1. Structure of the nucleus
advertisement
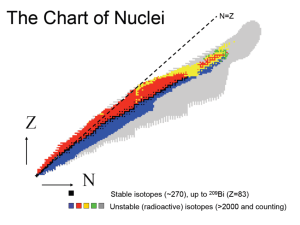
Structure of the nucleus A bit of History… ~400 BC: Greek Philosopher Democritus believed that each kind of matter could be subdivided into smaller and smaller bits until one reached the very limit beyond which no further division was possible. “atomos” = “that cannot be cut” 1895: Discovery of X-rays by Wilhelm Röntgen 1896: Discovery of the radioactivity in Uranium by Henri Becquerel 1898: Isolation of Radium by Pierre et Marie Curie Around 1896: Birth of Nuclear Physics Radioactivity ? Emission of energy stored in the material by the mean of “mysterious” rays ~1900: The structure of the atom is not yet known... But Chemistry is ! (Mendeleiv 1834 – 1907) The radiation emitted by Radium is identified to be the element Helium 1911: Discovery of the atomic nucleus by Ernest Rutherford The first experiment with a beam of particles: The α-particles are most of the time not deflected, but sometimes they are scattered, even in the backward hemisphere. the foil is almost “transparent” to the α’s But when interaction occurs, it is on a heavy partner in the foil (the nucleus) full analysis: size of the nucleus, mass, etc… Elementary Constituents of the Nucleus All the nuclei can be made with: p proton n neutron Z N A positively charge (+q) neutral (James Chadwick, 1932) number of protons in the nucleus number of neutrons in the nucleus atomic number = Z + N The atoms are neutral: Charge of the nucleus: + Z.q Electron cloud is made of Z electrons of charge (–q) The electrons determine the chemical behavior Z defines the Element Two nuclei with a same Z but different N (or A) are isotopes (of the same element) Discovery of the neutron • In 1930 the German physicists Bothe and Becker used a radioactive polonium source that emitted α particles. When these α particles bombarded beryllium, the radiation penetrated several centimeters of lead. In 1932 Chadwick proposed that the new radiation produced by α + Be consisted of neutrons. His experimental data estimated the neutron’s mass as somewhere between 1.005 u and 1.008 u, not far from the modern value of 1.0087 u. 1 atomic mass unit (a.m.u. or u) = 1.6605 x 10-27 kg = 931.49 MeV/c2 = (1/12) m(12C) Mass of the proton: mp = 1.6726 x 10-27 kg = 938.27 MeV/c2 Mass of the neutron: mn = 1.6749 x 10-27 kg = 939.57 MeV/c2 Notations The symbol of an atomic nucleus is . where Z = atomic number (number of protons) N = neutron number (number of neutrons) A = mass number (Z + N) X = chemical element symbol A Z XN 1 Ex: 1 H0 Hydrogen 2 1 H1 Deuterium 12 6 C6 Carbon U143 Uranium Other examples: 235 92 Isotopes of the same element 12 Simplified: C 235 U In practice, the Z number is redundant with the element symbol & the N number is obsolete The Chart of Nuclei N=Z Stable isotopes (~270), up to 209Bi (Z=83) Unstable (radioactive) isotopes (>2000 and counting) Beyond the nucleon… • Both neutrons and protons, collectively called nucleons, are constructed of other particles called quarks. • The (non-zero) magnetic moment of the neutron is evidence of an underlying structure in the nucleon. Mass of the proton Units and Dimensions -15 Size: the order of magnitude is 1fm = 10 m (a femtometer or fermi) 1/3 Radius (assuming nucleus = sphere): R = R0.A with R0 = 1.2 fm [ Nucleus made of A nucleons, each of them has a R0=1.2fm radius 3 Vnucleon = (4/3)πR0 ; Vnucleus = A . Vnucleon ] 17 Nuclear Density: 10 kg/m3 100 million tons per cm3 !!! density found in the core of a neutron star Nuclear matter is uncompressible (properties of the strong force) Binding energy =mass of the constituents – mass of the product Atom Nucleus Force Coulomb Strong Binding Energy The hydrogen atom: 13.6 eV 2H: 2.2 x 106 eV 2.2 MeV Enrico Fermi Exercise • Calculate the density of 238U (M=238.05 u) 238 3 1/3 Assuming that the U nucleus is a sphere: V = (4/3)πR and R = R0.A , 3 the mass is: M = ρ . (4/3)AπR0 Density of the nuclear Matter Volume V of the nucleus -27 -25 M = 238.05 x 1.6605 x 10 = 3.953 x 10 kg -15 3 -42 3 V = (4/3) x 238 x 3.14159 x (1.2 x 10 ) = 1.723 x 10 m 17 3 ρ = M/V = 2.3 x 10 kg/m • Based on this density, what would be the weight of a 1cm radius sphere of nuclear matter? 3 3 -6 3 1cm radius sphere: V = (4/3)πR = (4/3) x 3.14159 x (0.01) = 4.2 x 10 m 11 Mass of the sphere with the nuclear matter density: M = ρ x V = 9.6 x 10 kg That’s about the mass equivalent of a 1-km sphere of ordinary matter! The Nuclear / Strong Force Short Range, only a few fm Nuclear Matter is uncompressible ; repulsive at very short range (< 1 fm) Attractive over a range of a few fm a given nucleon only interacts with its next neighbors in the nucleus Negligible at long distances Same force for protons and neutrons Nuclear properties • Protons and Neutrons are Fermions (half-integer spin particles) Fermi-Dirac statistics • Density ~ constant “Skin” e.g. the nucleus has a diffuse surface (Valid for light nuclei) How to calculate the binding energy ? The hydrogen atom: we win energy by bringing together the proton and the electron (13.6 eV) Equivalence Energy Mass: E = m.c2 Binding energy (B) = mass of the constituents – mass of the product = (mpc2+mec2) – mHc2 = 13.6 eV with: mpc2 = rest mass of the proton = 938.28 MeV mec2 = rest mass of the electron = 0.511 MeV = 511 keV the Hydrogen atom weighs less than its constituents One needs to provide 13.6 eV to take the hydrogen atom apart Binding energy is also called Mass deficiency Any atom (defined by Z): B = mNucleusc2 + Z. mec2 – mAtomc2 How do we calculate mNucleusc2 ? Atomic Mass Unit (amu) • The atomic mass unit (amu or u) is defined as 1/12th of the mass of 12C with all its electrons, that is: – 1uc2 = (1/12) x M(12C)c2 = (1/12) x [6mp+6me-+6mn]c2 • All masses given in amu are the ATOMIC mass, i.e. they include Z electrons as well. • In order to take into account the electron mass in the calculations, we replace (mp+me-) by mH, the mass of the hydrogen atom including the electron. – B(AZX) = (Z.mpc2 + Z.mec2 + N.mnc2) – mXc2 becomes: – B(AZX) = (Z.mHc2 + N.mnc2) – mXc2 • Instead of using: mp = 1.007276u, use mH = 1.007825u Binding energy of the nucleus The nucleus: 1H nucleus = free proton Binding energy = 0 (but still has a rest mass) 2H (deuterium) = one proton and one neutron B = (mHc2 + mnc2) – mDc2 = 2.2 MeV A ZX : B = (Z.mHc2 + N.mnc2) – mXc2 Example: 56Fe (Z=26) m56Fe = 55.934969 u (or amu, atomic mass unit = 931.5 MeV/c2) with: mH = 1.007825 u & mn = 1.008665 u B(56Fe) = 26 x 1.007825u.c2 + 30 x 1.008665u.c2 – 55.934969u.c2 = 0.528461u.c2 = 492.3 MeV Very large number compared to B’s in atomic physics Manufacturing 1g of 56Fe from protons and neutrons per second would generate 8.5.1011 Watt !!! 850 GWatt Average Binding Energy Average binding energy produced by the strong force can be expressed by dividing the total Binding Energy of the nucleus by its mass number (B/A) B/A ~ 7-8 MeV is a typical value The Liquid Drop Model (I) • Goal: estimate the binding energy of a given nucleus with a “semi-empirical” model. – Nucleus = Collection of interacting particles in a liquid drop of nuclear matter Symmetry term: in the absence of the Coulomb force, the nucleus prefers to have N ≈ Z Volume term: The binding energy is approximately the sum of all the interactions between the nucleons. Surface term: Correction for the nucleons at the surface of the nucleus (interact with less neighbors) Pairing term Coulomb term: Adding more protons increases the Coulomb repulsion within the nucleus The Liquid Drop Model (II) One can show that the Coulomb term can also be written (See example 12.5 p436): -1/3 Ec = 0.72 Z(Z-1) A MeV -¾ With Δ = 33 A MeV The Liquid Drop Model (III) Example: Binding energy per nucleon (Ex. 12.7 p439): Liquid Drop Model 20Ne (Z=10, N=10) 8.03 MeV / nucleon 8.19 MeV / nucleon 56Fe (Z=26, N=30) 8.79 MeV / nucleon 8.35 MeV / nucleon 238U (Z=92, N=146) 7.57 MeV / nucleon 6.84 MeV / nucleon