Determination of Calcium by Titration with EDTA
advertisement

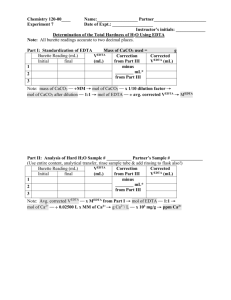
Determination of Calcium Oxide by Titration with a Chelating Ligand, Ethylenediamminetetraacetic Acid (EDTA) Ethylenediamminetetraacetic acid, more commonly known as EDTA, belongs to a class of synthetic compounds known as polyaminocarboxylic acids. Acting as a ligand that shows multiple coordination sites, EDTA forms very strong 1:1 stoichiometric complexes with all +2 and higher charged metal ions in aqueous solution. EDTA contains six sites that can be protonated. Since EDTA itself is quite insoluble in water, the disodium salt is normally used to make EDTA solutions. To form the strongest complexes, EDTA solutions are usually buffered in a region that ensures that protonation reactions do not compete 23with the complexation reaction. The important species in solution are generally H2Y and HY . Figure 13-4 in your text shows the ideal pH buffer ranges for the EDTA titration of various metal ions. The analytical reaction in this experiment can be written as: Ca 2+ 3- + HY 2+ CaY + H + Note that the reaction has a 1:1 stoichiometry. The overall procedure to be used involves the standardization of an EDTA solution by titration with a known amount of calcium followed by using the calibrated solution to determine an unknown amount of calcium. Before coming to lab: • Record the analytical reaction above in your lab notebook. • Record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium salt dihydrate) in your lab notebook. Reagents: • calcium carbonate • methyl red indicator • ethylenediamminetetracetic acid, disodium salt dihydrate • magnesium chloride hexahydrate • 6 M HCl • 6 M NaOH • Calmagite • Ammonia / ammonium chloride buffer Page 1 of 3 determination of calcium by titration with EDA.pdf Put your unknown in the oven at 150 °C for at least 30 minutes, while you prepare your EDTA solution and do your standardization titrations. Preparation of EDTA: 1. Add 500 mL of distilled water to your largest beaker (at least 600 mL). 2. Place the beaker on a magnetic stirrer and add 0.05 g of MgCl2•6 H2O or 20 mL of a 1% solution of magnesium chloride. 3. Using the top loading balance weigh approximately 2 g of disodium EDTA, Na2C10H14N2O8. 4. Slowly add the disodium EDTA. Use a magnetic stirrer to homogenize the solution. Mix until fully dissolved. Rinse a clean 1 L bottle with small volumes of the EDTA solution and then pour the solution into the bottle for storage. Preparation of primary standard calcium solution: 1. The primary standard calcium carbonate should be dried at 150 degrees °C for at least 1 hour. (Note the analytical purity on the bottle.) 2. Calculate the number of grams of pure calcium carbonate required to prepare a 100.0 mL standard calcium solution that would require ~35 mL of 0.01 M EDTA for titration of a 10.00 mL aliquot: g CaCO3 = M EDTA x 0.035L x 1 mol CaCO3/1 mol EDTA x MM CaCO3 x 100.0mL/10.00mL 3. Place the beaker in the hood and add 6M HCl dropwise until the sample completely dissolves. Make the volume up to about 50 mL with distilled water. 4. Gently boil the solution for a few minutes to expel excess CO2 being careful not to lose any of the solution. 5. Allow the solution to cool and add two drops of methyl red indicator. 6. Add drops of 6 M NaOH until the methyl red indicator turns yellow (the yellow may be difficult to see but there is no trace of red.) Any flocculant precipitate that forms may indicate that too much NaOH has been added. Add a drop or two of HCl to dissolve the precipitate. 7. Quantitatively transfer the solution from the beaker with distilled water into a 100.0 mL volumetric flask. Dilute to the mark in the volumetric flask. Mix the solution carefully before continuing. Standardization of 0.01 M EDTA: perform at least 3 times. 1. Pipet a 10.00 mL aliquot of the calcium standard into a 250 mL Erlenmeyer flask and add 15 mL of the ammonium-ammonium chloride buffer. 2. Add 10 drops of the calmagite indicator. 3. Add enough distilled water to have a total volume of 75-100 mL in the Erlenmeyer flask. 4. Titrate from the initial "wine red" color to an endpoint of "sky blue." At this point, the 2+ EDTA has completely replaced calmagite indicator as the chelate for Ca . You should obtain three results that agree to ±1%. Page 2 of 3 determination of calcium by titration with EDA.pdf Unknown calcium samples (treat in the same manner as the CaCO3 standard): perform at least 3 times. 1. Remove the unknown from the oven and allow it to cool before weighing. 2. Calculate the number of grams of your unknown required to use about 35 mL of your standardized EDTA solution: g unknown = M EDTA x 0.035L x 1 mol CaO x 1 mol Ca x MM CaO x 100.0mL/10.00mL 1 mol EDTA 1 mol CaO estimated % CaO in unknown 100% 3. Place the flask in the hood and add 6M HCl drop wise until the sample dissolves. Some samples may have a trace of very fine insoluble silica (sand) that appears transparent upon close observation. Make the volume up to about 50 mL with distilled water. 4. Gently boil the solution for a few minutes to expel excess CO2 being careful not to lose any of the solution. 5. Allow the solution to cool and add two drops of methyl red indicator. 6. Add drops of 6 M NaOH until the methyl red indicator turns yellow. 7. Any flocculant precipitate that forms may indicate that too much NaOH has been added. Add a drop or two of HCl to dissolve the precipitate. 8. Carefully rinse the beaker with distilled water into a 100.0 mL volumetric flask to quantitatively transfer all of the calcium solution. Dilute to the mark in the volumetric flask. Mix the solution carefully before continuing. 9. Pipet a 10.00 mL aliquot of the calcium unknown into a 250 mL Erlenmeyer flask and add 15 mL of the ammonium-ammonium chloride buffer. 10. Add 10 drops of the calmagite indicator. 11. Add enough distilled water to have a total volume of 75-100 mL in the Erlenmeyer flask. 12. Titrate from the initial "wine red" color to an endpoint of "sky blue." 13. Titrations should take between 30 and 40 mL of EDTA titrant to minimize the buret volume error. 14. Obtain at least three good reproducible titrations. Your results should agree to within ±1%. Report the result in terms of the percentage of calcium oxide (%CaO) in your unknown. The range on unknown values should be between 35% to 60%. Page 3 of 3 determination of calcium by titration with EDA.pdf