Calorimetry: Heat of Vaporization
advertisement

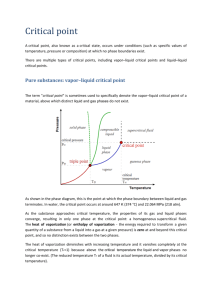
Calorimetry: Heat of Vaporization OBJECTIVES - Learn what is meant by the heat of vaporization of a liquid or solid. - Discuss the connection between heat of vaporization and intermolecular forces. - Become familiar with the concept of vapor pressure. - Measure the vapor pressure of water at several temperatures and use the results to obtain the molar heat of vaporization of water. - Estimate the molar heat of vaporization of water from its known boiling point using two semi-empirical equations. INTRODUCTION The heat of vaporization represents the amount of energy required to expand the volume of a liquid material to that of its gas. For 0.018 L of liquid water at 1 atm and 273 K, for example, the molecules must be separated far enough to produce about 22.4 L of vapor. Substantial energy is required for the vaporization process, as the strong attractive forces found between the molecules in the liquid must be overcome. The magnitude of the heat of vaporization reflects the strength of the intermolecular forces (Experiment Table 17.1). The heats are tabulated at the normal boiling points (Tnbp) of the liquids – that is, at the temperature at which each liquid boils when held at 1 atm pressure. A comparison of the heat of vaporization of the nonpolar CH4 (or C2H6) with that of the polar CH3OH (or C2H5OH) shows the effect of increased intermolecular forces due to the polar hydroxyl group. An examination of the data for the nonpolar methane, ethane, and octane shows that the intermolecular forces also increase with molecular size (due to the increasing importance of van der Waals forces). Although a general correlation between the normal boiling point and the heat of vaporization is also seen, this relationship is more complex. Notice the very high Tnbp of n-octane but its only moderately high heat of vaporization. EXPERIMENT TABLE 17.1 Liquid Formula Mercury Hg Water H2O Ethanol C2H5OH Methanol CH3OH n-Octane C8H18 Acetone (CH3)2CO Chloroform CHCl3 Diethyl ether (C2H5)2O Ethane C2H6 Methane CH4 Normal boiling temp (°C) 357.0 100.0 78.5 64.7 125.7 56.1 61.3 34.6 -88.6 -161.5 Heat of vaporization (kJ/mol) 59.0 40.7 38.6 35.3 35.0 30.3 29.4 26.0 14.7 8.2 Adapted from the experiment described by Gerald S. Levinson in J. Chem. Ed. 59 (337) 1982. Calorimetry: Heat of Vaporization The heat of vaporization of a solid material is called its heat of sublimation. If the solid is an ionic compound, the heat of vaporization reflects the amount of energy required to remove the electrically charged ions from their crystal lattice to form an ion gas. This energy is called the lattice energy. These heats are much larger than the heats of vaporization encountered with liquids. Sodium chloride, for example, has a lattice energy of 790 kJ/mol. A strong connection should exist between the volatility of a liquid and its heat of vaporization. Volatile materials, such as chloroform, evaporate readily at room temperature: nonvolatile liquids, such as vegetable oil, evaporate only slightly, if at all. The volatility of a liquid is readily determined by measuring its vapor pressure. The liquid is placed in a closed container and allowed to evaporate into an empty region. As the vapor of the liquid fills this empty space, its pressure increases; some of the vapor molecules collide with and are again trapped by the liquid. The escape rate from the liquid is primarily determined by the strength of intermolecular bonds: the weaker the bonds, the greater the rate of escape. The return rate, on the other hand, is determined by the density of molecules found in the vapor. This particle density (n/V) increases with vapor pressure according to the ideal gas law: n P V RT Volatile liquids, with their high escape rates, require high vapor densities (pressures) before an equilibrium with the return rate can be achieved. When the vapor is in equilibrium with its liquid, the pressure of the vapor is called the vapor pressure of the liquid. Vapor pressure is easily measured and found to increase with temperature. Rudolf Clausius (1822 – 1888), a German physicist, and Benoit Clapeyron (1799 – 1864), a French physicist, developed the theory for relating the temperature variation of the vapor pressure of a liquid to its ΔHvap. By assuming that the molar volume of a liquid (about 0.018 L/mol at 273 K and 1 atm for water vapor) is negligible compared with the molar volume of its vapor (about 22.4 L/mol at 273 K and 1 atm for water vapor), that the vapor obeys the ideal gas law, and that ΔHvap does not change rapidly with temperature it can be shown that the vapor pressure (P) is related to the heat of vaporization: ln( P) H vap RT cons tan t This is called the Clausius-Clapeyron equation, and is used routinely to find heat of vaporization. The temperature again must be expressed in Kelvin. The equation has the form of a straight line: y = mx + b If ln(P) is associated with y, 1/T with x, H vap , R with slope m, and the constant with yintercept b, a graph of log(P) versus 1/T should yield a straight line with a slope directly related to the heat of vaporization. Calorimetry: Heat of Vaporization PROCEDURE A known quantity of air is trapped under water and kept at constant pressure. The air quickly becomes saturated with the water vapor. The temperature of the liquid water is varied, and the changes in the volume of the trapped gas mixture are recorded. This volume change is caused by the thermal expansion of the constant amount of air and by the variation in the amount of water vapor. These data are sufficient to calculate the vapor pressure of water. CAUTION WEAR EYE PROTECTION! 1. Obtain a 10 mL graduated cylinder, a thermometer, and a tall 1000 mL beaker. Read the barometric pressure and record it on your Report Sheet. 2. Fill the beaker with 900 mL of water. 3. Fill the graduated cylinder to the 7 or 8 mL mark with water and completely cover the top of the cylinder with your finger. Now rapidly flip the cylinder over and place it into the tall beaker. The cylinder should be completely submerged (Experiment Figure 17.1) and the volume of trapped air should be 4 to 5 mL. 4. Carefully heat the water on a hot plate or by using an iron ring, wire gauze, and Bunsen burner. Heat the water to about 80°C, or until the air has expanded sufficiently to extend beyond the graduated scale on the cylinder. Turn off the heat source. 5. Gently stir the water in the beaker and watch the air in the cylinder. As soon as the air level reaches the 10 mL mark, record this volume to the nearest 0.1 mL and the temperature to the nearest 0.1 °C. 6. Move the hot beaker to the tray so that the tray will collect any water that spills out. Keep stirring the hot water, and record temperature and volume readings for each twoor-three degree drop down to 50 °C. 7. Finally, rapidly cool the water temperature to 5 °C or less with large quantities of ice. Record the temperature and air volume at the lowest temperature. Calorimetry: Heat of Vaporization CALCULATIONS 1. The 10 mL cylinder is graduated to give correct volume readings only when right side up. This is due to the shape of the water meniscus. Subtract 0.2 mL from each of your readings to correct for this effect. 2. Use the lowest temperature reading (in K), the associated air volume (in L), and the barometric pressure (in atm) in the ideal gas law to find the number of moles of air (nair) trapped in the cylinder. nair PatmV RT 3. The vapor pressure of the water at this low temperature is assumed to be negligible. Use the other temperature readings and the associated corrected volumes to calculate the partial pressure of air in the gas mixture at higher temperatures: nair RT V 4. The gas mixture in the cylinder is composed of water vapor and air with a total pressure equal to the atmospheric pressure when the levels of water (in the cylinder and beaker) are equal. The total pressure, according to Dalton’s Law, is equal to the sum of the two partial pressures of the air and the water vapor: Pair Patm Pair Pwater Pwater Patm Pair Calculate this partial pressure of water, which is the vapor pressure of the water. 5. Tabulate the vapor pressure, and 1/T on the x-axis. Draw the best straight fit line through the points and determine its slope. The Clausius-Clapeyron equation indicates that the slope is given by: slope H vap R Solve this equation for ΔHvap. Use R=8.314 J/K·mol so that the heat of vaporization is given in J/mol. 6. Use Trouton’s Rule and Kistiakowsky’s expression to estimate the heat of vaporization of water from its normal boiling point. Trouton’s Rule: ΔSvap (Tb) = 88 J/K·mol Kistiakowsky’s Expression: ΔSvap (Tb) = KF(36.6 + 8.31ln(Tb)) Calorimetry: Heat of Vaporization _________________________________ Name ___________ Section ____________ Date Data Sheet 1 Temp Corrected Vair Vair Temp Vair Corrected Vair °C mL mL °C mL mL °C mL mL °C mL mL °C mL mL °C mL mL °C mL mL °C mL mL Barometric Pressure _________________ Torr = ___________ atm Volume of Dry Air _________________ L Number of Moles of Dry Air __________________ mol Temp (K) Pair (atm) Slope = ________________ Pwater (atm) 1/T (1/K) Log Pwater ΔHvap (expected) = ________________ J/mol Calorimetry: Heat of Vaporization _________________________________ Name ____________ Section _____________ Date Post-Laboratory Questions 1. You have assumed the vapor pressure of water below 5 °C to be negligible. How would the inclusion of its actual vapor pressure affect your results? 2. Assume the graph of ln(P) versus 1/T results in a curved line instead of a straight line. What does this mean? 3. Other liquids may be examined by this experimental method to determine the heat of vaporization. What properties should these liquids exhibit? 4. Assume you make a mistake and plot ln(P) against 1/T and express T in °C instead of K. How does this affect your results? Calorimetry: Heat of Vaporization _________________________________ Name ____________ Section _____________ Date Pre-Laboratory Questions 1. In each pair, choose the compound expected to have the higher boiling point: 2. Why does chloroform (CHCl3) have such a large heat of vaporization compared to that of methane (CH4)? 3. Why must 0.2 mL be subtracted from the air volume readings taken from the graduated cylinder?