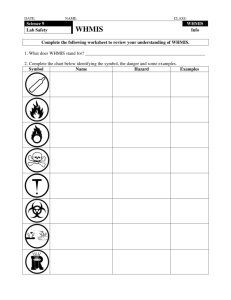
Unit Overview Lesson Topic 1 An introduction to WHMIS 2
advertisement

Unit Overview Lesson 1 2 3 4 5 Topic An introduction to WHMIS Common fire hazards and fire prevention methods in the home and workplace Toxins in the workplace An introduction to chemical reactions Wise management of hazardous materials in the home Suggested Time: approx. 20 periods (70 min per period) Table of contents Lesson 1.1 Ready to Work: It Happened in Ontario 2 Lesson 1.2 Smart Marks 10 Lesson 1.3 Taking Precautions 22 Lesson 1.4 First Day on the Job 28 Lesson 2.1 Fighting Fires 49 Lesson 2.2 Detection Systems 60 Lesson 2.3 Flammable materials 69 Lesson 2.4 Special Materials 78 Lesson 3.1 Dressed to Live 85 Lesson 3.2 How Much is Enough? 91 Lesson 4.1 Reaction Rates 95 Lesson 4.2 Reactivity Series 100 Lesson 4.3 The Air We Breathe 105 Lesson 5.1 Clean Up 109 Lesson 5.2 Off You Go! 117 References 118 1 Lesson 1.1 Ready to Work: It Happened in Ontario EXPECTATION CODES MSV.01 Time Suggested: 70 min EXPECTATIONS demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home BOTTOM LINE Make students aware how WHMIS exists to protect them in the workplace, through case studies and discussion. MATERIALS TEACHER "It Happened in Ontario" scenario sheet "Live Safe, Work Smart" WHMIS overheads STUDENT "It Happened in Ontario” question sheet SAFETY CONCERNS There is a need for balance in presenting these materials: there are hazards, and we have the means to understand and prevent such hazards. Safety is information and prevention, not avoidance. LESSON SEQUENCE Read "It Happened in Ontario" aloud, using overhead as written copy. Using prompt questions on student sheet, guide discussion about what happened, why, and how it could have been prevented. Encourage sharing of other incidents, statistics on fires and injuries at work and home. Use "Live Safe, Work Smart" overheads to introduce the aspects of WHMIS. Teachers should familiarize themselves with the components of the WHMIS system, and the responsibilities of supplier, employer, and employee. See background information. ASSESSMENT TOOLS AND STRATEGIES Observe participation in discussion. ACCOMMODATIONS None required EXTENSIONS Graph statistics by type of injury, location, age, etc. depending on availability. Survey class to establish type of hazards they may experience in their day: at home, at work, at school. 2 BACKGROUND INFORMATION WHMIS: An Introduction (Source: Saskatchewan Labour, Ready to Work) WHMIS (pronounced "wimis") stands for Workplace Hazardous Materials Information System. It is a Canada-wide information system set up to protect all Canadian workers and employers. A hazardous material is any substance that can cause illness, disease or death to unprotected people. Sometimes hazardous materials are called “hazardous products”, “controlled products “or “dangerous goods”. WHMIS provides vital information about any materials that pose a risk or hazard in the workplace. Students may have already been introduced to this system in secondary level science classes. WHMIS provides employers and workers with information about the hazardous materials they work with on the job. This information is necessary to protect the health and safety of everyone in the work place. The WHMIS information system is based on a law in Canada that came into effect in October, 1988. It states that everyone has a right to know about the hazardous substances that are being used in their workplace. It requires suppliers, employers and workers to use the system to identify and handle hazardous materials safely. WHMIS rules apply in every province and territory of Canada. People who do not follow the laws on hazardous materials can be charged with an offence and, if convicted, can be fined or jailed. Why is it needed? In our daily lives there are hundreds of materials and chemicals that have been developed to make our work easier and to allow us to make better products. In this process there are substances that are used or produced that can be dangerous to people if handled improperly. WHMIS lets us know a) which materials are dangerous, and b) how we can protect ourselves when we handle them. The danger of hazardous materials can come from explosion, fire, skin contact, inhalation or ingestion. How bad the danger is will usually depend on one or more of the following: • • • • • • the amount of pressure there is (gases) how easily the material burns or explodes the amount of material there is how toxic it is how it enters the body its concentration 3 Who developed WHMIS? Once the need for a national information system was recognized, WHMIS was developed by joint committees of employers, unions and governments. What problems does WHMIS try to solve? • unlabelled chemicals in workplaces • lack of awareness by employers about the identity and hazards of the chemicals they are using • inadequate information provided by suppliers to employers and workers, about the hazards of the chemicals they are using • differences between provinces and territories in the way hazardous materials are handled The three main parts of WHMIS WHMIS has three main parts to help identify and handle hazardous materials safely: 1. Labels: They are applied to the containers with materials inside. The labels supply vital warning information. 2. Material Safety Data Sheets (MSDS): Sheets of information stored separately from the material. These sheets give details for handling emergencies, clean-ups, and controls for the safe use of the hazardous materials. The law requires the employer to have a MSDS available for every hazardous material in the workplace. 3. Worker Education: Employers must provide instruction to each worker on how to use WHMIS, what hazardous materials are on site, and how to handle them properly. Employee Responsibility Workers have the responsibility to use the system to protect themselves from hazardous materials by: • • • recognizing labels checking the hazards following recommended procedures Employer and supplier responsibilities will be discussed in other activities. Exemptions of Products from WHMIS Some products are already covered by other legislation. These have been partially exempted from having to follow WHMIS requirements for labels and MSDS’s. 4 Employers must still follow WHMIS laws for these products by educating workers in the safe handling of the products and by using labels when the contents are transferred. These products include consumer products, cosmetics and drugs, explosives, pesticides and radioactive substances. Some products are covered by other laws and are completely exempted from WHMIS. These include wood and products of wood, tobacco and products made of tobacco, hazardous wastes and manufactured articles. HELPFUL HINTS Consider whole and small group facilitation of discussion. Have groups record their responses on paper to the accident that killed Sean Kells. Be sensitive to the emotional impact this may have on students. The emphasis is that understanding WHMIS has the potential to protect them and their colleagues from injury. RESOURCES It Happened in Ontario - Scenario Sheet and Question Sheet Live Safe, Work Smart - Text content of overheads in "Chemical Hazards: Grade 9" 5 It Happened in Ontario - Question Sheet It was his third day on the job, and a new worker was asked to pour a chemical product from a drum. While he was pouring the liquid, the drum exploded and the worker received third degree burns to 90% of his body. He was rushed to the hospital but he died the next day. The story told is about a young worker named Sean Kells. He died on November 18, 1994. Since Sean's death, his family has been active trying to increase health and safety awareness for young people. It can't bring Sean back, but they hope they can help prevent other deaths and injuries among young people. What the worker didn't know: The chemical contained a hazardous material called toluene Toluene can explode very easily. In this case, it exploded because of the static electricity charge that was created when he poured the material into the drum. Why did he not know it was a hazardous material? What could you do to prevent this from happening where you work? Taken from "Live Safe, Work Smart." Also available at: www.livesafeworksmart.net 6 It Happened in Ontario - Scenario Sheet (Teacher Copy) It was his third day on the job, and a new worker was asked to pour a chemical product from a drum. While he was pouring the liquid, the drum exploded and the worker received third degree burns to 90% of his body. He was rushed to the hospital but he died the next day. The story told is about a young worker named Sean Kells. He died on November 18, 1994. Since Sean's death, his family has been active trying to increase health and safety awareness for young people. It can't bring Sean back, but they hope they can help prevent other deaths and injuries among young people. What the worker didn't know: The chemical contained a hazardous material called toluene Toluene can explode very easily. In this case, it exploded because of the static electricity charge that was created when he poured the material into the drum. Why did he not know it was a hazardous material? The container was not marked or labelled. He had not received any training. He did not know his rights and responsibilities. What could you do to prevent this from happening where you work? Make sure you get the appropriate training before starting a new job or task. Check that there is a label on every product. (The law requires that all hazardous products have a label. This requirement is part of the WHMIS system.) Know your rights and responsibilities. MOST IMPORTANTLY, always ask for help if you are not sure if the job you are asked to do is hazardous or not. Taken from "Live Safe, Work Smart." Also available at: www.livesafeworksmart.net 7 W H M I S Workplace Hazardous Materials Information System WHY IS IT NEEDED? • Ensure every worker’s Right to Know about health and safety hazards • To help stop injuries, illnesses, deaths, medical costs, fires, explosions from unsafe use of hazardous chemicals. • To protect all workers. WHMIS IS LAW In Ontario coverage by: • Occupational Health and Safety Act • Ontario WHMIS Regulation • Enforced by the Occupational Health and Safety Branch of the Ontario Ministry of Labour 8 Taken from "Live Safe, Work Smart." Also available at: www.livesafeworksmart.net 9 WHMIS RESPONSIBILITIES Supplier • Provide labels and MSDS Employer • Make labels & MSDS’s available • Develop safe procedures • Train workers on WHMIS Worker • Follow safe work procedures • Inform employer of hazards FOUR MAIN PARTS OF WHMIS 1. Classification and Symbols 2. Labels 3. Material Safety Data Sheets (MSDS) 4. Worker Education Taken from "Live Safe, Work Smart." Also available at: www.livesafeworksmart.net 10 Lesson 1.2 Smart Marks Time Suggested: 70 min EXPECTATION CODES SIS.04 EXPECTATIONS SIS.05 select and use appropriate numeric, symbolic, graphical, and linguistic modes of representation to communicate scientific ideas, plans, and experimental results (e.g., present a detailed experimental report according to specified standards) SIS.06 compile and interpret data or other information gathered from print, laboratory, and electronic sources, including Internet sites, to research a topic, solve a problem, or support an opinion (e.g., research the uses of the most common products of the refining of petroleum) MSV.01 demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school la boratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MS1.01 categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS1.02 demonstrate an understanding of important safety legislation (e.g., WHMIS legislation, the Fire Code, the Building Code, the Occupational Health and Safety Act) MS1.04 identify some oxidizing agents by name and/or chemical formula, and describe their chemical reactivity with fuels and other oxidizable substances (e.g., write the chemical formula for oxygen gas and explain the reaction of oxygen gas with a fuel in terms of the products formed) MS1.08 demonstrate an understanding of the toxicity and hazards of some chemical substances (e.g., mercury) MS1.10 explain the meaning of the terms acute and chronic as they apply to the effect of hazardous materials on the body. MS2.02 demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) demonstrate a knowledge of emergency laboratory procedures 11 MS2.03 plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) BOTTOM LINE Students identify WHMIS hazard classifications and workplace products symbols MATERIALS TEACHER Large selection of empty product containers: domestic, industrial, and laboratory. Include some non-hazardous. STUDENT Master list of WHMIS symbols Quiz sheet on WHMIS symbols Vocabulary journal Safety gloves and goggles SAFETY CONCERNS Containers need to be empty and clean. Teacher should be responsible at this point to make collection. As students learn how to read and deal with the hazard warnings, they can add to the collection. Even so, introduce the students to wearing safety goggles and gloves when dealing with chemicals, to avoid the risk of coming into contact with any product remaining in the containers. Add this to the list of general lab safety rules. LESSON SEQUENCE Provide students with master WHMIS symbol list and selection of containers. Have them find the symbols on each product and place into groups of similar hazard. Close activity by discussing any pattern in the groups: does one type of product always have a similar hazard associated? Review WHMIS definitions of "hazards." Ask students to collect words from the product labels that give a warning of potential hazard, e.g.: flammable, combustible, etc. Students to record in an ongoing vocabulary journal and on large paper to display around lab. Discuss meanings by using associated words, group into hazard types. Complete “Designer Labels” exercise if review is required. ASSESSMENT TOOLS AND STRATEGIES Use vocabulary building exercise throughout course and review scope and correctness when unit complete. WHMIS symbol matching quiz sheet ACCOMMODATIONS Provide key word list for the hazard types prior to activity to assist reading Simplify the master list to only the symbol and hazard 12 EXTENSIONS As an alternative to providing empty containers, perform this lesson as a "field trip" to a hardware or automotive store. Carry out the symbol search along the shelves, with students recording product name, type and hazard. BACKGROUND INFORMATION See WHMIS Product Classification Symbols master sheet WHMIS Product Classification and Hazard Symbols WHMIS divides hazardous materials into six main classes based on their specific hazards. The classes are lettered A through F. Class D, which is poisonous and infectious materials, has 3 divisions for different types of poisons. Each of these 8 classes of materials has a symbol. Class B, flammable and combustible materials, is also divided into subclasses or divisions. Unlike Class D, however, it does not have separate symbols for each. Class B subclasses or divisions are: • Division 1 − flammable gas • Division 2 − flammable liquid (flash point below 37.8 2C) • Division 3 − combustible liquid (flash point greater than 37.8 2C) • Division 4 − flammable solid (can be ignited by heat or friction) • Division 5 − flammable aerosol (small drops of a liquid suspended in air) • Division 6 − reactive flammable material (flammable in air) Exemptions Some products such as pesticides, certain consumer products and explosives do not require the distinctive WHMIS label because they are already covered by other labelling laws. If those products are transferred to smaller containers, WHMIS requires that workplace labels be applied. HELPFUL HINTS Class B and C have very similar symbols: think of the "O" for "oxidizing." RESOURCES WHMIS symbol master sheet WHMIS symbol quiz sheet plus solution sheet 13 WHMIS Product Classification Symbols Symbol Class Type Description Examples A Compressed gases Products held under pressure Oxygen Propane B Flammable and combustible materials Products that will burn or catch on fire easily C Oxidizing materials D1 Materials causing immediate and serious toxic effects Products that can cause or promote combustion of another material (whether or not they are themselves combustible) or products that are organic peroxides Products that can rapidly cause harmful health effects, including death Propane Acetone Kerosene Magnesium Sodium Hydrogen peroxide Nitric acid D2 Materials causing other toxic effects D3 Biohazardous infectious materials E Corrosive materials F Dangerously reactive materials Carbon monoxide Phenol Products whose health effects generally appear over time following one or several exposures Living organisms or their toxins that can cause disease in people or animals Products that can corrode metal surfaces or cause burns to skin Benzene Diisocyanates Lead Products that can be health or safety hazards under certain conditions (pressure, temperature, impact, violent reaction with water or air) Fluorine Hydrogen cyanide B-Chloroprene AIDS virus Hepatitis B virus Rabies virus Caustic soda Hydrochloric acid Bleach 14 WHMIS Symbols Quiz Sheet Taken from: Saskatchewan Labour, Ready for Work V. An Introduction to WHMIS 15 WHMIS Symbols Quiz Sheet (Teacher’s Copy - solutions) Taken from: Saskatchewan Labour, Ready for Work V. An Introduction to WHMIS 16 Designer Labels Consumer Symbols Degree of Hazard Warning labels used for household products have an OUTER BORDER which INDICATES THE DEGREE OF HAZARD and a CENTRAL SYMBOL which INDICATES THE TYPE OF HAZARD. 17 FLAMMABLE $ Fire hazard $ Will ignite if exposed to a spark $ Store away from heat $ Use in ventilated area POISON $ Potentially fatal if inhaled or swallowed $ May have serious long term effects $ Wear gloves & face mask $ Wash after using EXPLOSIVE $ Handle container with care $ may explode if heated or dropped $ May react violently with other material $ Keep away from heat sources CORROSIVE $ Causes skin & eye burns $ Do not breath fumes $ Wear glove and eye protection $ May damage metals 18 DESIGN A SIGN Use the information to design a sign for the following materials. You need to think about two things: 1) The Degree of Hazard 2) The Type of Hazard Cut the shape of the sign out and glue it beside the description. Then cut and glue the type of hazard on top of the shape. The materials or equipment used could explode under certain conditions. The material is very poisonous. 19 The material, while less toxic, is still dangerous. There is some danger of the substance catching fire. The substance may eat away at skin, clothing, or other material. The substance will irritate the skin, eyes, nose, or lungs a lot. 20 The substance might readily ignite and burn when in contact with other substances. There is some danger of infection or poisoning from animals, plants, or microorganisms. The substance is strongly radioactive and gives off radiation. There is a slight danger of electric shock. 21 22 Lesson 1.3 Taking Precautions Time Suggested: 70 min EXPECTATION CODES SPECIFIC EXPECTATIONS MSV.01 demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MS1.01 c categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS1.02 demonstrate an understanding of important safety legislation (e.g., WHMIS legislation, the Fire Code, the Building Code, the Occupational Health and Safety Act) MS1.08 demonstrate an understanding of the toxicity and hazards of some chemical substances (e.g., mercury) MS1.10 explain the meaning of the terms acute and chronic as they apply to the effect of hazardous materials on the body demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) MS2.02 MS2.03 plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) BOTTOM LINE Students to use the precautions and emergency procedures given on WHMIS supplier labels to produce a summary set of safety guidelines. MATERIALS TEACHER Collection of empty product containers as in activity 1.2 STUDENT Vocabulary journal WHMIS General Precautions sheets WHMIS symbols master from lesson 1.2 Safety gloves and goggles 23 SAFETY CONCERNS As in 1.2 use gloves and goggles when handling hazardous product containers. LESSON SEQUENCE Introduction of concept that we can take precautions against harm from the hazardous products described by the WHMIS symbols (lesson 1.2). Recall lesson 1.1 and why we are learning about WHMIS. Part of the WHMIS system is to tell us what precautions are necessary and what to do in an emergency. The first place we can find that information is on the product label. In small investigative groups looking at one type of product, students are to find out what precautions and first aid procedures are described on the labels. They record their discoveries by adding vocabulary to their journals, now they have "safety" words to add to "warning words," and summarizing one group on a "WHMIS General Precaution Sheet." Using a jigsaw structure, each student then becomes part of a learning group, and will both provide and collect safety information on all the WHMIS categories. In closing the activity, whole group discussion as to what specific safety rules can now be added to the general laboratory guidelines as presented in the introductory lesson. ASSESSMENT TOOLS AND STRATEGIES Maintenance of vocabulary journal Assess completion of worksheets ACCOMMODATIONS Provide a cut and paste alternative with typical safety precautions and emergency procedures to match and add to the WHMIS General Precaution sheets. BACKGROUND INFORMATION Labels The Workplace Hazardous Materials Information System has labels that are used to identify hazardous materials. The purpose of the labels is to alert workers to the main hazards of products and provide procedures for working with them, as well as to direct workers to the second part of the information system, the Material Safety Data Sheet. There are three main types of WHMIS labels: • • • Supplier Labels which are placed on the container by the manufacturer or distributor. The materials are then shipped to the workplace. Workplace Labels which are placed on hazardous materials where needed on the job site. When any hazardous material is taken out of its supplier container and put into another container, workplace labels must be applied to the new container. Other means of identification in the workplace: pipes, tubes, pumps or vessels may be used to transport hazardous materials from one place to another. Since each work site may be different, the employer has to develop ways of warning the worker that 24 site may be different, the employer has to develop ways of warning the worker that there are hazardous materials present. Sometimes coloured flags or tapes are attached or the containers are coloured. As each employer has developed his/her own system for warning employees, it is necessary that the employee be trained to recognize this ‘other means of identification’ used by the employer. Supplier Labels When hazardous materials enter the workplace, the supplier label is the first warning sign that hazardous materials are present. The label may be placed on the container of hazardous materials by the supplier before shipping, or the supplier label may be included with the shipment and placed on the containers by the receiver when the shipment arrives at the workplace. The supplier label has a special "hatch" border to draw attention to it. The label will signal that hazardous materials are present. Suppliers must provide supplier labels on containers of products sold or imported into the workplace. The supplier label provides these 7 types of information: q q q q q q q product identifier - the name of the hazardous material supplier identifier - the name and address of the supplier MSDS statement - a statement indicating that a Material Safety Data Sheet for that material is available in the workplace hazard symbols - one or more of 8 WHMIS hazard symbols relevant to the hazardous material risk phrase - a brief description of the hazard and the effects of exposure on the body precautionary measures - brief instructions for the safe use of the materials first aid measures - how to treat persons who have been exposed to the material There is no specific rule for the size, shape or colour of the label, but it must contrast with the background colour of the container. In other words, a yellow label is not allowed on a yellow drum or a blue label on a blue bottle, and so forth. A problem arises when the container with the hazardous material is small. It is difficult to fit a label with all the above information on a small bottle. When the container is less than 100 millilitres, or one third of a can of pop, only the following information is required on the supplier label: q q q q product identifier supplier identifier a statement making reference to a MSDS hazard symbols showing the dangers associated with the material 25 HELPFUL HINTS The jigsaw structure is only one possible way to disseminate information through the group. A simple substitution is to have each expert group produce a handout or poster that can then be copied for or by the other students. The intent is to avoid unnecessary repetition of the same task, i.e. reading a label, but to maximize the information received. RESOURCES Sample supplier label WHMIS General Precautions sheet Sample precaution statements Sample WHMIS Supplier Label Taken from Saskatchewan Labour, Ready For Work 26 WHMIS General Precautions WHMIS Hazard Group (A - F) Type of hazard Take these Precautions: In emergency do this: 27 Sample Precaution Statements q Handle with care q Keep away from heat q May explode if dropped q Keep away from flame q Keep separate from flammable materials q Keep away from areas where food and drink is consumed q Avoid exposure to skin q Wear safety glasses q Wear gloves q Wear protective clothing q Ensure eyewash station is nearby q Ensure shower is nearby q Use in a well ventilated area 28 Lesson 1.4 First Day on the Job Time Suggested: 100 – 140 min EXPECTATION CODES SIS.04 EXPECTATIONS SIS.05 select and use appropriate numeric, symbolic, graphical, and linguistic modes of representation to communicate scientific ideas, plans, and experimental results (e.g., present a detailed experimental report according to specified standards) SIS.06 compile and interpret data or other information gathered from print, laboratory, and electronic sources, including Internet sites, to research a topic, solve a problem, or support an opinion (e.g., research the uses of the most common products of the refining of petroleum) MSV.01 demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MS1.01 categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS1.02 demonstrate an understanding of important safety legislation (e.g., WHMIS legislation, the Fire Code, the Building Code, the Occupational Health and Safety Act) MS1.06 describe the factors that increase the danger of flammable substances (e.g., flash point, auto-ignition) MS1.07 identify and explain common types of incompatibility between classes of chemicals (e.g., acids must not be stored on the same shelf as bases) MS1.08 demonstrate an understanding of the toxicity and hazards of some chemical substances (e.g., mercury) MS1.09 describe routes of entry of hazardous materials into the body (e.g., ingestion, inhalation, absorption through the skin) MS1.10 explain the meaning of the terms acute and chronic as they apply to the effect of hazardous materials on the body. MS2.02 demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, demonstrate a knowledge of emergency laboratory procedures 29 demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) MS2.05 demonstrate, in oral and in written reports, a thorough knowledge of the terminology and symbols used in WHMIS (e.g., correctly interpret material safety data [MSD] sheets, labelling symbols, and acronyms such as LD50, LC50, TWAEV, STEV, CEV). BOTTOM LINE In a workplace scenario, students extract information from the MSDS (Material Safety Data Sheet) to create a supplier label for a product. MATERIALS TEACHER Sample of product containers (lesson 1.2/1.3) with the product MSDS MSDS sample template (for accommodations) STUDENT Supplier label data sheet Art supplies WHMIS MSDS Basics Using MSDS for Common Products SAFETY CONCERNS As in 1.2, 1.3, wear goggles and gloves when handling the containers. LESSON SEQUENCE Present this scenario to students: you have arrived at your new job and been told that you will be working with product X. How do you find out what it is and how to work with it safely? The answer can be found on the product’s Material Safety Data Sheet or MSDS the ultimate source! Introduce sections using the MSDS sample template. Be aware that a variety of layout formats exist but ultimately all MSDS formats provide the same information. The students' tasks are: 1 Collect the necessary information from the MSDS onto the supplier label data sheet 2 Use this supplier label data to create a label that clearly presents the important details. Provide paper, colours, stencils, etc. for artistic presentation. If access is available, allow production on a computer. Alternative: make label poster size for display in lab. The MSDS contains numerous opportunities for students to continue adding to their vocabulary journals. Some may necessitate further research for meaning. Students independently complete the question sheets for homework or continuation tasks as determined by the teacher: WHMIS MSDS Basics Using MSDS for Common Products 30 ASSESSMENT TOOLS AND STRATEGIES Finished product assessed for technical accuracy and organization. Use independent question sheets as summative assessment. Completion of worksheets can be assessed for evidence of initiative ( a learning skill) ACCOMMODATIONS Either make the supplier label data sheet the final product, or provide this summary data for the students to design the label. Provide a the MSDS sample template and highlight sections required to complete by reading the product MSDS EXTENSIONS Students can use internet resources to track down MSDS sheets. Compare the 9 section and 16 section formats. Using the laboratory MSDS library, students can carry out a hazardous materials audit of the lab, reviewing safety measures. BACKGROUND INFORMATION See lesson 1.3 for details of WHMIS labels Material Safety Data Sheets (MSDS) The Material Safety Data Sheet is a very important technical document. There is a MSDS for every hazardous material on site. The MSDS is the second level of the right to know. Federal law requires that a supplier provide a MSDS for each controlled product. Saskatchewan law requires the employer to have a MSDS available for every hazardous material in the workplace. The MSDS must be readily accessible to all workers, worker representatives and members of the occupational health committee. Every MSDS must be current (up to a maximum of 3 years is allowed between updates). The MSDS must be revised within 90 days after new hazard information becomes known about the material. The MSDS has 9 main sections containing information that the employer should be aware of. None of these sections should be left blank, but their order may vary. 1. Product Identification and Use: the product name, identification number and use, as well as information on how to contact the supplier or manufacturer. 2. Hazardous Ingredients: the identity of the ingredients, their concentrations and estimates of immediate and severe health effects. 3. Physical Data: a physical description of the product. 31 4. Fire and Explosion Data: information on the ability of the product to catch fire or explode, and the means of extinguishing a fire. 5. Reactivity Data: the ability of the product to react dangerously. 6. Toxicological Properties: information on how materials enter the body and what the short and long-term health effects are. 7. Preventive Measures: information on control measures including ventilation, personal protective equipment (gloves, respirators, etc.) and work procedures. 8. First Aid Measures: information on immediate treatment in case of contact with the product. 9. Preparation Information: information on who prepared the MSDS and when. The MSDS can contain more information than the required 9 sections. For example, some MSDSs will include information about how to safely transport the product. This will be listed under “TDG” or “ Transportation of Dangerous Goods”. Exemptions Some companies do not want to disclose information on the MSDS because they would be giving away trade secrets. Some cleaners and soaps are examples of this. Those companies submit a request to a committee that approves or turns down the company’s request. Other situations arise where consumer products fall under other laws; when this occurs, the MSDS does not have to have all parts completed. Please note that there is also a 16 heading format for MSDS’s that is also acceptable. Many of the chemicals purchased from common suppliers of school science supplies come with MSDS’s of this format. HELPFUL HINTS This would be more relevant to students if prior to class, the teacher could find out any products they already work with, at home or in employment, and provide those MSDS. The extension task in lesson 1.1 would be a source of information. An important skill in this lesson is the ability to "skim read" the MSDS, skipping irrelevant or too complex information, looking for the key words. Remind students to use their vocabulary lists to help them find the right details. RESOURCES WHMIS Supplier Label Data Sheet MSDS Sample Template WHMIS MSDS Basics Using MSDS for Common Products MSDS for WD40 and Varsol 32 WHMIS Supplier Label Data Sheet Product name Supplier name and address MSDS Statement "Refer to the MSDS available for further information." WHMIS hazard symbol(s) Risk phrase Precautions necessary First aid 33 MSDS Sheets - Sample Template Material Safety Data Sheet (MSDS) Section 1: Product Information Product Identifier Manufacture’s Name Supplier’s Name Street Address Street Address City Province City Province Postal Code Emergency Telephone Postal Code Emergency Telephone Product Use Section 2: Hazardous Ingredients Chemical Identity Concentration CAS# PIN # LD50 Species & Route LC50 Species & Route Section 3: Physical Data Physical State Odour & Appearance Odour Threshold Specific Gravity Co-efficient of Water/Oil Distribution Vapour Pressure Boiling Point Freezing Point pH Evaporation Rate Percent Volatile (by volume) Section 4: Fire or Explosion Hazard Conditions of Flammability Means of Extinction Explosion Data: Sensitivity to Mechanical Impact Sensitivity to Static Discharge 34 Flashpoint & Method Upper Flammable Limit % Autoignition Temperature Lower Flammable Limit % Hazardous Combustion Products Section 5: Reactivity Data Stability Incompatible Materials Phone # Date of Preparation Conditions of Reactivity Hazardous Decomposition Products Section 6: Toxicological Properties Route of Entry ___ Skin Contact ___ Skin Absorption ___ Eye Contact ___ Inhalation ___ Ingestion Effects of Acute Exposure to Product Effects of Chronic Exposure to Product Exposure Limits Irritancy of Product Synergistic Products Evidence of Carcinogenicity, Reproductive Toxicity, Teratogenicity or Mutagenicity? Sensitization to Product 35 Section 7: Preventive Measures Personal Protective Equipment Gloves (specify) Respiratory (specify) Eye (specify) Footwear (specify) Other Equipment (specify) Engineering Control (e.g., ventilation, enclosed process, specify) Leak and Spill Procedure Waste Disposal Handling Procedures & Equipment Storage Requirements Special Shipping Information Section 8: First Aid Measures Inhalation Ingestion Eye contact Skin Contact Section 9: Preparation Information Prepared by: ( group, department) Phone number Date: Source: Saskatchewan Labour "Ready for Work, Introduction to WHMIS" 36 WHMIS MSDS Basics 1. How many sections must be provided on a MSDS? 2. What is the title of the section of the MSDS that tells whom to contact if you have questions about the product? 3. Which section of the MSDS lists the special protective measures you can take to avoid harmful contact with the product? 4. How often must a MSDS be updated? 37 WHMIS MSDS Basics (Teacher’s Copy with Answers) 1 How many sections must be provided on a MSDS? 9 sections 2 What is the title of the section of the MSDS that tells whom to contact if you have questions about the product? product identification and use 3 Which section of the MSDS lists the special protective measures you can take to avoid harmful contact with the product? preventive measures 4 How often must a MSDS be updated? Every three years or 90 days after new hazard information becomes known. 38 Using MSDS with Common Products WD40 What is the telephone number of the supplier? Under what section of the MSDS did you find this information? What problems can occur if you get WD40 in your eyes? In what section did you find this information? What should you do if you get WD40 in your eyes? What could you have done to prevent it from happening? What engineering controls may be needed for this product? Are the ingredients of this product carcinogenic (cancer causing)? Varsol What is the WHMIS classification for this product? Based on this classification, what precautions will you have to take with product? What may happen if this product is breathed in? Under what section was this found? What should be done if the person breathes the product in? What personal protection may be needed when using this product? What handling and storage precautions are required with this product? 39 Using MSDS with Common Products (Teacher's copy with responses) WD40 What is the telephone number of the supplier? Under what section of the MSDS did you find this information? What problems can occur if you get WD40 in your eyes? In what section did you find this information? What should you do if you get WD40 in your eyes? What could you have done to prevent it from happening? What engineering controls may be needed for this product? Are the ingredients of this product carcinogenic (cancer causing)? Varsol What is the WHMIS classification for this product? Based on this classification, what precautions will you have to take with product? What may happen if this product is breathed in? Under what section was this found? What should be done if the person breathes the product in? What personal protection may be needed when using this product? What handling and storage precautions are required with this product? The emergency telephone number is (613) 996-6666 Look under Product Identification to find the contact information. WD40 may cause eye irritation. Look in Toxicological Properties. Flush eyes immediately with plenty of water for at least 15 minutes. Then get medical attention. You should have worn safety glasses when using this product. Proper ventilation if used frequently indoors. No, the ingredients are not listed as carcinogens. Class B: Flammable and Combustible material; Division 3: Combustible liquid. Keep the product away from anything that could ignite the liquid - electric sparks, flames, etc. High vapour concentrations are irritating to the eyes and respiratory tract. This may cause headaches, dizziness, anesthesia, drowsiness, unconsciousness, and other central nervous system effects, including death. Look in the Health Hazard Information section. Remove the person from exposure to the fumes. Administer artificial respiration if the person has stopped breathing. Call for medical attention. If there is prolonged and/or repeated skin and eye contact, wear safety glasses with side shields, long sleeves, and chemical resistant gloves. Where ventilation is not adequate and concentrations in the air may exceed safe limits, approved respirators may be needed. Handle open containers with care. Keep the container closed as much as possible. Store varsol in a cool, wellventilated place away from incompatible materials. Do not handle or store near an open flame, heat, or other sources of ignition. Protect material from direct sunlight. Use proper grounding procedures as varsol will accumulate static charges which may cause an electrical spark. 40 * * * * * * * * * * * * * * * * * * * * * * * * * * * * * M S D S * * Canadian Centre for Occupational Health and Safety * * * * * * * * * * * * Issue : 2000-1 (February, 2000) * * * * *** IDENTIFICATION *** MSDS RECORD NUMBER PRODUCT NAME(S) PRODUCT IDENTIFICATION DATE OF MSDS CURRENCY NOTE form on 1998-04-27 : : : : : 1671684 3958-1020 WD40 FULL PALLET 3.785L & SPRAY MATERIAL SAFETY DATA SHEET : 00002589 1996-07-11 This MSDS was provided to CCOHS in electronic *** MANUFACTURER INFORMATION *** MANUFACTURER ADDRESS : K-G PACKAGING : 8001 Keele Street Post Office Box 89 Concord Ontario Canada L4K 1B2 EMERGENCY TELEPHONE NO. : 613-996-6666 (24 HRS) (CANUTEC) *** MATERIAL SAFETY DATA *** MATERIAL SAFETY DATA SHEET : 00002589 K-G PACKAGING 8001 KEELE STREET P.O. BOX 89 CONCORD, ONTARIO CANADA L4K 1B2 CANUTEC EMERGENCY #:1-613-996-6666(24HR) --------------------------------------------------------------------Product: 3958-1020 WD40 FULL PALLET 3.785L & SPRAY ---------------------------------------------------------------------SECTION 01: MANUFACTURER INFORMATION --------------------------------------------------------------------MANUFACTURER...................... K-G PACKAGING 8001 KEELE STREET P.O. BOX 89 CONCORD, ONTARIO CANADA L4K 1B2 CANUTEC EMERGENCY #:1-613-9966666(24HR) PRODUCT NAME...................... 3958-1020 WD40 FULL PALLET 3.785L & SPRAY CHEMICAL FAMILY................... ORGANIC MIXTURE. TRADE NAMES & SYNONYMS............ SAME AS PRODUCT NAME. PRODUCT USES...................... LUBRICANT/PENETRANT. CHEMICAL FORMULA.................. NOT APPLICABLE. MOLECULAR WEIGHT.................. NOT APPLICABLE. T.D.G. CLASSIFICATION............. NOT REGULATED. WHMIS CLASSIFICATION.............. B3. NFCC SECTION 4.1.................. CLASS II. NFPA CODE 30B..................... CLASS II. 41 HMIS RATING FLAMMABILITY.......... 2 MODERATE HAZARD. HMIS RATING HEALTH................ 1 SLIGHT HAZARD. HMIS RATING REACTIVITY............ 0 MINIMAL HAZARD. ---------------------------------------------------------------------SECTION 02: HAZARDOUS INGREDIENTS ---------------------------------------------------------------------% CAS / TLV LD/50, ROUTE, SPECIES LC/50, ROUTE, SPECIES ---------------------------------------------------------------------STODDARD SOLVENT 10-30 8052-41-3 5g/kg 5g/m3 1OO ppm ORAL-RAT INHAL-RAT PETROLEUM BASE OIL 5-10 64742-65-0 5 mg/m3 MINERAL SPIRITS 60-100 64742-47-8 NOT AVAILABLE NOT AVAILABLE NOT AVAILABLE >5000 mg/kg ORAL - RAT 1400 ppm/4H INHAL - RAT ---------------------------------------------------------------------SECTION 03: PHYSICAL DATA ---------------------------------------------------------------------PHYSICAL STATE.................... LIQUID. BOILING POINT (DEG C)(CONC)....... >149. VAPOUR PRESSURE(PSIG)-AEROSOL..... NOT AVAILABLE. @ 20 C VAPOUR DENSITY (AIR=1)............ GREATER THAN 1. (BY WEIGHT) SOLUBILITY IN WATER............... NEGLIGIBLE. APPEARANCE........................ LIGHT AMBER. ODOR.............................. CHARACTERISTIC. ODOR THRESHOLD.................... NOT AVAILABLE. SPECIFIC GRAVITY (LIQUID)......... 0.78-0.82. PERCENT VOLATILE (BY WEIGHT)...... 72-75. EVAPORATION RATE.................. LESS THAN 1. n-BUTYL ACETATE = 1 pH................................ NOT APPLICABLE. FREEZING POINT: (C)............... NOT AVAILABLE. COEFFICIENT OF WATER\OIL.......... NOT AVAILABLE. DIST. ---------------------------------------------------------------------SECTION 04: FIRE & EXPLOSION DATA ---------------------------------------------------------------------FLAMMABILITY...................... COMBUSTIBLE. IF YES, UNDER WHICH............... EXCESSIVE HEAT, SPARKS, OPEN FLAME CONDITIONS ? EXTINGUISHING MEDIA............... CARBON DIOXIDE, DRY CHEMICAL, FOAM. SPECIAL PROCEDURES................ WATER FROM FOGGING NOZZLES MAY BE USED TO COOL CLOSED CONTAINERS TO PREVENT BUILD-UP. IF EXPOSED TO EXTREME TEMPERATURES.FULL PROTECTIVE EQUIPMENT INCLUDING SELF CONTAINED BREATHING APPARTATUS SHOULD BE WORN IN A FIRE INVOLVING THIS MATERIAL. FLASH POINT(C),TAG CLOSED-CUP..... >42. (CONCENTRA TE) AUTO IGNITION TEMPERATURE (C)..... NOT AVAILABLE. LOWER FLAMMABLE LIMIT............. 1. 42 (% BY VOLUME) UPPER FLAMMABLE LIMIT............. 6. (% BY VOLUME) HAZARDOUS COMBUSTION PRODUCTS.......................... HYDROCARBON FUMES AND SMOKE. CARBON MONOXIDE WHERE COMBUSTION IS INCOMPLETE. EXPLOSION DATA SENSITIVITY TO STATIC....... NOT APPLICABLE. DISCHARGE SENSITIVITY TO IMPACT....... NOT APPLICABLE. -------------------------------------------------------------------SECTION 05: REACTIVITY DATA --------------------------------------------------------------------CHEMICAL STABILITY: YES......................... UNDER NORMAL CONDITIONS. NO, WHICH CONDITIONS?....... NOT APPLICABLE. COMPATABILITY WITH OTHER SUBSTANCES : NO, WHICH ONES?............. STRONG OXIDIZING AGENTS. HAZARDOUS PRODUCTS OF DECOMPOSITION..................... HYDROCARBON FUMES AND SMOKE. CARBON MONOXIDE WHERE COMBUSTION IS INCOMPLETE. REACTIVITY CONDITIONS?............ NOT APPLICABLE. ---------------------------------------------------------------------SECTION 06: TOXICOLOGICAL PROPERTIES ---------------------------------------------------------------------ROUTE OF ENTRY: SKIN CONTACT................ MAY CAUSE IRRITATION. SKIN ABSORPTION............. NO DATA AVAILABLE FOR THIS PRODUCT MIXTURE. EYE CONTACT................. MAY CAUSE IRRITATION. INHALATION.................. INHALATION OF SOLVENTS MAY CAUSE IRRITATION. INHALATION, CHRONIC......... UNKNOWN. INGESTION................... MAY CAUSE HEADACHE, NAUSEA, VOMITING AND WEAKNESS. EFFECTS OF ACUTE EXPOSURE......... DIZZINESS, NAUSEA. IRRITATION TO SKIN & EYES. EFFECTS OF CHRONIC EXPOSURE....... SOLVENTS MAY CAUSE DEFATTING DERMATITIS. EXPOSURE LIMIT OF MATERIAL........ SEE SECTION II. IRRITANCY OF MATERIAL............. SLIGHT. SENSITIZING CAPABILITY OF......... UNKNOWN. MATERIAL CARCINOGENICITY OF MATERIAL....... THE INGREDIENTS OF THIS PRODUCT ARE NOT LISTED AS CARCINOGENS BY NTP, (NATIONAL TOXICOLOGY PROGRAM), NOT REGULATED AS CARCINOGENS BY OSHA, (OCCUPATIONAL SAFETY AND HEALTH ADMINISTRATION), AND HAVE NOT BEEN EVALUATED BY IARC,(INTERNATIONAL AGENCY FOR RESEARCH ON CANCER), NOR BYACGIH (AMERICAN CONFERENCE OF GOVERNMENTALINDUSTRIAL HYGIENISTS). REPRODUCTIVE EFFECTS.............. NO INFORMATION IS AVAILABLE AND NO ADVERSE REPRODUCTIVE EFFECTS ARE ANTICIPATED. TERATOGENICITY.................... NO INFORMATION IS AVAILABLE AND NO ADVERSE TERATOGENIC EFFECTS ARE ANTICIPATED. MUTAGENICITY...................... NO INFORMATION IS AVAILABLE AND NO ADVERSE MUTAGENIC EFFECTS ARE ANTICIPATED. 43 SYNERGISTIC MATERIALS............. NONE KNOWN. ---------------------------------------------------------------------SECTION 07: PREVENTIVE MEASURES --------------------------------------------------------------------GLOVES/ TYPE................ WEAR CHEMICAL RESISTANT GLOVES. RESPIRATORY/TYPE............ IF USED INDOORS ON A CONTINUOUS BASIS, USE OF A CARTRIDGE TYPE RESPIRATOR (NIOSH/MSHATC 23C OR EQUIVALENT) IS RECOMMENDED. EYE/TYPE.................... SAFETY GLASSES. FOOTWEAR/TYPE............... RUBBER SAFETY BOOTS. OTHER/TYPE.................. NOT REQUIRED. ENGINEERING CONTROLS.............. VENTILATION - LOCAL (MECHANICAL IF USED INDOORS ON A CONTINUOUS BASIS). LEAK/SPILL........................ REMOVE ALL SOURCES OF IGNITION. USE AN INERT ABSORBENT MATERIAL, AND NON-SPARKINGTOOLS. AVOID BREATHING FUMES. VENTILATE AREA. PREVENT FROM ENTERING A WATERCOURSE. HANDLING PROCEDURES AND EQUIPMENT......................... STORE IN A COOL, WELL VENTILATED AREA NOTTO EXCEED 50 DEG C. WASTE DISPOSAL.................... DO NOT PUNCTURE OR INCINERATE CONTAINERS,EVEN WHEN EMPTY.DISPOSE OF IN ACCORDANCE WITH LOCAL, PROVINCIAL AND FEDERAL REGULATIONS. STORAGE NEEDS..................... KEEP AWAY FROM HEAT, SPARKS, AND OPEN FLAMES. --------------------------------------------------------------------SECTION 08: FIRST AID MEASURES --------------------------------------------------------------------EMERGENCY FIRST AID PROCEDURE......................... IN CASE OF EYE CONTACT, FLUSH IMMEDIATELY WITH PLENTY OF WATER FOR AT LEAST 15 MINUTES AND GET MEDICAL ATTENTION. FOR SKIN, WASH THOROUGHLY WITH SOAP AND WATER. IF AFFECTED BY INHALATION OF VAPOUR OR SPRAY MIST, REMOVE TO FRESH AIR. IF SWALLOWED; DO NOT INDUCE VOMITING, GET MEDICAL ATTENTION. --------------------------------------------------------------------SECTION 09: ADDITIONAL INFORMATION --------------------------------------------------------------------NOTICE FROM CCL INDUSTRIES INC............................... THE INFORMATION ON THIS MATERIAL SAFETYDATA SHEET IS PROVIDED BY CCL INDUSTRIES INC. FREE OF CHARGE. WHILE BELIEVED TO BE RELIABLE, IT IS INTENDED FOR USE BY SKILLED PERSONS AT THEIR OWN RISK. CCL INDUSTRIES INC. ASSUMES NO RESPONSIBILITY FOR EVENTS RESULTING OR DAMAGES INCURRED FROM ITS USE. THE INFORMATION ON THIS MATERIAL SAFETY DATA SHEET RELATES ONLY TO THE SPECIFIC MATERIAL DESIGNATED HEREIN AND DOES NOT RELATE TO USE IN COMBINATION WITH ANY OTHER MATERIAL OR IN ANY PROCESS. DATED............................. 071196 44 * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * M S D S * * Canadian Centre for Occupational Health and Safety * * * * * * * * * * * * Issue : 2000-1 (February, 2000) * * * * *** IDENTIFICATION *** MSDS RECORD NUMBER PRODUCT NAME(S) PRODUCT IDENTIFICATION DATE OF MSDS CURRENCY NOTE form on 1999-12-08 : 2179769 : HP VARSOL SOLVENT : MSDS Number: 07163 CAS Number: 8052-41-3 : 1998-05-26 : This MSDS was provided to CCOHS in electronic *** MANUFACTURER INFORMATION *** MANUFACTURER : Imperial Oil Chemicals Division : 111 St Clair Avenue West Toronto Ontario Canada M5W 1K3 ADDRESS *** SUPPLIER/DISTRIBUTOR INFORMATION *** SUPPLIER/DISTRIBUTOR ADDRESS : Imperial Oil Chemicals Division : 111 St Clair Avenue West Toronto Ontario Canada M5W 1K3 *** MATERIAL SAFETY DATA *** Date Prepared: May 26, 1998 Supersedes: March 14, 1998 MSDS Number: 07163 ___________________________________________________________________ 1. PRODUCT INFORMATION Product Identifier: HP VARSOL SOLVENT Application and Use: solvent, diluent, chemical feedstock, or fuel. Product Description: Aliphatic hydrocarbon. CAS number: 8052-41-3 -------------------------------------------------------------------REGULATORY CLASSIFICATION WHMIS Information: Class B, Division 3: Combustible Liquids TDG Information (Rail/Road): PIN Number: UN 1256 Shipping Name: Naphtha, solvent Packing Group: III Primary TDG: Class 3 Canadian Environmental Protection Act (CEPA): All components of this product are either on the Domestic Substances List (DSL) or exempt. National Pollutant Release Inventory (NPRI): 45 This product contains the following NPRI reportable substances: COMPONENT CAS # APPROX. % 1,2,4- Trimethylbenzene 95-63-6 4.2 --------------------------------------------------------------------EMERGENCY TELEPHONE NUMBER MANUFACTURER/SUPPLIER Health/Transportation Imperial Oil Chemicals Division 24 Hour Service (519) 339-2145 111 St. Clair Avenue West, Toronto, Ontario, M5W 1K3 ____________________________________________________________________ 2. REGULATED COMPONENTS The following component data is defined in accordance with subparagraph 13(a)(i) to (iv) or paragraph 14(a) of the Hazardous Products Act. NAME Stoddard solvent orl rat % (v/v) 100 CAS 8052-41-3 LD50: >5 g/kg LC50: >5 g/m3 rat ____________________________________________________________________ 3. TYPICAL PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid Spec. Gravity: 0.79 at 15.5 deg C Vap. Pres.: 0.3 kPa at 20 deg C Approximate Solubility in Water: < 0.01% at 25 deg C Boiling Point: 158 to 195 deg C Typical Freezing/Melting Point.: -58 deg C Viscosity: 1.14 cST at 25 deg C Vapour Density (air=1): 5 Evaporation Rate, n-Butyl Acetate=1: 0.1 Approximately % Volatile: 100 pH: Not applicable. Odour: Mild petroleum odor. Appearance: Clear, colorless liquid. ____________________________________________________________________ 4. HEALTH HAZARD INFORMATION NATURE OF HAZARD INHALATION:high vapour/aerosol concentrations (greater than approximately 1000 ppm) are irritating to the eyes and the respiratory tract, and may cause headaches, dizziness, anesthesia, drowsiness, unconsciousness, and other central nervous system effects, including death. EYE CONTACT: Slightly irritating, but will not injure eye tissue. SKIN CONTACT: Low toxicity. Frequent or prolonged contact may irritate the skin and cause a skin rash (dermatitis). Skin contact may aggravate an existing dermatitis condition. INGESTION: Minimal toxicity. Small amounts of liquid aspirated into the respiratory system during ingestion or from vomiting may cause mild to severe pulmonary injury and possibly death. CHRONIC: This product contains ethylbenzene. A study conducted by the National Toxicology program states that lifetime inhalation exposure of rats and mice to concentrations of ethylbenzene (750 ppm) resulted in increases in certain types of cancer, including kidney tumors in rats 46 and lung and liver tumors in mice. These effects were not observed in animals exposed to lower concentrations of ethylbenzene (75 ppm or 250 ppm). The study does not address the relevance of these results to humans. High exposures to xylenes in some animal studies have been reported to cause health effects on the developing embryo/fetus. These effects were often at levels toxic to the mother. The significance of these findings to humans has not been determined. SPECIAL HEALTH PRECAUTIONS: Health studies have shown that many petroleum hydrocarbons pose Potential human health risks which may vary from person to person. As a precaution, exposure to liquids, vapors, mists or fumes should be minimized. -------------------------------------------------------------------OCCUPATIONAL EXPOSURE LIMIT ACGIH RECOMMENDS: For Trimethylbenzene, 25 ppm (123 mg/m3). For Stoddard Solvent, 100 ppm (525 mg/m3). MANUFACTURER RECOMMENDS: 100 ppm based on composition. Local regulated limits may vary. ______________________________________________________________________ 5. FIRST AID MEASURES INHALATION: In emergency situations use proper respiratory protection to immediately remove the affected victim from exposure. Administer artificial respiration if breathing has stopped. Keep at rest. Call for prompt medical attention. EYE CONTACT: Flush eyes with large amounts of water until irritation subsides. If irritation persists, get medical attention. SKIN CONTACT: Immediately flush with large amounts of water. Use soap if available. Remove contaminated clothing, including shoes, after flushing has begun. INGESTION: If swallowed, DO NOT induce vomiting. Keep at rest. Get prompt medical attention. ____________________________________________________________________ 6. PREVENTIVE AND CORRECTIVE MEASURES PERSONAL PROTECTION: The selection of personal protective equipment varies depending upon conditions of use. Where prolonged and/or repeated skin and eye contact is likely to occur, wear safety glasses with side shields, long sleeves, and chemical resistant gloves. Where eye contact is unlikely, but may occur as a result of short and/or periodic exposures, wear safety glasses with side shields. Where concentrations in air may exceed the occupational exposure limits given in Section 4 and where engineering, work practices or other means of exposure reduction are not adequate, approved respirators may be necessary to prevent overexposure by inhalation. ENGINEERING CONTROLS: The use of local exhaust ventilation is recommended to control emissions near the source. Laboratory samples should be handled in a lab hood. Provide mechanical ventilation of confined spaces. 47 ELECTROSTATIC ACCUMULATION HAZARD: Yes, use proper ground procedure. Additional information regarding safe handling of products with static accumulation potential can be ordered by contacting the American Petroleum Institute (API) for API Recommended Practice 2003, entitled "Protection Against Ignitions Arising Out of Static, Lighting, and Stray Currents" (American Petroleum Institute, 1220 L Street Northwest, Washington, DC 20005), or the National Fire Protection Association (NFPA) for NFPA 77 entitled "Static Electricity" (National Fire Protection Association, 1 Batterymarch Park, P.O. Box 9101, Quincy, MA 02269-9101). HANDLING, STORAGE AND SHIPPING: Keep container closed. Handle and open containers with care. Store in a cool, well ventilated place away from incompatible materials. DO NOT handle or store near an open flame, heat, or other sources of ignition. Protect material from direct sunlight. Material will accumulate static charges which may cause an electrical spark (ignition source). Use proper grounding procedures. DO NOT pressurize, cut, heat, or weld containers. Empty product containers may contain product residue. DO NOT reuse empty containers without commercial cleaning or reconditioning. SPILL CONTROL AND DISPOSAL: Consult an expert on the disposal of recovered material. Ensure disposal in compliance with government requirements and ensure conformity to local disposal regulations. Notify the appropriate authorities immediately. Take all additional action necessary to prevent and remedy the adverse effects of the spill. LAND SPILL: Eliminate source of ignition. Keep public away. Prevent additional discharge of material, if possible to do so without hazard. Prevent spills from entering sewers, watercourses or low areas. Contain spilled liquid with sand or earth. Do not use combustible materials such as sawdust. Recover by pumping (use an explosion proof motor or hand pump), or by using a suitable absorbent. WATER SPILL: Eliminate sources of ignition. Warn occupants and shipping in Surrounding and downwind areas of fire and explosion hazard and request all to stay clear. Remove from surface by skimming or with suitable absorbents. If allowed by local authorities and environmental agencies, sinking and/or suitable dispersants may be used in unconfined waters. _____________________________________________________________________ 7. FIRE AND EXPLOSION HAZARD Flashpoint and Method: 43 deg C TCC Typical Autoignition Temperature: 229 deg C Approximate Flammable Limits: 1 to 13.3 % by volume Approximate GENERAL HAZARDS: Combustible Liquid; may form combustible mixtures at or above the flash point. Toxic gases will form upon combustion. 48 FIRE FIGHTING: Use water spray to cool fire exposed surfaces and to protect personnel. Shut off fuel to fire. Use foam, dry chemical or water spray to extinguish fire. Respiratory and eye protection required for fire fighting personnel. Avoid spraying water directly into storage containers due to danger of boilover. A self-contained breathing apparatus (SCBA) is recommended for indoor fires and any significant outdoor fires. For small outdoor fires, which may easily be extinguished with a portable fire extinguisher, use of an SCBA is optional. This liquid is volatile and gives off invisible vapors. Either the liquid or vapor may settle in low areas or travel some distance along the ground or surface to ignition sources where they may ignite or explode. HAZARDOUS COMBUSTION PRODUCTS: Fumes, smoke and carbon monoxide _________________________________________________________________ 8. REACTIVITY DATA GENERAL: This product is stable and hazardous polymerization will not occur. INCOMPATIBLE MATERIALS AND CONDITIONS TO AVOID: Strong oxidizing agents HAZARDOUS DECOMPOSITION: Not applicable ___________________________________________________________________ 9. NOTES In containers of 454 litres capacity or less this product is exempt from TDG regulations. REVISION SUMMARY: Since March 14, 1998 this MSDS has been revised in Section(s): 3, 4, 7 ______________________________________________________________________ 10. PREPARATION DATE PREPARED: MAY 26, 1998 SUPERSEDES: MAR 14, 1998 Prepared By: Solvents (416) 968-4415 CAUTION: The information contained herein relates only to this product or material and may not be valid when used in combination with any other product or material or in any process. If the product is not to be used for a purpose or under conditions which are normal or reasonably foreseeable, this information can not be relied upon as complete or applicable. For greater certainty, uses other than those described in "Application and Use" of section 1 must be reviewed with the supplier. The information contained herein is based on information available at the indicated date of preparation. 49 Lesson 2.1 Fighting Fires Time Suggested: 140 - 210 min EXPECTATION CODES SIS.01 EXPECTATIONS SIS.02 select appropriate instruments and use them effectively and accurately in collecting observations and data (e.g., use a balance to accurately measure the mass of a precipitate) SIS.03 demonstrate the skills required to plan and carry out investigations using laboratory equipment safely, effectively, and accurately (e.g., plan and carry out an investigation to determine the percentage composition of a compound) MS1.01 categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS2.03 plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) MS3.01 identify and analyse the different aspects of fire safety, including fire prevention and inspection in the home, school, and workplace (e.g., the use of appropriate sources of heat in the kitchen or laboratory the appropriate use of various types of fire extinguishers and other methods for extinguishing fires the need for a planned evacuation route at home and at school) demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) BOTTOM LINE Students will see by experimentation that extinguishing a fire requires the removal of one part of the "fire triangle." 50 MATERIALS TEACHER Examples of Fire Triangles STUDENT Lights Out! Experiment materials for each group: 250 mL beaker candle (tealight is most suitable) (modeling clay) Test tube 2 tablespoons of baking powder 50 mL vinegar 50 mL sand 500 mL beaker or glass plate large enough to cover top of 250 mL beaker Matches and splint Safety goggles for every person SAFETY CONCERNS This lab introduces heat and flame into the lab. Students need to be reminded of lab fire procedures, and introduced to specific safety for flames, for example: q Keep all materials away from experiment area q Place the heat source on a stable, heat proof base. Preferably clamp it in position before lighting q Do not leave the flame unattended q Wear safety goggles when heating: spluttering and sparks q Test to see if a piece of equipment is hot with a drop of water, not your fingers q Remove one match at a time from the box, and keep the box closed LESSON SEQUENCE Introduce this section of the course that is going to focus on WHMIS type B hazards: flammables and combustibles. Discuss what is necessary for a fire to burn. Introduce the concept of the "Fire Triangle." See examples in the resources. Students work in small groups experimenting with different methods of extinguishing a fire. Experiment procedure in resource sheet "Lights Out!" Apply experiment results to professional fire fighting equipment. Show and discuss "Types of Fires and Fire Extinguishers." Students independently complete "The Right Tools for the Job" ASSESSMENT TOOLS AND STRATEGIES Observe experiment procedures and laboratory practice Responses to "Right Tools for the Job" ACCOMMODATIONS Reduce steps in experiment. Design working groups to support those needing accommodations. 51 EXTENSIONS Invite a custodian or firefighter and give students the opportunity to activate an extinguisher for a simulated fire. Support this with guidance on when NOT to fight a fire and call for help. HELPFUL HINTS The experiment could be set up as activity centres, where students try one method at each station. RESOURCES Examples of the "Fire Triangle" Types of Fires and Fire Extinguishers The Right Tool for the Job Lights Out! 52 Examples of the "Fire Triangle" 53 Types of Fires and Fire Extinguishers. There are four common types of fires that are classified by the letters A, B, C, and D depending on what type of material is burning. Fire extinguishers are also classified with the same letters to show which type of fire they are best suited to be used for. Type of Fire Class A Class B Class C Class D Type of Material Involved common combustible materials like paper, wood, plastic flammable or combustible liquids like gasoline, oil, kerosene overloaded electrical equipment, appliances, power tools combustible metals like magnesium, sodium and metallic powders Type of Fire Extinguisher Required Water extinguishers Dry chemical extinguishers Dry chemical extinguishers CO2 extinguishers Dry chemical extinguishers CO2 extinguishers Metal sand extinguishers A fire extinguisher works best when it separates one of the components of the Fire Triangle from the other two. There are four common types of fire extinguishers. Type of extinguisher Air-Pressurized Water extinguishers (APW) Type Code A How they extinguish a flame remove heat from the flame Dry chemical extinguishers CO2 extinguishers AB ABC Metal sand extinguishers D smother the flame smother the flame and remove heat from the flame because compressed CO2 is very cold smother the flame 54 The Right Tool for the Job! How does water extinguish a campfire? Why would water poured onto an oil fire cause the fire to spread? What part of the Fire Triangle does a CO2 extinguisher remove? A carbon dioxide ( CO2 ) fire extinguisher is the best choice if an electric clothes dryer is on fire. Why would a water extinguisher not be suitable for the clothes dryer fire? 55 The Right Tool for the Job! (Teacher sheet with answers) How does water extinguish a campfire? It takes heat away from the fire as liquid water is converted into steam Why would water poured onto an oil fire cause the fire to spread? It sink below the burning oil and the fire would spread through the oil on the surface What part of the Fire Triangle does a CO2 extinguisher remove? It isolates oxygen and makes it unavailable for the fire. A carbon dioxide ( CO2 ) fire extinguisher is the best choice if an electric clothes dryer is on fire. Why would a water extinguisher not be suitable for the clothes dryer fire? Because tap water is a good conductor of electricity, the person operating the extinguisher may be electrocuted. 56 Lights Out! Materials 250 mL beaker Candle (tealight is most suitable) (modeling clay) Test tube 2 tablespoons of baking powder 50 mL vinegar 50 mL sand 500 mL beaker or glass plate large enough to cover top of 250 mL beaker Matches and splint Safety goggles Safety Wear your goggles and tie back long hair Clear all your things from the bench, except your instructions and experiment materials. Collect all the materials and read all the instructions before you begin. Step 1 q Place the candle into the 250 mL beaker. If using a regular taper candle, make it stand firmly using modeling clay. Position the candle in the middle of the bottom so the flame will not touch the glass. q Light the candle using a match or a splint if you have to reach into the beaker. Do not try to lift the candle to light it. Use a longer splint if you need. q Put the 500 mL beaker upside down, completely over the smaller beaker. It should rest safely on the bench. Or place the glass plate over the top of the 250 mL beaker to completely cover the hole. How long does the candle continue to burn? Which part of the fire triangle have you removed? Step 2 q Remove the large beaker or glass plate and relight the candle. q Pour the sand onto the flame from above the beaker top. Do not try to reach into the flame. 57 What happens to the candle? Which part of the fire triangle have you removed? Step 3 q With the candle out, clean out the sand from the beaker and put the candle back firmly in the bottom. Relight the candle. q Using the large beaker, fill it with 2 cm of cold water and pour it onto the candle to extinguish the flame. Which part of the fire triangle have you removed? Step 4 q Pour away any water and replace the candle firmly in the bottom of the beaker. Relight the candle. q In the test tube, put 2 teaspoons of baking powder. Make sure everyone in your group is wearing their safety goggles. Hold the test tube very close to the side of the beaker and be ready to move quickly. Half fill the test tube with vinegar. It will fizz and make bubbles that rise up the tube! As it does, let the bubbles overflow into the beaker, onto the candle. Do not pour in the liquid - keep that to use your fire extinguisher again! Which part of the fire triangle have you removed? If you have baking powder and vinegar left, you may try this step again and see if changing the amounts make more or less foam. 58 Lights Out! (Teacher copy - responses and comments) Materials: The quantities and equipment used is open to variation. When substituting other materials, concern should be taken that the candle can be held securely, and the flame is enclosed to avoid accidental contact yet is still accessible for lighting and extinguishing. Safety: Do not overprotect students. This experiment does involve a naked flame, but it is of low temperature and is easily extinguished. Allow students to demonstrate good laboratory skills and they will be prepared for the more complex experiments to come. Step 1 Placing the beaker or glass plate over the candle removes the air and hence oxygen supply. The candle will burn until it has used up the available oxygen in the enclosed space. Step 2 Sand "smothers" the fire if placed over the flame. This also removes the oxygen supply available from the air. Step 3 Water cools the candle and flame, removing heat from the equation. Step 4 It might appear that again cooling is the method of extinguishing here, but also the foam is created by the reaction producing carbon dioxide. When poured over the flame, it introduces large amounts of CO2 that displace the oxygen, and the fire cannot burn. 59 Lesson 2.2 Detection Systems Time Suggested: 70 - 100 min EXPECTATION CODES SIS.05 EXPECTATIONS SIS.09 select and use appropriate SI units (units of measurement of the Système international d’unités, or International System of Units) MSV.03 describe practices that promote fire safety, as well as safety in the handling and disposal of materials, in everyday living in the home and workplace. MS3.01 identify and analyse the different aspects of fire safety, including fire prevention and inspection in the home, school, and workplace (e.g., the use of appropriate sources of heat in the kitchen or laboratory the appropriate use of various types of fire extinguishers and other methods for extinguishing fires the need for a planned evacuation route at home and at school) select and use appropriate numeric, symbolic, graphical, and linguistic modes of representation to communicate scientific ideas, plans, and experimental results (e.g., present a detailed experimental report according to specified standards) BOTTOM LINE The workings of domestic smoke detectors and research flame detectors are revealed to students through research opportunities. MATERIALS TEACHER Resources of smoke detector diagrams (available online) Domestic smoke detector STUDENT "Seeing What Can't Be Seen" article Smoke Alarms Worksheet Helping Smoke Alarms Do Their Job Home Smoke Alarm Survey SAFETY CONCERNS If smoke detectors are used for hands-on investigation, product warnings should be carefully followed as some contain harmful materials. 60 LESSON SEQUENCE Use the "Seeing What Can't Be Seen" article as a reading activity about fire detection at NASA. Guided reading may be useful, including new vocabulary and summarizing content. Using either diagrams of smoke detectors on display, or by their own research (internet), students complete the "Smoke Alarms Worksheet." A dismantled smoke alarm may be interesting to see inside - see safety precautions. Teacher led discussion based on the information on "Helping Smoke Alarms Do Their Job." This is the link into the students' lives: how their knowledge can protect them and their families. A homework exercise is recommended in "Home Smoke Alarm Survey." Be sensitive to varying living arrangements of your students. ASSESSMENT TOOLS AND STRATEGIES This mainly text-based activity would suit a visual summary: students could produce a chart, diagram, poster or graphic organizer of the safety information contained in the lesson. ACCOMMODATIONS The level of assistance provided in the research and reading activities is easily varied to suit the individual students. EXTENSIONS Direct students to continue their research into sprinkler systems. BACKGROUND INFORMATION Review the additional material presented in the unit Fire Safety's My Job, from the Texas Fire Marshall's Office at: http://www.tdi.state.tx.us/fire/fmcurric.html HELPFUL HINTS Throughout this lesson, make good use of the students' vocabulary journals, display lists, keywords, etc. to support their reading. RESOURCES Seeing What Can't Be Seen Helping Smoke Alarms Do Their Job Smoke Alarms Worksheet Home Smoke Alarm Survey 61 Seeing What Can't Be Seen Read the original document with illustrations at: http://nasaexplores.com/lessons/01-029/fullarticle.pdf There's more to fire than meets the eye. Because humans have a very limited range of vision, there are times when they need to see what can't be seen. A typical house fire is hard to miss with its thick, choking black smoke - the result of burning wood, paper and lots of waste - and bright orange, red and blue flames. Rockets don't use wood and paper for fuel, though; they burn liquid hydrogen, a substance that burns so cleanly that there's no visible flame or smoke. A hydrogen fire wouldn't be easy to spot. NASA employees who work with rockets had to come up with a way to detect these fires to prevent dangerous situations. The first answer was a simple corn straw broom. Since workers couldn't see hydrogen leaks or fires, they held a broom in front of them when entering areas where hydrogen was stored. If the broom caught on fire, they knew there was a hydrogen leak. The broom method of fire detection was simple and effective, but danger was only a broomstick's length away. There had to be a better way. "Technology is good when it helps us do our job in a safer way," says John Wilson, manager of the Virtual Learning Center at Stennis Space Center in Mississippi. "Here at Stennis, we test engines for the Space Shuttle and other projects. We use millions of gallons of hydrogen fuel, so it's inevitable that there will be a leak somewhere. But with no combustion byproducts, a hydrogen flame is almost invisible. We needed to see what couldn't be seen." The solution might have been an infrared viewing system, much like the night vision goggles you see in movies. In the movies, the hero can spot people hiding by the heat they emit. While that sounds good, the drawback is that there are many sources of heat— people, sunlight, and steam— so it would be easy for an infrared fire detector to give a false reading. Researchers know that hydrogen emits energy in the ultraviolet range, so they combined an ultraviolet sensor with an infrared detector. By combining both forms of light detection, the fire detector could more accurately zero in on hydrogen leaks and fires. "The imager we developed sees ultraviolet light, which human eyes can't," says Wilson. "And that means helps us spot a fire up to 250 feet away. It works like binoculars, only in reverse. It expands our range of vision to help us see things that are close, rather than far away." 62 The fire detection unit looks like binoculars, too. The user looks through a headpiece that shows the hydrogen flame pattern in a coloured image. Other items in the room will be colorless shapes, so the fire source will stand out vividly. This technology could be used anywhere hydrogen fuels are stored. There's a down-to-Earth adaptation already in use. Many types of race cars use alcoholbased fuels, and alcohol is another clean-burning fuel where leaks spell danger. The pit crews can now use a similar fire detection unit to spot fuel fires on the racetrack. "All technology— and technology is science put to work— is made in incremental steps," says Wilson. "We start small and increase from there. As we tune our detector to see hydrogen leaks, we may be able to develop it to see other things. In the past, firefighters dealt mainly with wood fires, but with the many composite products now on the market, there's no way to tell what type of hazardous materials could be burning in a house fire. Developing more refined detectors could be very useful right here on Earth to save many lives. "Science fiction is just one or two steps ahead of reality," Wilson continues. "Infrared night vision was a fantasy idea for expanding our range of vision, but now we use it daily. The hydrogen fire detection system builds on very similar ideas to help us see what can't be seen." Courtesy of NASA Aerospace Technology Enterprise Article Published by NASAexplores: March 15, 2001 63 64 Smoke Alarms (Adapted from Fire Safety's My Job, from the Texas Fire Marshall's Office) Examine diagrams of a smoke alarms to answer the following questions. Name the two types of smoke alarms commonly found in homes. _____________________ _____________________ What are the two most common power sources for smoke alarms? 1. _____________________ 2. _____________________ Describe one advantage and disadvantage for each. Power source 1. 2. Advantage Disadvantage Describe how smoke particles activate a photoelectric smoke alarm. _________________________________________________________________ _________________________________________________________________ Describe how smoke particles activate an ionization smoke alarm. _________________________________________________________________ _________________________________________________________________ What type of fires is a photoelectric smoke alarm is best suited for? ___________________________________________ What type of fires is an ionization smoke alarm best suited for? ______________________________________________ 65 Smoke Alarms (Teacher copy) Examine diagrams of a smoke alarms to answer the following questions. Name the two types of smoke alarms commonly found in homes. photoelectric ionization What are the two most common power sources for smoke alarms? 1. _batteries 2. _a direct connection to the house electrical power Describe one advantage and disadvantage for each. Power source 1. Battery Advantage No wiring required 2. House power No batteries to replace Disadvantage Needs to be checked and replaced when necessary. Smoke alarm does not work during a power outage. Describe how smoke particles activate a photoelectric smoke alarm. When smoke enters the sensing chamber, light reflects off the smoke particles and hits the light sensor which sets off the alarm. Describe how smoke particles activate an ionization smoke alarm. In clean air, electrons from a small radioactive source keep the electrical circuit closed. Smoke particles interfere with the flow of the electrons, breaking the circuit. A sensor in the circuit tells the alarm to sound. What type of fires is a photoelectric smoke alarm is best suited for? This detector responds better to large smoke particles produced during a smouldering fire. What type of fires is an ionization smoke alarm best suited for? This detector responds best to small smoke particles produced during a flaming fire. 66 Helping Smoke Alarms Do Their Job Do Their Job ý Place at least one smoke alarm on each level (story) of the building. Fire experts ý Place a smoke alarm outside each sleeping area. say that having a ý If your family sleeps with bedroom doors closed, place a smoke alarm in each bedroom. working ý Test each smoke alarm once a month. (Match to an smoke alarm important date, such as pay day or the day the electric bill triples your arrives.) chances of ý Change the batteries once a year. Suggested dates: surviving a daylight savings time clock change, birthday, anniversary of fire. alarm installation or moving. ý For the best warning system, have alarm smoke alarms interconnected so that if one sounds, they all sound. Have the alarms wired to house wiring, with backup batteries. Smoke alarms come in a variety of options. Match the description to the type. 1. Good early warning for smoke and fires 2. Should be tested once a month 3. More effective at detecting smoke from flaming fire 4. More effective at detecting smouldering fires 5. Should be placed outside sleeping areas 6. Uses a small light sensor 7. Uses a small radioactive cell A. battery-operated B. hard-wired C. both A. battery-operated B. hard-wired C. both A. photoelectric B. ionization C. both A. photoelectric B. ionization C. both A. photoelectric B. ionization C. both A. photoelectric B. ionization C. both A. photoelectric B. ionization C. both Taken from: Fire Safety's My Job, Texas Fire Marshall 67 Home Smoke Alarm Survey Draw a plan of your home on a separate sheet of paper. Draw a blackened circle to show the location of each smoke alarm. If needed, draw an open circle where other smoke alarms should be located. Check each smoke alarm using the steps in the table below. Test by pressing test button Did the alarm sound? 1. Check when done Circle one: Yes No Circle one: Alarm sounded Batteries were changed Batteries were not changed Circle one: Alarm sounded after changing batteries Should be replaced because alarm did not sound 2. Check when done Circle one: Yes No Circle one: Alarm sounded Batteries were changed Batteries were not changed Circle one: Alarm sounded after changing batteries Should be replaced because alarm did not sound 3. Check when done Circle one: Yes No Circle one: Alarm sounded Batteries were changed Batteries were not changed Circle one: Alarm sounded after changing batteries Should be replaced because alarm did not sound Location If the alarm did not work, were the batteries changed? Test again. If the alarm still does not sound, the smoke alarm should be replaced. Taken from: Fire Safety's My Job, Texas Fire Marshall 68 Lesson 2.3 Flammable materials Time Suggested: 100 – 140 min EXPECTATION CODES SIS.01 EXPECTATIONS SIS.02 select appropriate instruments and use them effectively and accurately in collecting observations and data (e.g., use a balance to accurately measure the mass of a precipitate) SIS.03 demonstrate the skills required to plan and carry out investigations using laboratory equipment safely, effectively, and accurately (e.g., plan and carry out an investigation to determine the percentage composition of a compound) MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MS1.01 categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS1.02 demonstrate an understanding of important safety legislation (e.g., WHMIS legislation, the Fire Code, the Building Code, the Occupational Health and Safety Act) MS1.03 describe factors that affect the rate of chemical reaction, paying special attention to what makes reactions dangerous (e.g., increasing the temperature at which a reaction takes place can cause an explosion volatile liquids and dispersed powders have a greater rate of reaction) describe the factors that increase the danger of flammable substances (e.g., flash point, auto-ignition) MS1.06 MS2.03 demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) 69 BOTTOM LINE Students will experience the variability of flammable and combustible materials. MATERIALS TEACHER Fume hood STUDENT deep fireproof (glass or metal) pan lit candle or long matches metal tongs heat proof gloves safety goggles 2 cm squares of fabrics (natural: wool, cotton, linen, silk, and synthetic: polyester, acrylic, nylon) large bowl of water stop watch hand lens Fabric Flammability SAFETY CONCERNS See the experiment procedure for safe execution. The students are to burn only natural materials and the teacher demonstrates the synthetics in the fume hood due to the potential for hazardous gases to be produced. LESSON SEQUENCE Introduction links from the WHMIS classification of flammable materials. Some burn easily, others not at all. Prepare students with safety procedures for their experiment looking at "Fabric Flammability." Allow investigation in small groups. Follow up the student experiment with a demonstration of how synthetic materials burn. Follow the same procedure as the students used, but this must be done in a working fume hood due to hazardous vapours. Use the analysis questions to close the activity with group discussion of the consequences. Link their observations to the properties that contribute to flammability: composition, texture (air content) and how this is important when handling materials. Consider now liquid fuels such as we use at home and in vehicles. Burn a small volume of ethanol in a teaspoon as a demonstration of a flammable liquid in action. Caution: Always move the stock bottle of any flammable liquid to another room (preferably) or to the far side of the room before demonstrating combustion. Compare this relatively safe use with something like gasoline, around which you NEVER want a flame or spark. Explain the difference with the concept of "Flashpoint" and have students complete the worksheet. ASSESSMENT TOOLS AND STRATEGIES Observational rubrics for laboratory practice Worksheet responses can be assessed for knowledge and making connections 70 ACCOMMODATIONS The experiment on flammability may be better performed as a teacher demonstration with the students actively involved as observers and recorders. EXTENSIONS Introduce auto-ignition as another dangerous property of flammable substances. Instruct students to search the Internet for the auto-ignition temperatures of the liquids given in the "Flashpoint" worksheet. BACKGROUND INFORMATION The auto-ignition temperature of a substance is the minimum temperature at which a substance will ignite without a spark or flame. The auto-ignition temperature is usually much higher than the flash point. For example, diethyl ether has an auto-ignition temperature of 1600 C but a flash point of -45° C. If heated on a hot plate, diethyl ether could quite easily ignite. HELPFUL HINTS Create a large thermometer on the board and have students place the flash points of these substances as well as others they wish to research on the thermometer. This will give them a strong visual message of the range of flash points. RESOURCES Fabric Flammability Flashpoint 71 CAUTION: Check that your school fire regulations permit this experiment Fabric Flammability Materials: deep fireproof (glass or metal) pan to catch burning material a lit candle or long fireplace-type matches metal tongs safety goggles 2 cm squares of fabrics (natural only: wool, cotton, linen, silk) large bowl of water stopwatch hand lens Safety: Remove all other materials from the experiment location Wear safety goggles Tie back long hair Perform each stage of the experiment separately Immerse any remaining fabric completely in the water Procedure: Read the questions in the results section to be ready to make your observations. Position the bowl of water next to the heatproof pan. q Hold one fabric swatch using the tongs over the heat resistant pan, no higher away than 10 cm. q Use the candle or matches to light the corner of the fabric furthest from the tongs. q Carefully observe the fabric. Time how long before burning stops. Let any pieces fall into the glass dish. AT ANY TIME YOU CAN STOP THE BURNING BY PUTTING THE FABRIC INTO THE BOWL OF WATER. q When all the flames have gone, look closely at any remains with the hand lens. They will still be hot - do not touch with your fingers. q Push the remains to one side and try with the next fabric sample q Wait until the remains have completely cooled before throwing away. q q 72 Results For each sample, answer the questions on the observation chart. Add extra columns as necessary. Fabric: What texture does this fabric have? How easily did the fabric start burning? Did the fire go out or stop spreading when the lighter was removed? How quickly did the fire spread? Was there a tendency for the fabric to burst into flames? Did the material seem to melt and disappear or turn to ash? How long did it continue to burn? What effect would this material have if clothes made of it were on fire? Analysis Which of the natural fibres burned fastest in the test? Which of the synthetic fibres burned fastest in the teacher demo? Which fabric would be best suited for skiwear? For summer wear? For children’s sleepwear? For clothing for the elderly or handicapped individuals? What differences did you see between the natural and synthetic fabrics? 73 Flashpoint There are more organic compounds found in nature than any other. In science, the word “organic” does not mean something that’s natural like apples that have been grown without pesticides. The word “organic” means that these compounds are made up mostly of the elements carbon and hydrogen. Find these elements on the periodic table and record their chemical symbol. Carbon______ Hydrogen________ Questions: C2 H6 C2 H6 O C3 H8 C3 H7 F CO2 H2 O 1. Which of the compounds above are organic. Explain. 2. The opposite of organic is “inorganic.” Which compounds listed in the box are inorganic compounds? There are millions of organic compounds in nature, everything from crude oil to polyester that is used to make fabric. As well as having carbon and hydrogen, most organic compounds have another thing in common – they burn. Some organic compounds are serious fire hazards while many others are safe to have at home. Here’s an example: Home heating oil is a dark, thick organic liquid with an awful tar-like odour. Ether is also an organic compound that is colourless, flows like water, and has a slight solvent odour. Both substances are flammable, i.e., they will burn. A match held 2 cm above a dish containing ether will start a fire. However, you can drop a match into a dish of home heating oil and watch the match safely go out. What’s the difference? Ether is an example of a liquid with an extremely low flash point – the temperature at which vapours from the liquid can catch fire or explode. Oil has a high flash point, producing very little combustible vapour. The lower the flashpoint, the greater the risk of vapours being produced. A low flash point increases the risk the flammable substance will ignite. 74 Here is a table of different flammable liquids and their flash points: Common liquids and their flash points Liquid Intended Use butane gasoline acetone toluene rubbing alcohol turpentine home heating oil charcoal barbeque lighter mineral spirits cigarette lighters fuelling an engine ONLY! nail polish remover cleaning solvents massage thinning paint burn in the furnace only ignite barbeque coals thinning paint and cleaning brushes Flash point o C -88 -42 -18 4 15 35 45 70 70 Use the chart to answer these questions: Butane has a very low flash point. Why is this property ideal for its intended use? Why is it dangerous to use nail-polish remover in a poorly ventilated room? Which is the greater fire hazard, a spill of gasoline or a spill of barbeque lighter? Explain. Which is the safest to use to clean paintbrushes, turpentine or mineral spirits? Explain. 75 Flashpoint ( Teacher copy with answers) There are more organic compounds found in nature than any other. In science, the word “organic” does not mean something that’s natural like apples that have been grown without pesticides. The word “organic” means that these compounds are made up mostly of the elements carbon and hydrogen. Find these elements on the periodic table and record their chemical symbol. Carbon__C___ Hydrogen__H______ Questions: C2 H6 C2 H6 O C3 H8 C3 H7 F CO2 H2 O 1. Which of the compounds above are organic. Explain. C2 H6 C2 H6 O C3 H8 C3 H7 F. These molecules are organic because they contain the elements carbon and hydrogen. 2. The opposite of organic is “inorganic.” Which compounds listed in the box are inorganic compounds? Inorganic compounds: CO2 H2 O There are millions of organic compounds in nature, everything from crude oil to polyester that is used to make fabric. As well as having carbon and hydrogen, most organic compounds have another thing in common – they burn. Some organic compounds are serious fire hazards while many others are safe to have at home. Here’s an example: Home heating oil is a dark, thick organic liquid with an awful tar-like odour. Ether is also an organic compound that is colourless, flows like water, and has a slight solvent odour. Both substances are flammable, i.e., they will burn. A match held 2 cm above a dish containing ether will start a fire. However, you can drop a match into a dish of home heating oil watch the match safely go out. What’s the difference? Ether is an example of a liquid with an extremely low flash point – the temperature at which vapours from the liquid can catch fire or explode. Oil has a high flash point, producing very little combustible vapour. The lower the flashpoint, the greater the risk of vapours being produced. A low flash point increases the risk the flammable substance will ignite. 76 Here is a table of different flammable liquids and their flash points: Common liquids and their flash points Liquid Intended Use butane gasoline acetone toluene rubbing alcohol turpentine home heating oil charcoal barbeque lighter mineral spirits cigarette lighters fuelling an engine ONLY! nail polish remover cleaning solvents massage thinning paint burn in the furnace only ignite barbeque coals thinning paint and cleaning brushes Flash point o C -88 -42 -18 4 15 35 45 70 70 Use the chart to answer these questions: Butane has a very low flash point. Why is this property ideal for its intended use? A small spark from the lighter flint will cause butane to ignite immediately. Why is it dangerous to use nail-polish remover in a poorly ventilated room? Because it has such a low flash point, any spark or flame could ignite the acetone in nail polish remover. Which is the greater fire hazard, a spill of gasoline or a spill of barbeque lighter? Explain. Gasoline is a far greater hazard because its flash point is much lower than that of barbeque lighter. Which is the safest to use to clean paintbrushes, turpentine or mineral spirits? Explain. Mineral spirits is much safer to use because its flash point is higher than that of turpentine, implying that it would not ignite as readily. 77 Lesson 2.4 Special Materials Time Suggested: 140 min EXPECTATION CODES SIS.01 EXPECTATIONS SIS.03 demonstrate the skills required to plan and carry out investigations using laboratory equipment safely, effectively, and accurately (e.g., plan and carry out an investigation to determine the percentage composition of a compound) MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MSV.03 describe practices that promote fire safety, as well as safety in the handling and disposal of materials, in everyday living in the home and workplace. MS1.01 categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS1.03 describe factors that affect the rate of chemical reaction, paying special attention to what makes reactions dangerous (e.g., increasing the temperature at which a reaction takes place can cause an explosion volatile liquids and dispersed powders have a greater rate of reaction) MS1.06 describe the factors that increase the danger of flammable substances (e.g., flash point, auto-ignition) MS2.01 formulate scientific questions, in qualitative terms, about rates of chemical reaction (e.g., How do the rates of combustion of some fuels in air differ? What happens to the rates of combustion of fuels in pure oxygen or when mixed with a solid oxidant?) MS2.03 plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) 78 BOTTOM LINE Students look at two specific hazardous materials in research and experiment: gasoline and hydrogen, to summarize the study of the nature of danger from flammables. MATERIALS TEACHER STUDENT Gasoline Do's and Don'ts A Real Explosion! Test tube Test tube rack Test tube stopper Microspatula size piece of zinc metal 15mL of 1 mol/L hydrochloric acid Wooden splint Matches SAFETY CONCERNS See experiment procedure. Review safety rules for handling chemicals. LESSON SEQUENCE A product we all deal with but many disregard it's hazardous nature is gasoline. In completing the "Gasoline Do's and Don'ts" worksheet, students will both have an opportunity to review safe handling techniques and apply the technical aspects of flammable materials discussed in lesson 2.3 As flammability and combustibility increases, what is the next step? Explosive. It is not only the material type that controls this change in danger level but also the external conditions. In a safe set of conditions, students will experiment with "A Real Explosion" in producing and detecting hydrogen gas. Link this back to the reading exercise in lesson 2.2. ASSESSMENT TOOLS AND STRATEGIES Completion of the gasoline worksheet can be assessed for Making Connections A Real Explosion observation table can be assessed for Inquiry ACCOMMODATIONS Provide sample responses to worksheet and match to best procedure. EXTENSIONS Create instructional material for safe gasoline handling: poster, brochure, labels, etc. Research special handling procedures for other familiar products, for example propane containers. 79 BACKGROUND INFORMATION Hydrogen is Greek for "water-father." A French chemist, Antoine Lavoisier named it hydrogen. Hydrogen is used in high temperatures for cutting, melting, and welding metals. It is also used in rocket fuel, production of hydrochloric acid, and hydrogenation of fats and oils. Hydrogen is the lightest and most abundant element, making up about 90% of the universe. It is a reactive, colorless, odourless, and tasteless gaseous element. Hydrogen in the form of water (H2 O) is needed for everyday life and is found in almost all organic compounds. The Sun and the stars consist mostly of hydrogen. Pockets of pure hydrogen gas can collect in underground mines and can cause explosions. The most common method of producing hydrogen is through steam reforming natural gas. Another method is electrolysis, where electricity is used to split water (H2 O) into its component elements, hydrogen and oxygen. HELPFUL HINTS The NASA Explores source has an extension to the experiment where the teacher fills a balloon with hydrogen and explodes it using a lit candle. Attach the balloon to the end of a metre stick with tape. Warn your students that the upcoming “bang” may be quite loud. RESOURCES Gasoline Do's and Don'ts A Real Explosion! 80 Gasoline Do’s and Don’ts Gasoline has an extremely low flash point that makes it ideal for it's only use - to fuel an engine. Here are some tips on using gasoline safely. Use the information in the section on flammable materials to explain these rules. Gasoline Safety Tip Always fuel gas-powered tools like lawn mowers outside. Why? Never fuel a lawn mower when the engine is still hot. Gasoline should always be stored in a tightly sealed container and in a wellventilated area. Never keep gasoline in the basement Gasoline is usually cold when it comes out of a service station fuel tank. Never fill your gas container right to top. Never use gasoline to remove grease from engine parts. 81 Gasoline Do’s and Don’ts (Teacher Copy with answers) Gasoline has an extremely low flash point that makes it ideal for it's only use - to fuel an engine. Here are some tips on using gasoline safely. Use the information in the section on flammable materials to explain these rules. Gasoline Safety Tip Always fuel gas-powered tools like lawn mowers outside. Why? This ensures there is adequate ventilation to disperse the vapours. Never fuel a lawn mower when the engine is still hot. Gasoline vapours could easily ignite. Gasoline should always be stored in a tightly sealed container and in a wellventilated area. A tightly sealed container prevents the vapours from escaping. Good ventilation allows any vapours that do escape to disperse. Never keep gasoline in the basement. There are many possible ignition sources in the basement, e.g., the pilot light in a hot water heater and sparks produced when operating power tools. Gasoline is usually cold when it comes out of a service station fuel tank. Never fill your gas container right to top. This allows room for the gasoline to expand. Otherwise, excessive pressure could build up causing either the cap to blow off or the can to rupture. Never use gasoline to remove grease from engine parts. There are other liquids that do the job just as well but have much higher flash point. 82 A Real Explosion! Seeing What Can't Be Seen: Testing for Hydrogen Materials: plastic baby bottle and rubber nipple pea-size piece of zinc metal 15mL of 1 mol/L hydrochloric acid Wooden splint Matches scissors Safety : Wear eye goggles at all times. Hydrochloric acid is severely corrosive to eyes and skin. Do not eat or breathe in chemicals Hydrogen gas is explosive. Do not have open flames or sparks near gas production. Procedure: Cut off the top half of the bottle nipple. Place a pea-size portion of zinc into the baby bottle. Pour 10 mL of 1 mol/L hydrochloric acid over the zinc. Immediately, screw the nipple and bottle cap back onto the bottle Place an inverted test tube over the cut nipple to collect the gas. Touch the bottom of the bottle with your fingertips. After 30 seconds, remove the test tube. The mouth of the test tube must always be pointed down! Place a burning splint into the mouth of the test tube. Observations How did the bottom of the bottle feel? What happened when the burning splint was placed near the hydrogen? The product of this reaction was water, H2 O. Where did the oxygen come from? Why do rockets contain huge tanks of hydrogen? Why did helium replace hydrogen in large air ships in 83 the 1920s and 1930s? Taken from: NASA Explores (Teacher’s copy ) Observations How did the bottom of the bottle feel? warm to the touch What happened when the burning splint was placed near the hydrogen? A loud “pop” was heard. The product of this reaction was water, H2 O. Where did the oxygen come from? The oxygen was supplied by the air. Why do rockets contain huge tanks of hydrogen? Hydrogen is an ideal fuel for rockets because it is lightweight and produces a great deal of energy. Why did helium replace hydrogen in large air ships in the 1920s and 1930s? Helium does not burn. Taken from: NASA Explores 84 Lesson 3.1 Dressed to Live Time Suggested: 70 min EXPECTATION CODES EXPECTATIONS SIS.04 demonstrate a knowledge of emergency laboratory procedures MSV.01 demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home demonstrate an understanding of important safety legislation (e.g., WHMIS legislation, the Fire Code, the Building Code, the Occupational Health and Safety Act) MS1.02 MS1.08 demonstrate an understanding of the toxicity and hazards of some chemical substances (e.g., mercury) MS1.09 describe routes of entry of hazardous materials into the body (e.g., ingestion, inhalation, absorption through the skin) explain the meaning of the terms acute and chronic as they apply to the effect of hazardous materials on the body. MS1.10 MS2.02 demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) BOTTOM LINE With the aid of a simple demonstration and visual props, students will identify how hazardous materials may enter the body and how to avoid the risk with appropriate personal protection equipment. MATERIALS TEACHER Safety goggles STUDENT How do hazardous materials enter the body? Protective gloves Lab coat/overalls Respirator/mask Hearing protectors Safety boots Onion Knife Coloured card/labels SAFETY CONCERNS Allow sensitive students to stay distant from the demonstration 85 LESSON SEQUENCE Demo: peel and cut an onion. Prompt discussion: Why do my eyes water? What if I wiped my eyes? Can you smell it? How is the irritating onion juice getting into my body? What if this wasn't a simple onion? Link to lab safety rules: no food, wash hands, wear gloves, etc. Student activity on outline of person or whole class using lab skeleton/mannequin: use coloured flags to identify routes into body (eye, mouth, nose, skin) with technical vocabulary (absorption, ingestion, inhalation) and words to journal lists. How can we protect ourselves? Add onto figure the personal protective equipment (PPE) available. If using the skeleton dress it in the PPE. Extend to all possible, including physical dangers (work boots, hearing protectors) Students independently complete "How do hazardous materials enter the body." ASSESSMENT TOOLS AND STRATEGIES Completion of the worksheets can be assessed for knowledge ACCOMMODATIONS Use workplace video resources to make the assignment more relevant. EXTENSIONS Use a safety equipment supplier catalogue to make a summary list of the range and types of PPE available. BACKGROUND INFORMATION See the information cards in Live Safe! Work Smart! HELPFUL HINTS This is intended to bring humour into a very serious topic. If a student volunteer is willing, "dress" them instead of the skeleton. Make sure at the closure, students appreciate the underlying important message: toxins are often invisible and protection is available. RESOURCES Live Safe! Work Smart! 86 How Do Hazardous Materials Enter the Body Many common household materials like soap and bleach can be toxic under certain conditions. Even ordinary table salt can be toxic if there is too much of it in our blood. SECTION 6 - TOXICOLOGICAL PROPERTIES of the MSDS contains health hazard information and describes the possible health effects, which may result from overexposure to a specific material. Route of Entry This part of Section 6 describes the ways in which hazardous materials can enter the body. This is important so we know how best to protect ourselves from exposure. The three routes of entry into the body are: ingestion, skin or eye absorption, and inhalation. Ingestion - means taking the material into the body by mouth (swallowing). Describe how a worker could accidentally ingest a toxic material. _________________________________________________________________ _________________________________________________________________ Describe two ways in which type this of hazard can be avoided. _________________________________________________________________ _________________________________________________________________ Absorption - Some chemicals may contact the eyes and the skin and are either absorbed into the body or cause local burns or irritations. A very common example of this irritation is the substance that irritates your eyes when you chop onions. Chopping onions, for example, causes certain chemicals in the onion to undergo a chemical change, producing a vapour that irritates the eye. Also, many organic chemicals are absorbed through the skin and the mucosal membranes, e.g., pesticides. Describe two ways in which type this of hazard can be avoided. _________________________________________________________________ _________________________________________________________________ Inhalation – This is probably the most common way that hazardous materials enter the body. Inhalation means taking the material into the body by breathing it in. Very tiny blood vessels in the lungs are in constant contact with the air you inhale. Airborne contaminants can be easily absorbed through this tissue. Airborne contaminants could be solids (dusts), liquids ( mists), and gases ( or vapours) 87 List two solids that could be inhalation hazards. Solid Workplace ( e.g., restaurant kitchen) List two liquids that could be inhalation hazards. Liquid Workplace ( e.g., restaurant kitchen) List two gases that could be inhalation hazards. Gas Workplace ( e.g., restaurant kitchen) Describe two precautions that could protect the worker from inhalation hazards. ________________________________________________________________ ________________________________________________________________ 88 How Do Hazardous Materials Enter the Body (Teacher copy) Many common household materials like soap and bleach can be toxic under certain conditions. Even ordinary table salt can be toxic if there is too much of it in our blood. SECTION 6 - TOXICOLOGICAL PROPERTIES of the MSDS contains health hazard information and describes the possible health effects, which may result from overexposure to a specific material. Route of Entry This part of Section 6 describes the ways in which hazardous materials can enter the body. This is important so we know how best to protect ourselves from exposure. The three routes of entry into the body are: ingestion, skin or eye absorption, and inhalation. Ingestion - means taking the material into the body by mouth (swallowing). Describe how a worker could accidentally ingest a toxic material. A toxic material could be ingested if the worker handles the material and then consumes something without washing his/her hands in the correct way. Describe two ways in which type this of hazard can be avoided. 1. Wearing gloves if required. 2. Washing hands and face using the guidelines required for the jobsite ( Absorption - Some chemicals may contact the eyes and the skin and are either absorbed into the body or cause local burns or irritations. A very common example of this irritation is the substance that irritates your eyes when you chop onions. Chopping onions, for example, causes certain chemicals in the onion to undergo a chemical change, producing a vapour that irritates the eye. Also, many organic chemicals are absorbed through the skin and the mucosal membranes, e.g., pesticides. Describe two ways in which type this of hazard can be avoided. 1. Working in a properly ventilated area. 2. Wearing appropriate personal protective equipment such as gloves, protective clothing and respirators or masks. Inhalation – This is probably the most common way that hazardous materials enter the body. Inhalation means taking the material into the body by breathing it in. Very tiny blood vessels in the lungs are in constant contact with the air you inhale. Airborne contaminants can be easily absorbed through this tissue. Airborne contaminants could be solids (dusts), liquids ( mists), and gases ( or vapours) 89 List two solids that could be inhalation hazards. Solid Workplace ( e.g., restaurant kitchen) wood dust wood shop/furniture manufacturing flour -bakeries List two liquids that could be inhalation hazards. Liquids Workplace ( e.g., restaurant kitchen) cleaning mists janitorial work hair products hair salon List two gases that could be inhalation hazards. Gas Workplace ( e.g., restaurant kitchen) volatile solvents painting vapours from fuels auto shop Describe two precautions that could protect the worker from inhalation hazards. 1. Respirator of dust mask. 2. Ensuring that the workplace ventilation system is operating efficiently. 90 Lesson 3.2 How Much is Enough? Time Suggested: 70 min EXPECTATION CODES EXPECTATIONS SIS.04 demonstrate a knowledge of emergency laboratory procedures MSV.01 demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home demonstrate an understanding of important safety legislation (e.g., WHMIS legislation, the Fire Code, the Building Code, the Occupational Health and Safety Act) MS1.02 MS1.08 demonstrate an understanding of the toxicity and hazards of some chemical substances (e.g., mercury) MS1.09 describe routes of entry of hazardous materials into the body (e.g., ingestion, inhalation, absorption through the skin) explain the meaning of the terms acute and chronic as they apply to the effect of hazardous materials on the body MS1.10 MS2.02 demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) BOTTOM LINE Students complete a worksheet to practice their understanding of LC50 and LD50- terms used on the MSDS to describe toxicity. MATERIALS TEACHER prepare worksheet STUDENT SAFETY CONCERNS no concerns LESSON SEQUENCE Display an overhead of a blank MSDS. Ask students to locate which section contains route of entry information. Provide sample MSDS’s and have students identify routes of entry. (see lesson 1.4) Arrange for a tour of a local shop or medical lab to observe the personal safety equipment and ventilation standards required by these professions. Locate the acronyms LC50 and LD50 on an MSDS and discuss what they mean. (see "What Do LD50 and LC50 Tell Us?") Some students will perhaps question the appropriateness of using lab animals in this 91 manner. Allow some time for discussion. Stress that a material with a large LD50 or LC50 value is less toxic than a material with a small value. For example, discuss these substances: varsol - LD50 >5 g/kg orl rat ; caffeine LD50 192 mg/kg or 0.192 g/kg To help students visualize these quantities pour out 5 g of water in a graduated cylinder (5 mL) and 0.19 g of any white powder (e.g., flour) into a clear plastic vial. Stress that although the amounts are quite different, both will kill 50% of laboratory rats. Provide What Do LD50 and LC50 Tell Us? Otherwise, provide samples of MSDS sheets and ask students to rank the substances in order from least to most toxic. ASSESSMENT AND EVALUATION TOOLS Completion of the worksheets can be assessed for knowledge ACCOMMODATIONS What Do LD50 and LC50 Tell Us? This worksheet requires students to be able to convert from mg to g and vice versa. Be prepared to review this conversion. Otherwise, modify the worksheet so that all units are identical. Consider decreasing the number of materials if students find the worksheet too challenging. EXTENSIONS Absorption : Research the properties of materials that absorb through the skin. Dimethyl sulfoxide DMSO is a particularly interesting one that appears to have medical applications. A search on the Internet for DMSO should provide lots of information. Research and report on the acronyms TWAEV, STEV, and CEV BACKGROUND INFORMATION Ingestion: Toxic materials may be transferred from contaminated fingers as a result of eating and drinking in a contaminated work area. Good personal hygiene and other protective measures are required to prevent ingestion. Workers should also avoid placing personal objects like pens and the tips of eyeglasses frames in their mouths. The MSDS may also provide exposure limits depending on the type of chemical. Some examples include: TWAEV Time-Weighted Average Exposure Value: The average airborne concentration of a biological or chemical agent to which a worker may be exposed in a workday or a workweek. STEV Short Term Exposure Value: - The maximum airborne concentration of a chemical or biological agent to which a worker may be exposed in any 15 minute period, provided the TWAEV is not exceeded. CEV Ceiling Exposure Value: The maximum airborne concentration of a biological or chemical agent to which a worker may be exposed at any time. 92 What Do LD50 and LC50 Tell Us? MSDS information comes in a variety of formats. For example, LD50 information is sometimes given in mg/kg and other times is in g/kg. Because of this, the safe worker must be able to read and interpret MSDS information. Here is the LD50 information taken from the MSDS sheets of these materials aspirin ( a pain reliever) benzene ( a solvent which is banned from use in schools because it is so toxic) caffeine ( a stimulant in coffee) rubbing alcohol ( massages) sucrose ( table sugar) Goop ( a hand cleaner) air freshener See if you can match the substance with its LD50 information Substance LD50 Information ORAL LD50 (rat) 4710 mg/kg 29700 mg/kg Oral rat LD50: 5045 mg/kg; skin rabbit LD50: 12.8 gm/kg LD50 (IPR-RAT)(MG/KG) - 390 LD50 (IPR-RAT)(MG/KG) - 2.9 ORAL LD50 (RATS): 19.7 G/KG 93 What Do LD50 and LC50 Tell Us? (Teacher copy with answers) This worksheet illustrates the variety of forms that MSDS information comes in. A safe worker, for example, needs to be able to distinguish between milligrams and grams when examining LD50 information. The information in the right column was taken directly from different MSDS sheets. Be prepared to assist students with the conversion of mg to grams if necessary. You will notice a lack of consistency for which case to use for mg and kg. Substance LD50 Information air freshener ORAL LD50 (rat) 4710 mg/kg sucrose 29700 mg/kg rubbing alcohol Oral rat LD50: 5045 mg/kg; skin rabbit LD50: 12.8 gm/kg aspirin LD50 (IPR-RAT)(MG/KG) - 390 benzene LD50 (IPR-RAT)(MG/KG) - 2.9 Goop ( a hand cleaner) ORAL LD50 (RATS): 19.7 G/KG 94 Lesson 4.1 Reaction Rates Time Suggested: 100 – 140 min EXPECTATION CODES EXPECTATIONS SIS.01 demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) SIS.02 select appropriate instruments and use them effectively and accurately in collecting observations and data (e.g., use a balance to accurately measure the mass of a precipitate) SIS.03 demonstrate the skills required to plan and carry out investigations using laboratory equipment safely, effectively, and accurately (e.g., plan and carry out an investigation to determine the percentage composition of a compound) SIS.07 communicate the procedures and results of investigations for specific purposes by displaying evidence and information, either in writing or using a computer, in various forms, including flow charts, tables, graphs, and laboratory reports (e.g., draw a graph of the relationship between the volume and pressure of a fixed amount of gas at constant temperature) MS1.03 describe factors that affect the rate of chemical reaction, paying special attention to what makes reactions dangerous (e.g., increasing the temperature at which a reaction takes place can cause an explosion; volatile liquids and dispersed powders have a greater rate of reaction); MS2.01 formulate scientific questions, in qualitative terms, about rates of chemical reaction (e.g., How do the rates of combustion of some fuels in air differ? What happens to the rates of combustion of fuels in pure oxygen or when mixed with a solid oxidant?); MS2.03 plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher); BOTTOM LINE Students will change simple experimental variables and observe how this affects the reaction rate. 95 MATERIALS TEACHER STUDENT Safety goggles 5 Alka Seltzer tablets plastic cup iced water tap water hot water 100 mL graduated cylinder stopwatch pestle and mortar empty film canister plastic bowl/bucket Reaction Rate Proposal Film Can Pop Off SAFETY CONCERNS The rates of reaction activity involves pressure changes. Eye protection must be worn. LESSON SEQUENCE Propose the puzzle to students that we need to know how temperature or surface area will affect the rate of a chemical reaction. Provide them with the "Reaction Rates Proposal" sheet and ask them to design a suitable experimental test. They must write a sequenced procedure, include safety considerations, and plan for a "fair test." Once the proposal is approved, they can carry out the experiment. The concept is continued in "Film Can Pop-Off", using the same equipment. ASSESSMENT TOOLS AND STRATEGIES The student lab design can be assessed for Inquiry with an appropriate rubric ACCOMMODATIONS Provide the experiment procedure. EXTENSIONS Do not provide the list of materials. Allow for independent investigation. BACKGROUND INFORMATION The reaction of Alka-seltzer and water releases a considerable volume of carbon dioxide gas. If you allow students to vary the temperature, the lid of the film canister may pop off very quickly creating a potentially dangerous situation. HELPFUL HINTS Start with the canister half-filled with water and then increase or decrease depending on 96 the pop time desired. RESOURCES Consult the rates of reaction section of any grade 12 chemistry text for more background on the factors affecting reaction rates. 97 Reaction Rates - Proposal Materials Safety goggles 4 Alka Seltzer tablets plastic cup iced water tap water hot water 100 mL graduated cylinder stopwatch pestle and mortar plastic bowl/bucket Caution: This activity involves pressure changes. Eye protection must be worn. Purpose Part 1: To see how temperature affects how fast a reaction happens Part 2: To see how surface area affects how fast a reaction happens Considerations to be answered in your proposal: List all the variables in your experiment you are going to keep the same Name one variable that you are going to change during your experiment What safety factors must be considered? Write out a step-by-step procedure for both part 1 and part 2 of the experiment and have it approved by your teacher 98 Film Can Pop-Off Task: To make the lid of the film canister pop off in not less than 10 seconds and not more than 20 seconds using only the Alka-Seltzer and water reaction. Challenge: Students have only ONE Alka-Seltzer tablet to complete all the testing and launching in this experiment. Hint: break up the tablet into smaller pieces to try the experiment more than once. Teacher notes: Suggest the students break the tablets into pieces of similar size for each of their trials. Tricks: if the can is completely full of water when the tablet is added, not enough gas is produced to pop off the lid. A can that is 25% full of water may produce too much CO2 , causing the lid to pop off too quickly. 99 Lesson 4.2 Reactivity Series Time Suggested: 70 – 110 min EXPECTATION CODES EXPECTATIONS SIS.01 demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) select appropriate instruments and use them effectively and accurately in collecting observations and data (e.g., use a balance to accurately measure the mass of a precipitate) demonstrate the skills required to plan and carry out investigations using laboratory equipment safely, effectively, and accurately (e.g., plan and carry out an investigation to determine the percentage composition of a compound) demonstrate a knowledge of emergency laboratory procedures SIS.02 SIS.03 SIS.04 MS1.05 MS2.03 predict the reactivity of metal elements with other chemical substances, using the activity series of metals (e.g., predict the reactivity of metals with acids and oxygen) plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) BOTTOM LINE Students conduct an experiment to compare the reactivity of a given set of common metals. MATERIALS TEACHER Steel wool or sandpaper STUDENT safety goggles Spot plate (minimum 5 rows, 5 columns) Dropping bottles of 0.5 mol/L solutions: Magnesium nitrate Zinc nitrate Iron (III) nitrate Copper (II) nitrate Hydrochloric acid Small strips of metals, 4 pieces each: magnesium, zinc, iron, stainless steel, copper 100 SAFETY CONCERNS This experiment is presented using microscale equipment: a method that uses very small quantities of reactants and non-glass labware. This minimizes many of the typical hazards in school laboratories: spills, breakages, cross-contamination of reactants. The equipment is of minimal expense, and chemical supply costs are reduced. Most laboratory supply companies now include micro scale kits and supplies. LESSON SEQUENCE Review safety rules for handling chemicals and experiments whilst introducing the activity. Brainstorm where metals are used, particularly those included in the experiment. Students complete the "Activity Series" experiment. Closing discussion should link their discoveries to the danger of substitutions, and the practical application of choosing metals for different purposes ASSESSMENT TOOLS AND STRATEGIES Student inquiry skills can be assessed using an appropriate inquiry rubric. ACCOMMODATIONS Reduce the number of metals and metal ion solutions used. EXTENSIONS Have students write the word and perhaps chemical equations for the reactions involved. Show video clips of the reaction of the alkali metals with water. BACKGROUND INFORMATION A metal activity series is a qualitative ranking of metals in terms of their reactivity. Metals to the left on the periodic table tend to be more reactive than those on the right. However, this is a very “rough” rule of thumb. Relate the observations of this experiment to relevant reactions your students are likely to see, e.g., corrosion of steel. HELPFUL HINTS In preparing metals for the experiment, clean all surfaces with steel wool or sandpaper before cutting into small strips. The magnesium nitrate solution does not react with any of the metals. To save time and expense, you may wish to use distilled water as a placebo. 101 Activity Series Materials safety goggles Spot plate (minimum 5 rows, 5 columns) Dropping bottles of 0.5 mol/L solutions: Magnesium nitrate Zinc nitrate Iron (III) nitrate Copper (II) nitrate Hydrochloric acid Small strips of metals, 4 pieces each: Magnesium Zinc Iron Stainless steel Copper Procedure q q q q q Wear eye protection Using the chart below, place a piece of metal strip into each well. Each 'column' is one type of metal. Following the pattern on the chart, add 5 drops of solution onto the metal strips. Each 'row' is one type of solution. Observe the appearance of the metals after the solutions are added. If you believe a chemical reaction has taken place, write "R" in the chart on the spot that matches the metal/solution combination. Dispose of the contents of the spot plate into the special waste container provided. 102 Observations Copper nitrate Iron (III) nitrate Hydrochloric acid Zinc nitrate Magnesium nitrate Magnesium Zinc Iron Stainless steel copper Magnesium Zinc Iron Stainless steel Copper Copper nitrate Copper nitrate Copper nitrate Copper nitrate Copper nitrate Magnesium Zinc Iron Stainless steel Copper Iron (III nitrate Magnesium Iron (III) nitrate Zinc Iron (III) nitrate Iron Iron (III) nitrate Stainless steel Iron (III) nitrate Copper Hydrochloric acid Magnesium Hydrochloric acid Zinc Hydrochloric acid Iron Hydrochloric acid Stainless steel Hydrochloric acid Copper Zinc nitrate Magnesium Zinc nitrate Zinc Zinc nitrate Iron Zinc nitrate Stainless steel Zinc nitrate Copper Magnesium nitrate Magnesium nitrate Magnesium nitrate Magnesium nitrate Magnesium nitrate Rank the metals in order: Most reactive Least reactive Based on this experiment, which metal could be used to build a totally rust proof car? 103 Observations (Teacher copy) R –reaction Copper nitrate NR – no reaction Magnesium Zinc Iron Stainless steel copper Magnesium Zinc Iron Stainless steel Copper R R R NR Copper nitrate Copper nitrate Copper nitrate Copper nitrate Copper nitrate Magnesium Zinc Iron Stainless steel Copper R R NR NR NR Iron (III nitrate Magnesium Iron (III) nitrate Zinc Iron (III) nitrate Iron Iron (III) nitrate Stainless steel Iron (III) nitrate Copper R R R NR NR Zinc Hydrochloric acid Iron Hydrochloric acid Stainless steel Hydrochloric acid Copper R NR NR NR NR Zinc nitrate Magnesium Zinc nitrate Zinc Zinc nitrate Iron Zinc nitrate Stainless steel Zinc nitrate Copper NR NR NR NR NR Magnesium nitrate Magnesium nitrate Magnesium nitrate Magnesium nitrate Magnesium nitrate R Iron (III) nitrate Hydrochloric acid Zinc nitrate Magnesium nitrate Hydrochloric acid Magnesium Hydrochloric acid Rank the metals in order: Most reactive magnesium zinc iron stainless steel, copper Least reactive Based on this experiment, which metal could be used to build a totally rust proof Stainless steel or copper could be used because they were the least reactive. 104 Lesson 4.3 The Air We Breathe Time Suggested: 70 min EXPECTATION CODES EXPECTATIONS SIS.01 demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) SIS.03 demonstrate the skills required to plan and carry out investigations using laboratory equipment safely, effectively, and accurately (e.g., plan and carry out an investigation to determine the percentage composition of a compound) MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MS1.04 identify some oxidizing agents by name and/or chemical formula, and describe their chemical reactivity with fuels and other oxidizable substances (e.g., write the chemical formula for oxygen gas and explain the reaction of oxygen gas with a fuel in terms of the products formed) MS1.05 predict the reactivity of metal elements with other chemical substances, using the activity series of metals (e.g., predict the reactivity of metals with acids and oxygen) MS2.01 formulate scientific questions, in qualitative terms, about rates of chemical reaction (e.g., How do the rates of combustion of some fuels in air differ? What happens to the rates of combustion of fuels in pure oxygen or when mixed with a solid oxidant?) MS2.02 demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) BOTTOM LINE Students carry out an experiment to oxidize a small ball of steel wool in a flame and then set up a 24-hour experiment to demonstrate the oxidation of steel wool in water under different conditions. 105 MATERIALS TEACHER Flame (Bunsen, candle) Safety goggles Steel wool STUDENT Steel wool (~ 15 grams) 8 test tube Tongs Safety goggles Boiled water Salt solution (50 g NaCl in 1000 mL of water) White vinegar Vegetable oil SAFETY CONCERNS See the experiment procedure. As with all experiments, it is highly recommended that the teacher practice the demonstration prior to the lesson. LESSON SEQUENCE Introduce the process of oxidation and relate it to the weakening of metal. Review the safety rules for using flame in the laboratory. Explain the two part structure of the experiment "Oxidization" before students start ASSESSMENT TOOLS AND STRATEGIES Have students self-assess their inquiry skills using an appropriate skills checklist or rubric. ACCOMMODATIONS Setup one set of test tubes 2 days prior to this class. Show students the results of the experiment immediately. EXTENSIONS Have students research and experiment with different ways in which corrosion can be prevented. BACKGROUND INFORMATION The corrosion of iron is a complicated process that is accelerated by the presence of an electrolyte like salt. Consult a grade 12 chemistry textbook for more details regarding this process. HELPFUL HINTS The reaction of iron in oxygen is available on video or CDROM from a variety of sources. Consult your AV catalogue for further details. RESOURCES Oxidization 106 Oxidization Materials Steel wool (~ 15 grams) 8 test tubes Tongs Flame (Bunsen, candle) Safety goggles Boiled water Salt solution (50 g NaCl in 1000 mL of water) White vinegar Vegetable oil Procedure Teacher demonstration Wear safety goggles throughout this experiment. q Divide the steel wool into nine equal portions. Part 1 q Take one piece of steel wool and observe and record the physical appearance. q Using tongs, hold the steel wool in an open flame. CAUTION: The small drops of molten iron can burn skin. Hold the steel wool well away from your hands and body. q Observe and record the physical appearance of what is left after oxidization has occurred. q Part 2 Student Experiment q Roll the remaining eight portions of steel wool tightly to form a ball and put into a test tube. Roll it smaller if it will not fit easily. q Half fill the test tubes with solution: 2 each of tap water, boiled water, salt water and vinegar. Label each tube with its contents. q In one test tube for each solution, pour a thin layer of vegetable on top of the liquid to make a seal and add "+ oil" to the label. q You should now have 8 test tubes as listed on the observation chart below. q After 24 hours, observe and record what has happened in each tube. 107 Observations Part 1 Appearance of the steel wool before Appearance of the steel wool after Part 2 Observations after 24 hours Tap water Boiled water Salt water Vinegar Tap water + oil Boiled water + oil Salt water + oil Vinegar + oil Adapted from Ontario Curriculum Centre 108 Lesson 5.1 Clean Up EXPECTATION EXPECTATIONS CODES Time Suggested: 70 min SIS.01 demonstrate an understanding of safe laboratory practices by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., safely disposing of hazardous solutions correctly interpreting Workplace Hazardous Materials Information System [WHMIS] symbols), and using appropriate personal protection (e.g., wearing safety goggles) SIS.05 select and use appropriate numeric, symbolic, graphical, and linguistic modes of representation to communicate scientific ideas, plans, and experimental results (e.g., present a detailed experimental report according to specified standards) SIS.06 compile and interpret data or other information gathered from print, laboratory, and electronic sources, including Internet sites, to research a topic, solve a problem, or support an opinion (e.g., research the uses of the most common products of the refining of petroleum) MSV.01 demonstrate an understanding of WHMIS legislation and general safety procedures as they apply to materials in the workplace and the home MSV.02 demonstrate safe handling, storage, and disposal procedures for a variety of materials, including some hazardous materials, in the school laboratory (e.g., safely handle solvents, oxidizing agents, acids, bases) MS1.01 categorize hazardous chemicals as flammable, as reactive, or as harmful to health MS2.02 demonstrate an understanding of WHMIS legislation by selecting and applying appropriate techniques for handling, storing, and disposing of laboratory materials (e.g., use appropriate personal protection, and demonstrate proper housekeeping and knowledge of emergency procedures, when handling chemicals in the laboratory) MS2.03 plan and carry out investigations using laboratory equipment effectively, safely, and accurately (e.g., compare the corrosive action of acids on various metals, and collect and test the hydrogen produced by this action; prepare and use a foam fire extinguisher) MS3.02 investigate and report on a topic related to the safe handling, storage, and disposal of hazardous materials, focusing on some specific examples (e.g., the hazards of disposing of chemicals and drugs in rural and urban water systems local means of disposing of hazardous materia ls hazardous materials in the home application of WHMIS in the use of materials in a local workplace). 109 BOTTOM LINE In a safe simulation, students will practice techniques for cleaning up a hazardous material spill. MATERIALS TEACHER Safety goggles Gloves Masks Lab coats/aprons STUDENT Aluminum pie plate (or similar dish) Coloured oil (motor, chain saw, etc.) Water Liquid detergent Kitty litter (or laboratory absorbent pad) Glass rod or spoon Clean Up Procedures Household Hazardous Waste SAFETY CONCERNS The use of protective gloves may be considered, more for cleanliness than because of health hazards. LESSON SEQUENCE Have waiting at each student station a pie plate containing coloured oil. Upon entry into lab, alert students there has been a spill of a caustic material. Place warning signs at doorway. Provide students with "Clean Up Procedures" and observe them in action. Consider all the hazardous materials (review lessons 1.1, 1.2, 1.3, 1.4) we encounter daily. What principles should be applied for the storage and disposal of these products? Use the information contained in "Household Hazardous Waste" sheets for students to create instructions for safe use of at least one product. Use scenarios common in everyday life: changing oil in the car, finishing a painting job, using a darkroom. Have students brainstorm what do we tend to do? What should we do? ASSESSMENT TOOLS AND STRATEGIES Observation of choices and lab skills during experiment ACCOMMODATIONS If students may overreact to the spill scenario, be specific that this is a simulation using safe oil. EXTENSIONS The DEQ Oregon site has tables for products typically found in workshops, gardens, etc. Students could extend their summary activity to other areas using this resource. BACKGROUND INFORMATION The website of the Oregon Department of Environmental Quality contains a great deal of useful information for dealing with household hazardous wastes. HELPFUL HINTS You may wish to have students conduct a hazardous household chemical inventory to see 110 what items are most common. Ask students to inquire about the location of the nearest Household Hazardous Waste disposal centres in their neighbourhood. RESOURCES Clean Up Procedures 111 Clean up Procedures Emergency - WHMIS Class D2 and E Material has spilled in the lab. You are being observed as waste control officers and your performance evaluated Step 1: Personal Safety Put on appropriate PPE for dealing with a Class E material. Recommend any other safety precautions you think necessary: The spill has occurred at your workspace and is fortunately contained on the dish there. This sheet guides you through three types of clean up methods. Today you are going to model each one in sequence. Usually you would choose just the most efficient for the material and situation. Step 2: Dilution There are products that become less hazardous when diluted with water. Apply this clean up method to the chemical. Stir the solutions to increase mixing. Do not allow any material to spill. Describe the appearance of the mixture: Step 3: Neutralization We can change the chemical properties of a hazardous material by reacting it with something else, where the products are less dangerous. Apply this clean up method to the diluted chemical by adding liquid detergent. Do not allow any material to spill. Describe the appearance of the mixture: Step 3: Absorption Collecting up a liquid is tricky, so this final clean up method uses an absorbent solid material to soak up the chemical. Apply this method to the neutralized mixture by adding kitty litter or placing the absorbent pad into the liquid. How long does it take for all the liquid to be absorbed? The chemical has now been properly contained and the solid waste can be swept or dropped into the garbage can. Congratulations - you have succeeded in making the area safe. 112 Household Hazardous Waste Why Is It A Problem? Many products found in your home can pose a health or environmental hazard if you don't dispose of them properly. Anything labelled as toxic, flammable, corrosive, reactive, infectious or radioactive can threaten family health and safety. According to national estimates, each home contains 3 - 8 gallons of hazardous materials in kitchens, bathrooms, garages and basements. Throwing these materials into the garbage can result in sanitation workers who may be injured by fires or explosions or poisoned by acids. Hazardous wastes that reach our landfills can leach into the soil, polluting water and threatening all living things. Substances poured into household drains and toilets enter into the sewage treatment process, eventually impacting fish and wildlife. Substances poured on soil or streets or into storm drains are carried to our streams. As little as one pint of solvent can cause measurable fish kills. 113 How To Minimize Hazardous Waste In Your Home q q q q Use safer alternatives. Read labels before purchasing. Watch for the words "caution," "warning," and "danger." Follow label directions. Buy only what you need and will use up. If you do have products left over, give them to friends, neighbours, or charitable institutions to use up. Handle Hazardous Waste The Recommended Way Until you use up or safely dispose of these materials, you can q Keep containers upright, tightly closed, with labels intact. q Keep unused portions and empty containers (check labels to see if an empty container can be triple-rinsed and safely discarded in your household garbage.) q Never mix substances or pour into other containers. q Avoid burning or reusing empty containers. q Keep out of reach of children, pets, and wildlife. If Something Spills ... Your first concern must be for your own safety. If you have been exposed to toxic materials, call your local Poison Control Centre. For medical emergencies or large spills, call 911 or your fire department. q q q q q q q Read the product label for exposure and spill information. Keep the area well ventilated. Keep children and pets away. Wear gloves and protective clothing. Contain and cover the spill with absorbent material such as cat litter, clay, or sand. Sweep and scoop the material into a container with a lid or doubled plastic bags. Secure well. Finally, wash the surface well with soap and water. 114 Product Aerosols Batteries: Household (mercury, cadmium, lithium, silver, lead) Bleach: Chlorine Disposal Suggestions Best: Put only empty cans in trash. 2nd Best: Take full or partially full cans to HHW collection site. Best: Take to HHW collection site. Substitutions and Precautions Instead: Use non-aerosol products. Safe Use: Store in cool place. Do not burn or put in trash compactor. Best: Use up/give away. 2nd Best: Flush small amounts down drain with plenty of water. Take large amounts to HHW collection site. Instead: Use ½cup borax per washe r load, or use hydrogen peroxide in a 3% solution. Safe Use: Never mix bleach with strong acids such as toilet bowl cleaner The combination produces hazardous fumes. Instead: Use non-toxic alternatives Safe Use: Liquid dishwashing detergent is mildest, laundry detergent is moderate, automatic dishwasher detergent is harshest. Use mildest product for your needs. Instead: Use non-toxic alternatives. Safe Use: Do not pour grease down the drain. Pour boiling water down drain weekly. Use plunger or plumber’s snake. Pour ½cup baking soda and ½cup vinegar down drain. Let stand 15 minutes. Pour boiling water down drain. Safe Use: Check contents of medicine chest regularly. Old medications may lose their effectiveness, but not their toxicity. Instead: Use non-toxic alternatives. Safe Use: Use only in wellventilated area Detergent Cleaners Best: Use up/give away. 2nd Best: Dilute and wash down sink or take to HHW collection site. Drain Cleaners Best: Use up/give away. Put empty container in trash. 2nd Best: Dilute small amounts and wash down sink or take to HHW collection site. Medicines: Unneeded or expired Best: Take to HHW collection site 2nd Best: Flush down drain Best: Use up/give away 2nd Best: Take to HHW collection site 3rd Best: Expose to air to evaporate solvents, then put in garbage. Best: Use up/give away. 2nd Best: Take to HHW collection site. Metal Polishes Mildew Remover Instead: Use rechargeable batteries. Avoid battery-operated products. Instead: Scrub with a mixture of vinegar and salt 115 Mothballs Best: Use up/give away. 2nd Best: Take to HHW collection site. Oven Cleaner Best: Use up/give away 2nd Best: Flush with lots of water Toilet Bowl Cleaner Best: Use up/give away. 2nd Best: Take to HHW collection site. Best: Use up/give away. 2nd Best: Take to HHW collection site. Window Cleaner Wood Cleaners, Polishes and Waxes, Best: Use up/give away 2nd Best: Take to HHW collection site. Instead: Before storing clean articles, double wrap in tightly sealed plastic bags or in tight container (such as a cedar chest). Safe Use: Don’t use in living areas. Air out clothing before use Instead: Use a non- chlorinated scouring powder, pumice stick or a copper or steel wool scrubbing pad. Use cleaner without lye. Safe Use: Do not use aerosols; they can explode Instead: Use a paste of borax and lemon juice and scrub with a stiff brush. Instead: Spray on solution of ½ water and ½vinegar; wipe dry with newspaper or squeegee. Safe Use: Ventilate room. Instead: Damp mop wood floors with mild vegetable oil soap. Rub black heel marks with a paste of baking soda and water. For wood furniture, apply olive or almond oil. Let stand for several hours. Polish with a soft dry Taken from: Oregon Department of Environmental Quality 116 Lesson 5.2 Off You Go! EXPECTATION CODES Time Suggested: 45-70 min EXPECTATIONS See previous activities BOTTOM LINE Summary lesson applying concepts covered in the unit. MATERIALS TEACHER none required STUDENT none required SAFETY CONCERNS none LESSON SEQUENCE Students use their course notes, including the vocabulary journal to create a concept map or flow chart of the material covered in this unit. A brainstorming session of "What did we do?" may be useful first. With their knowledge of WHMIS legislation, students write a set of questions they should ask an employer before accepting or starting work. Review the employee (student) role and rights under WHMIS laws. Using research media, students look at accidents that have occurred involving hazardous materials and analyze them in the context of cause and prevention. Students write and carry out a safety inspection for the laboratory, their workplace or their home, including recommendations for improvement. ASSESSMENT TOOLS AND STRATEGIES The strategy for assessment here is to emphasize how this unit relates to the students' lives. Their understanding and recall of content is measured as they apply it to situations of importance to them ACCOMMODATIONS Have a copy of class notes from a well-organized student available as reference HELPFUL HINTS The concept summary map needs to be carried out by all students, otherwise there can be a measure of choice and selection for the remaining tasks. A good opportunity to have groups present to the class their individual applications. RESOURCES Internet, newspapers, magazines, etc. "Live Safe, Work Smart!" contains examples of interview questions, surveys, etc. 117 References DND ( Department of National Defense) General Safety Program -lots of good general information about WHMIS and workplace safety issues http://www.vcds.dnd.ca/dsafeg/pubs/vol4/intro_e.asp Fire Safety for Texans -an excellent compilation of background information and worksheets designed specifically for grade 8 students http://www.tdi.state.tx.us/general/download/fmcurrguide8.doc Health Canada -MSDS sheets as well as general WHMIS information http://www.hc-sc.gc.ca/ehp/ehd/psb/whmis/msds.htm Live Safe! Work Smart. Health and Safety Resources for Ontario Secondary School Teachers. Ontario Ministry of Labour -an excellent collection of safety resources and classroom ready materials. A must have for SNC 3E. A copy of this document should be in each secondary school. Consult your science or tech department head. Other copies are available from: Government of Ontario and Canadian Centre for Occupational Health and Safety Ontario Ministry of Labour, 2000 Binder Format (also available on CD-Rom for PC, Website) $Free to Ontario Secondary School teachers through their schools and boards OCC No. 1085 SCIENCE SAFETY: A Kindergarten to Senior 4 Resource Manual for Teachers, Schools, and School Divisions -excellent background material on WHMIS -good specific lesson plans and resources -a source of blank MSDS sheets http://www.edu.gov.mb.ca/metks4/docs/support/scisafe/index.html The Fairfax County (Virginia) Fire & Rescue Department -has an excellent website dealing with fire safety (especially on the use of fire extinguishers) -a downloadable PowerPoint presentation is available at http://www.ilpi.com/safety/downloads/ARESfireRevFINAL22mar01.ppt 118