C hem is try - Wood County Schools
advertisement

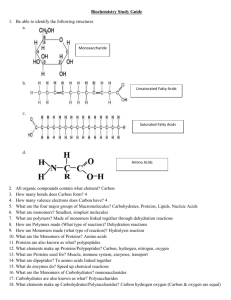
Study Guides Big Picture Biochemistry is the study of the structure and properties of molecules in living organisms. Our body is made out of four basic classes of biomolecules: carbohydrates, lipids, proteins, and nucleic acids. Each class performs a unique role and is vital for the function of life. Key Terms Biomolecule: A molecule created within an organism. Carbohydrate: A biomolecule containing carbon, hydrogen, and oxygen that supply the energy living systems need Chemistry Biochemistry to survive. Lipid: A biomolecule produced for storing energy. Protein: A biomolecule making up muscles, enzymes, and other organs in the body. Nucleic Acid: A biomolecule used to store genetic information. Monomer: One basic building block of a biomolecule. Polymer: A long chain of monomers. Fatty Acid: A long hydrocarbon chain ending in a carboxylic acid group (-COOH). Amino Acid: This is the monomer of a protein, which consists of an amine group (NH2-), a carboxylic acid group (-COOH), and a carbon side-chain. Four Main Types of Biomolecules There are 4 main types of biomolecules responsible for cellular functions within cells. These are carbohydrates, lipids, proteins, and nucleic acids. • Each biomolecule is made up of monomers. • Monomers lose a water molecule when they A table of the monomers and polymers of biomolecules Biomolecule combine into polymers in dehydration synthesis. • The breakdown of polymers into monomers for digestion in the body occurs in hydrolysis, where water is added to the polymer. Monomer Polymer carbohydrate monosaccharide polysaccharide lipid fatty acid triglyceride protein amino acid polypeptide (protein) nucleic acid nucleotide nucleic acid (DNA and RNA) This is why you need to drink water when you eat! Carbohydrates Carbohydrates are sugars used to provide energy to your body. They perform specific functions depending on their structure. The basic formula for carbohydrates is (CH2O)n, where n can be any number of repeating monosaccharides. • Monosaccharide: The monomer of a carbohydrate. Examples are glucose, fructose, and galactose. • Polysaccharide: A polymer chain of monosaccharides connected with glycosidic linkages (bonded through an oxygen). Examples are sucrose (table sugar) which is glucose and fructose, and lactose, which is glucose and galactose. Longer chains also exist, such as starch, which is a chain of glucose, and is broken down and digested by the body for energy. Figure: Starch polymer showing individual glucose monomers connected by glycosidic linkages. Lipids • Monounsaturated fatty acid: A fatty acid with one double bond. • Polyunsaturated fatty acid: A fatty acid with more than one double bond. Image Credit: Wolfgang Schaefer, Public Domain Figure: The general formula of a fat triglyceride molecule. On the left is the glycerol molecule (the 3-carbon chain and the three oxygens it is attached to), and three different fatty acids attached to the molecule. This guide was created by Steven Lai, Rory Runser, and Jin Yu. To learn more about the student authors, visit http://www.ck12.org/about/about-us/team/ interns. Page 1 of 2 v1.11.4.2011 Disclaimer: this study guide was not created to replace your textbook and is for classroom or individual use only. Lipids are fats. A triglyceride is the most common type of lipid and consists of three fatty acids attached to a glycerol molecule. They are used to store energy in our body. Lipids also include phospholipids, which have a phosphate group attached to a fatty acid and make up cell linings, and steroids, which have four carbon rings bounded together with branches and functional groups. • Saturated fatty acid: A fatty acid with no double bonds. Every carbon is saturated with hydrogens. Chemistry Biochemistry cont . Four Main Types of Biomolecules (cont.) Proteins Proteins, also called polypeptides, are made out of amino acids and are connected with peptide bonds. • There are 20 amino acids, which, like the alphabet, can be arranged in any order and number to form a nearinfinite number of polypeptides. Structure of a Protein • The shape of a protein is important for function. There are four different structures that proteins can form in the body. Large enough proteins fold, twist, and bend in certain ways to give them the right shape and structure. Nucleic Acids Figure: This shows the four steps that a polypeptide chain undergoes to become a fully-functioning protein. Nucleic acids include DNA, which is double-stranded, and RNA, which is single-stranded. Nucleic acids are made up of nucleotides joined with phosphodiester bonds. There are five different types of nucleotides, which are split into the pyrimidines and the purines. The pyrimidines are cytosine (C) and thymine (T). In RNA, thymine is replaced with uracil (U). The purines are adenine (A) and guanine (G). Adenine bonds only with thymine (uracil in RNA), and cytosine only with guanine. A clever way to remember that A goes with T and C goes with G is the phrase Chicken Gravy, Apple Tart. Another way to remember this is the phrase All Tigres Can Growl. • DNA: A double-stranded chain of deoxyribonucleic acids that forms a double-helix through hydrogen bonding. It holds the genetic information of most complex cells. • RNA: A single-stranded chain of ribonucleic acids. It holds the genetic information of some less complex organisms and is involved with protein synthesis in complex cells. Figure: This picture shows the differences between DNA and RNA, and the constituent nucleic acids associated with each one. Image Credit: Sponk, CC-BY-SA 3.0 Page 2 of 2 Image credit