Curriculum Map Advance Chemistry-Chemical Reactions
advertisement
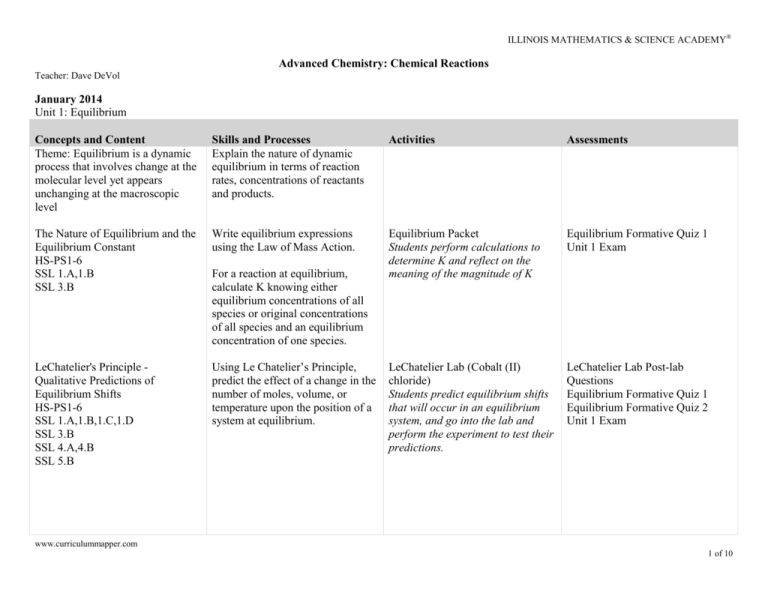
ILLINOIS MATHEMATICS & SCIENCE ACADEMY® Advanced Chemistry: Chemical Reactions Teacher: Dave DeVol January 2014 Unit 1: Equilibrium Concepts and Content Theme: Equilibrium is a dynamic process that involves change at the molecular level yet appears unchanging at the macroscopic level Skills and Processes Explain the nature of dynamic equilibrium in terms of reaction rates, concentrations of reactants and products. Activities Assessments The Nature of Equilibrium and the Equilibrium Constant HS-PS1-6 SSL 1.A,1.B SSL 3.B Write equilibrium expressions using the Law of Mass Action. Equilibrium Packet Students perform calculations to determine K and reflect on the meaning of the magnitude of K Equilibrium Formative Quiz 1 Unit 1 Exam LeChatelier's Principle Qualitative Predictions of Equilibrium Shifts HS-PS1-6 SSL 1.A,1.B,1.C,1.D SSL 3.B SSL 4.A,4.B SSL 5.B Using Le Chatelier’s Principle, predict the effect of a change in the number of moles, volume, or temperature upon the position of a system at equilibrium. LeChatelier Lab (Cobalt (II) chloride) Students predict equilibrium shifts that will occur in an equilibrium system, and go into the lab and perform the experiment to test their predictions. LeChatelier Lab Post-lab Questions Equilibrium Formative Quiz 1 Equilibrium Formative Quiz 2 Unit 1 Exam For a reaction at equilibrium, calculate K knowing either equilibrium concentrations of all species or original concentrations of all species and an equilibrium concentration of one species. www.curriculummapper.com 1 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® Concepts and Content Q and Ice Tables - Quantitative Calculations of Equilibrium Shifts HS-PS1-6 Skills and Processes Given the value of K, predict: a) the direction of a reaction after finding Q from original concentrations. b) the equilibrium concentration of one specie given those of all others. c) the equilibrium concentration of all species given their original concentrations. Activities Equilibrium Packet Students perform Q calculations and use ICE tables to find equilibrium concentrations of species. Ksp - the relationship between solubility and equilibrium constants HS-PS1-6 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Calculate Ksp knowing solubility Ksp Problems Worksheet or calculate solubility knowing Ksp Students work Ksp problems to investigate the relationship between Ksp and solubility Assessments Equilibrium Formative Quiz 2 Unit 1 Exam Ksp Lab Summary Equilibrium Formative Quiz 2 Unit 1 Exam Ksp of Copper (II) Tartrate Lab Students spectrophotometrically determine the Ksp of copper (II) tartrate. Teacher: Dave DeVol www.curriculummapper.com 2 of 10 Advanced Chemistry: Chemical Reactions DeVol ILLINOIS MATHEMATICS & SCIENCE ACADEMY® February 2014 Unit 2 - Acid-Base Chemistry and Buffers Concepts and Content Theme: Acid-base chemistry in the integration of stoichiometry and equilibrium, and is all about hydronium ions, hydroxide ions, and water. The Nature of Strong and Weak Acids and Bases HS-PS1-2 HS-PS1-3 HS-PS1-6 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Skills and Processes Given a chemical equation describing acid-base equilibria: a) Write an equation showing how a molecular or ionic substance behaves as an acid or base in water. b) Write an equation showing the dissociation of water. c) Write a net ionic equation to describe the reaction of a strong or weak acid with a strong or weak base. d) Given an equation for an acidbase reaction, select BronstedLowry acid and base, the Lewis acid and base, and the conjugate acid-base pair. Understand the concept of acid and base strength. Activities Indicators-pH Lab Activity Students use acid -base indicators to predict an approximate pH for various solutions; they then measure the pH with pH paper. Assessments Acid-base Homework Check Acid-base Quiz Unit 2 Exam pH of salts lab activity Students predict whether different salts should be acidic, basic, or neutral and then measure the pH with pH paper. Acid-base practice questions Students review fundamental acidbase concepts from SI Chem to prepare for more advanced problems. Strong acid-strong base titration lab Students titrate a strong acid (HCl) with a strong base (NaOH) and generate a titration curve using a pH electrode. Key points on the titration curve are discussed. Calculations are performed to determine the pH at critical points on the curve. Acid-base Homework Check, Acid-base Quiz Unit 2 Exam Strong and weak acid titration lab summary www.curriculummapper.com 3 of 10 DeVol Concepts and Content Calculating pH and pOH SSL 1.A SSL 3.B Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® Skills and Processes Calculate one of two quantities knowing the other: a) Ka or Kb for a weak acid/conjugate base b) [H+] , [OH-] and/or pH for acids and bases Activities Effectiveness of an antacid lab Students use a back-titration procedure in order to determine the effectiveness of antacid tablets (in mmol of acid neutralized per gram of antacid tablet). Assessments Unit 2 Exam Calculate the [H+], [OH-], and pH in a) a mixture of a strong acid and base b) a mixture of strong acid and weak base or strong base and weak acid c) a buffered solution d) a buffered solution to which strong acid or base is added Weak acid-strong base titration lab Students titrate a weak acid (acetic acid) with a strong base (NaOH) with a pH electrode, and generate a titration curve. Key points on the titration curve are discussed and compared to a strong acid-strong base titration. Calculations are performed to determine the pH at different critical points on the curve. Acid-base Homework Check, Acid-base Quiz Unit 2 Exam Buffers SSL 1.A SSL 3.B Understand what a buffer is and how it works Acid-base Titrations SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Use a pH meter or probe to find unknown [H+] and then generate a titration curve. Strong and weak acid titration lab summary Strong and weak acid titration lab summary Strong and weak acid titration lab summary, Unknown weak acid lab write-up Strong and weak acid titration lab summary, Unknown weak acid lab write-up www.curriculummapper.com 4 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® March 2014 Unit 2: Acid-Base Chemistry and Buffers (Continued) Concepts and Content Titrations SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Skills and Processes Given a titration curve of a weak acid determine: the equivalence point, pKa, and a suitable indicator. Activities Unknown weak acid lab Students perform a titration on an unknown weak acid with the goal of using the titration curve to determine the Ka and molar mass of the acid. Assessments Unknown weak acid lab write-up Unit 2 Exam Buffers SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Choose an acid-base pair and calculate concentrations of each to make a buffered solution at a given pH value. Preparation of a Buffer lab Students are presented with the challenge of creating a buffer of known pH using one of three possible buffer systems. They must choose the appropriate buffer system, create and test the buffer. Unit 2 Exam Buffer problems Students practice buffer problems applying the concepts learned in the weak acid-strong base titration and buffer lab. www.curriculummapper.com 5 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® Unit 3: Thermochemistry Concepts and Content Theme: Energy cannot be created or destroyed but can change forms; thermochemistry is the study of the conversions of energy from one form to another during chemical processes. Constant pressure and constant volume calorimetry HS-PS1-4 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Skills and Processes Be able to calculate heat flow from calorimetric data obtained from either a coffee-cup or bomb calorimeter. Given a series of thermochemical equations that occur in a stepwise fashion, use Hess’s Law to determine Delta H and calculate the magnitude of Delta H for a specific amount of reactant or product. Connecting bond energies and changes in enthalpy HS-PS1-4 SSL 3.B Given a table of bond energies (enthalpies), estimate Delta H of reaction. Entropy SSL 3.B Define entropy and explain the significance of positive and negative changes in entropy. The Laws of Thermodynamics HS-PS1-4 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Define and apply the first, second, and third laws of thermodynamics. Activities Determining the enthalpy change for a combustion reaction (lab activity) Students make the appropriate measurements and do the calculations to determine the enthalpy change for burning a candle (in kJ/mol) Assessments Thermochemistry Quiz Unit 3 Exam Thermochemistry Quiz Unit 3 Exam Introduction to thermochemistry questions Students perform basic thermochemical calculations using methane and propane as examples. Specific heat of metals lab Students perform an experiment two different ways in order to determine the specific heat of different metals and speculate on which procedure should give the best results. Unit 3 Exam Hess's law lab Students collect the necessary data and perform calculations (using Hess's Law) to determine the enthalpy change of a chemical reaction. Hess's Law Lab Write-up Unit 3 Exam Unit 3 Exam www.curriculummapper.com 6 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® April 2014 Unit 3: Thermochemistry (Continued) Concepts and Content Free Energy and Spontaneity SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B,4.C SSL 5.B Skills and Processes Use a table of calculated values to determine Delta H, Delta S, and Delta G at standard conditions. Describe how the sign of Delta H, Delta S, and Delta G relate to the spontaneity of a reaction. Use the Gibbs-Helmholtz equation to calculate Delta H, Delta S, Delta G, or T given three of the four variables. Predict the spontaneity of precipitation reactions two ways: a. Using solubility rules b. Using free energy change Activities Free energy study guide Students work through a packet that relates free energy to entropy, enthalpy, temperature, spontaneity, and the equilibrium constant. Assessments Unit 3 Exam Unit 3 Exam Unit 3 Exam Free energy lab Students calculate the free energy change for a series of potential chemical reactions and use their calculations to predict if a reaction will occur. They then perform the experiment to determine if their predictions are correct. Free Energy lab Summary www.curriculummapper.com 7 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® Unit 4: Electrochemistry Concepts and Content Theme: Electrochemistry is the study of reactions in which electrons are transferred, and can range from the obvious (batteries) to the not so obvious ( burning a log) Oxidation-Reduction Reactions and galvanic cells HS-PS1-2 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Skills and Processes Be able to identify a redox reaction, assign oxidation numbers to each element in the reaction, determine what species is oxidized and reduced, identify the oxidizing and reducing agents, and properly balance the equation in acid or base solutions. Carry out a redox titration in order to determine an equivalence point . Given a diagram of a voltaic or electrolytic cell, be able to determine the net balanced redox reaction, identify the anode and cathode, determine the direction the ions and electrons move, and the standard potential of the cell. Properly use a table of standard reduction potentials to determine relative strengths of oxidizing and reducing agents, cell voltages, and spontaneity. Activities Creating a Galvanic cell - Lab activity Students perform an open-ended activity where they create a galvanic cell and measure the voltage given appropriate materials. Assessments Unit 4 Exam Electrochemical Concepts practice sheet Students practice the basic concepts of galvanic cells including the anode, cathode, direction of Unit 4 Exam electron, cation, and anion flow, and where reduction and oxidation occur. Oxidation Numbers and Balancing Redox Practice Students practice assigning oxidation numbers and balancing redox reactions in acid and base. Oxidizing Agent Lab Summary Unit 4 Exam Ranking Oxidizing Agents - Lab activity Students use the reactivity of metals in different solutions to rank oxidizing agents (generate a metal activity ranking). www.curriculummapper.com 8 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® A redox titration in microscale Lab activity Students perform a microscale titration using a redox reaction to determine the concentration of Iron (II) in a solution. www.curriculummapper.com 9 of 10 DeVol Advanced Chemistry: Chemical Reactions ILLINOIS MATHEMATICS & SCIENCE ACADEMY® May 2014 Unit 4: Electrochemistry (Continued) Concepts and Content The Nernst Equation HS-PS1-5 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Skills and Processes Use the Nernst equation to calculate how changes in concentration will affect the voltage of a cell. Activities Assessments The Nernst Equation - Lab activity Unit 4 Exam Students construct a galvanic cell Nernst Lab Summary and change solution concentrations to investigate how changing concentration affects voltage. Electrolysis HS-PS1-2 HS-PS1-7 SSL 1.A,1.B,1.C,1.D SSL 3.A,3.B SSL 4.A,4.B SSL 5.B Relate the number of electrons or coulombs passing through an electrolytic cell to the amounts of products formed at the electrodes. Electrolysis Pre-lab activity Students perform calculations to prepare for the Electrolysis lab. Free energy and cell voltage SSL 3.B Relate cell voltage to Delta G and cell spontaneity Application of a redox reaction to nutritional chemistry SSL 1.A,1.B,1.C,1.D SSL 3.B SSL 4.A SSL 5.B Electrolysis Lab Students construct an electrolytic cell and perform an experiment to compare the moles of metal lost at the anode to the moles of hydrogen gas formed at the cathode; the concept of the Faraday is explored. Vitamin C titration Lab Students end the year of Ad Chem by utilizing a redox reaction to determine the concentration of Vitamin C in various beverages (Hi-C, Orange Juice, Orange Gatorade are examples). Unit 4 Exam Electrolysis Lab Summary Unit 4 Exam Note: Most Content, Skills and Processes that are assessed on unit exams are also assessed on the semester final exam. www.curriculummapper.com 10 of 10