the electrostatic valence rule would express the
satisfaction of the valences of the nonmetal atoms.', Pauling,s statement implies
[1] that the strength of an electrostatic bond, s, can be equated with bond
number, rz (Pauling, 1947,J. Amer. Clrcm. Soc. 69, 542-559); t2l that the correlations between f (O) and the length of the Si-O bond (Smith, 1953,Amer. Mineral.
38, 643-661; Ba-ur, 1970) are similar to the well known bond-length bond-number
curve for carbon-carbonbonds; and t3l ihat the chargeson bonded atoms are
small in agreement with his electroneutrality principle (Pauling, 19ffi, General
Chemistry, W. II. Freeman and Co.,286-287). The inference that z and s are
equivalent is consistent with the observation ihat f(O) and z(Si-O) are inversely
correlated (Louisnathan and Gibbs, l972b). Although Baur calls his model the
extended electrostatic valence rule, he is careful to note that the correlation between observed Si-O bond length and f(O) could be interpreted in terms of
Cruickshank's (1961) double bonding model as well as the Born model for ionic
crystals.